b University of Chinese Academy of Sciences, Beijing 100049, China
During the last two decades, organic photovoltaics and field- effect transistors based on conjugated π-functional materials have attracted considerable attention due to their potential advantages of easy fabrication, low cost, light weight, and high flexibility [1]. For bulky-heterojunction organic photovoltaics (OPVs), rational design of donor materials in the active layer is recognized as the most effective way to push up power conversion efficiency (PCE). Generally speaking, the ideal donor material should have a suitable optical bandgap with a wide absorption in the visible and nearinfrared region, the suitable highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels, a coplanar molecular structure with proper solubilizing substituents, which in combination can lead to a high open-circuit voltage (Voc) [2], short-circuit current (Jsc), and fill factor (FF) [3, 4]. Meanwhile, organic semiconductors also require suitable frontier orbital energy levels and proper packing mode for the realization of high-πerformance ambient-stable OFETs [5].
The regiochemistry of conjugated molecules have been confirmed to be crucial in the development of organic π-functional materials. As regioregular polythiophenes, poly(3-alkylthiophene) (P3HT) as the most typical example, showed highly ordered thin- film morphology, people started to notice the important influence of regiochemistry on the performance of electronic devices about 25 years ago [6]. Indeed, regioregular P3HT exhibited better performance both in OPVs and OFETs than that of regiorandom counterpart [7]. Recently, it has been found that the introduction of some unsymmetrical conjugated building blocks into conjugated backbones may significantly improve the device performance [8]. Especially, the unsymmetrical electron-deficiency moieties could effectively regulate HOMO and LUMO energy levels and packing modes in thin films. In this short review, we will summarize some of the recent applications of unsymmetrical electron-deficiency moieties and the positive influence of structural regioregularity on OPV and OFET devices.
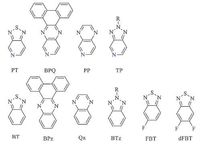
2. Unsymmetrical moieties related to nitrogenFor organic solar cells, the proposed “ideal” conjugated donor materials should exhibit a low HOMO energy level of -5.4 eV and a small bandgap of 1.5eV [9] in order to better match the most commonly used electron acceptors, fullerene derivatives. Nitrogen atom has been frequently utilized to replace carbon atom of electron-deficiency moieties to optimize the photoelectric properties, which may help establish a comprehensive understanding on the structure-πroperty relationships. By replacing the benzene ring of 2, 1, 3-benzothiadiazole (BT), dibenzo[a, c]phenazine (BPz), quinoxaline (Qx), and 2H-benzo[d][1, 2, 3]triazole (BTz) with pyridine, [1, 2, 5]thiadiazolo[3, 4-cpyridine (PT), dibenzo[f, h]pyrido [3, 4-bquinoxaline (BPQ), pyrido[3, 4-bpyrazine (PP), and 2H- [1, 2, 3]triazolo[4, 5-cpyridine (TP) (Fig. 1) become more electron- deficiency moieties and can be utilized for the design of low- bandgap (LBG) materials with low HOMO energy level [8]. Compared to their benzene counterparts, those pyridine-based materials normally showed better PCEs. For instance, the PCE of 1b [10] was significantly higher than that of 1a, 6.7% vs. 0.18% [11], which can be attributed to its wider absorption band, better crystallinity, and higher carrier mobility. Similarly, compound 2b showed a higher PCE, 3.15% than 2a, 1.73% in a planar heterojunction device: ITO/C60/donor/Di-NPB/Di-NPB:NDP9/
|
Download:
|
| Figure 1. Chemical structure of benzene- or pyridine-based electron-deficiency moieties | |
NDP9/Au [12], which was attributed to the better stacking in the solid state. This phenomenon is also observed in polymer solar cells. Quite a few PT-based polymers have delivered considerably high PCEs. Compared with 3a, 3.75%, regiorandom polymer 3b exhibited slightly higher PCE 3.93% with the same device configuration [13]. C.-G Wu and co-authors discussed the difference of polymers 4a and 4b without/with nitrogen and found that 4b had better planarity and stronger intermolecular stacking. Thus, the PCE of benzene-based polymer 4a was 2.45% which is smaller than that of pyridine-based 4b, 4.40% [14]. W. You and co-authors designed and synthesized three different PT-based polymers 5a, 5b, and 5c, and the PCEs of 6.20%, 5.57%, and 6.32% have been achieved with the typical device configuration: ITO/PEDOT:PSS/polymer:PC61BM/Ca/Al [15]. A. K.-Y. Jen and coauthors developed two PT-πolymers 6a and 6b without/with linkers. Compared with 6b, 3.41%, polymer 6a showed a relatively higher PCE of 3.91% [16]. Interestingly, W. You and co-authors developed a novel cyan-substituted TP moieties and the PCE of polymer 7 can reach as high as 8.4% with a device configuration of ITO/CuSCN/7:PC61BM/Ca/Al with 300 nm active layer. Besides, the PCE only decreased to 6.44% when the thickness was increased to 530 nm, which may be applicable for the fabrication of roll-to-roll solar cells [17]. Although these polymers had suitable optical bandgaps and HOMO/LUMO energy levels, they are not structurally perfect, i.e., regiorandom, which might be the bottleneck to further enhance the performance.
Based on the different reactivity of proximal/distal bromines (referring to nitrogen in pyridine) in microwave reaction, G.C. Bazan [18] and co-authors designed and synthesized a series of regioregular and regiorandom polymers for comparison. Compared to that of regiorandom 8b, the absorption of regioregular 8a in thin film was narrower in width, bathochromically shifted, and exhibited vibronic-like features, which was consistent with a greater degree of structural order within the polymer backbone and multichain aggregate. Grazing incidence wide-angle X-ray scattering (GIWAXS) measurements showed that 8a exhibits both face-on and edge-on orientations in the thin film. However, an amorphous hump on either the out-of-πlane or in-πlane direction could be observed for 8b, indicating that 8a was more morphologically order than 8b. As a result, the PCE of 8a was 6.13% that is much higher than that of 8b, 1.67% [19]. Regioregular polymer 9a and regiorandom polymer 9b showed similar results: polymer 9a had high PCEs of up to 6.7% and hole carrier mobilities of up to 0.2 cm2 V-1 s-1, while the PCE and hole carrier mobility of polymer 9b were just 4.5% and 0.04 cm2 V-1 s-1, respectively [20]. Differing from polymers, small- molecule materials do not have the regioregular problem. Instead, orientational isomers do exhibit. Different nitrogen orientations deeply affected the device performance. For instance, compound 1b with proximal nitrogen orientation exhibit high PCEs up to 6.70% [10], while 10a with distal nitrogen orientation had relatively low PCE 5.56%, and 10b showed the lowest PCE of 3.16% [11]. The performance difference comes from peculiar inter-crystallite ordering which might affect the local transport, rate of recombination, and so on. Besides, compounds 11 and 12 with proximal nitrogen orientation [21] and extended molecular structure exhibit a wide absorption and good π-π stacking. The PCEs of 11 and 12 were 5.8% and 6.5%, respectively, with the device configuration of ITO/MoOx/11 or 12: PC61BM/Al.
The regioregularity of conjugated polymers containing unsymmetrical electron-deficiency moieties can also significantly affect the performance of field-effect transistors. Similarly, regioregular polymers generally exhibit higher mobilities than their regioran- dom counterparts. A classic example comes from regioregular P3HT with a head-to-tail arrangement [7, 22, 23], from which higher crystallinity, red-shifted optical absorption, and larger charge carrier mobilities were observed than those ofregiorandom P3HT. In order to explore the influence of the structure-πroperty relationships related to the regiochemistry of conjugated backbones, G. C. Bazan and co-authors synthesized two regioregular polymers, 13a and 13b, and regiorandom polymer, 13c. The maximum absorption peaks of 13a, 13b, and 13c were 930, 885 and 880 nm, respectively. Compared with 13c, the larger bathochromical shift of 13a suggested the stronger intermolecular interactions. From GIWAXS examinations, polymer 13a had an overall higher population and larger correlation length of the lattice planes of π-π stacking than that of 13c. The orientation analysis showed that the regiorandom polymer, 13c, adopted mainly an edge-on orientation while the high-πerformance regioregular polymer, 13a, retained a mixed orientation character [24], which induced the different hole mobilities, 0.4 cm2 V-1 s-1 for 13a and 0.005 cm2 V-1 s-1 for 13c [18], the authors considered that a mixture of ordered lamellar stacking of edge-on and face-on orientations could be advantageous because there may exhibit three-dimensional network for charge transport. Besides, G.C. Bazan and co-authors found that the carrier mobilities of 13b were highly dependent on the molecular weight [25]. After fine tuning of the molecular weight, the mobilities of 13b can be improved from 0. 6 cm2 V-1 s-1 (15 kDa) to 23.7 cm2 V-1 s-1 (50 kDa), which is among the highest for polymer semiconductors [26] (Fig. 2, Table 1).
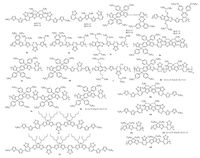
|
Download:
|
| Figure 2. Chemical structure of 1–13. | |
|
|
Table 1 Optical, electrochemical, charge transporting and photovoltaic properties of 1-13. |
3. Unsymmetrical moieties related to fluorine
Compared with nitrogen, fluorine is the weakerbut the smallest electron-withdrawing substituent. The introduction ofF atom into the conjugated backbones can lower the HOMO and LUMO energy levels, but does not cause much steric hindrance, and benefits the stability, which may improve molecular packing and charge carrier transport capability. W. You and co-authors [27] synthesized three polymers, 14a, 14b, and 14c, for OPVs with varied number of F atoms. With increasing number of F atom, the PCEs were improved with the decreased HOMO energy levels, which is mainly ascribed to the increased Voc from 0.78 V to 0.91V with comparable Jsc of about 12mAcm-2. Besides, fluorine atoms helped improve the morphology of bulky-heterojunction (BHJ) thin films. The authors found that the doubly fluorinated polymer 14c shows the greater face-on polymer crystalline orientation with improved π-π stacking and larger polymer/fullerene domains of higher purity, which possibly reduced bimolecular recombination and improved device performance. The PCEs of 14a, 14b, and 14c were 4.53%, 5.28% and 7.16%, respectively. Furthermore, the PCEs of some other mono-fluorinated polymers, 15b [28], 16b [29], and 17b [30], were unexceptionally higher than those of none-fluorinated polymers, 15a, 16a, and 17a, but lower than those of di-fluorinated polymers, 15c, 16c, and 17c. The detailed data were summarized in Table 2.
|
|
Table 2 Optical, electrochemical, charge transporting and photovoltaic properties of 14–35. |
However, for regiorandom fluorinated polymers, there were still exceptional materials with PCEs higher than those of none fluorinated or doubly fluorinated polymers. W.H.Jo and co-authors [31] designed and synthesized three fluorinated D-A polymers, 18a 18b and 18c with different number of fluorine atom, consisting of 3, 30-difluoro-2, 20-bithiophene (D) and BT (A) without/with fluorine atoms. The PCEs of mono-fluorinated polymer 18b is the highest among three materials, 9.14% with the device configuration of ITO/ZnO/PEIE/polymer:PCy1BM/Mo0°3/ Ag, which could be ascribed to its strongest tendency to take a face- on orientation when blended with PC71BM. Similarly, the PCE of mono-fluorinated polymer 19b [32], 6.16%, was higher than none fluorinated 19a 3.59%, and doubly fluorinated 19c [33], 3.37%. Compared with 19a and 19c the higher PCE and Jsc of 19b may be resulted from more suitable energy level matching PC71BM, less field dependence of free-carrier generation, and less non-geminate recombination. Besides, regiorandom mono-fluorinated polymers 20 [34], 21 [35], 22 [36] and 23 [37] all had relative high PCEs of 6.21%, 6.88%, 6.41%, and 4.29% respectively with a conventional device structure. Compared with fluorine-substituted BT moieties, fluorine-substituted TP unit was also promising for organic electronics because of the appropriate HOMO/LMUO energy level and enhanced solubility from N-alkyl substitution. Based on TP moiety, Q. Peng and co-authors [38] developed a novel kind of polymers, 24a and 24b . Polymer 24b with thiophene linkers had a relative higher PCE of 7.74% as compared with furan-containing polymer 24a, 6.25%. Moreover, Tandem solar cells with 24b blended with PC71BM as the front cell could reach high PCEs of up to 9.40%.
S. E. Watkins and co-authors [39] systematically investigated the F-substituted regiochemistry. The authors enabled the control of both the regiochemistry and the amount of nonchromophoric components and synthesized four polymers with two regiorandom and two regioregular. After device optimization, regioregular polymer 25b showed higher PCE of 5.92% than that of regiorandom 25a, just 3.91%. Another regioregular polymer 25c had the best performance with a PCE of 7.80%. For those polymers with similar absorption and HOMO and LUMO energy levels, regioregular polymers exhibit stronger intermolecular π-π stacking interaction and high ratio of face-on orientation, which may explain their higher PCEs as compared with regiorandom polymers (Fig. 3).

|
Download:
|
| Figure 3. Chemical structure of polymers 14–25. | |
Fluorinated conjugated small molecules also exhibited good device performance. J. Wang and co-authors designed and synthesized symmetrical di-fluorinated small molecules 26a [40] and compared with 26b, 26c, and 26d [41] with furan, thiophene, and selenophene π-bridge, respectively. The PCEs could be increased from just 3.4% (26a) to 8.7% (26b) under the same device structure because of the increased intensity and vibrational (Fig. 4, Table 2) feature of absorption, the balanced charge carrier mobility, and better phase domain features. Besides, Z. Wei and coauthors [42] investigated a symmetrical fluorinated oligomeric donor material 27 and the PCE could reach as high as 7.81% after using diiodohexane as additive in a conventional device structure. Similar to nitrogen, fluorine atoms in small molecules also had two basic directions to the symmetry axis of molecule: proximal and distal. Two molecules, proximal 28a and distal 28b, showed similar absorption spectra and HOMO/LUMO energy levels, however, the PCEs were completely different, 7.0% for 28a [43] and 1.3% for 28b [44] under the same device structure. The authors found no apparent indication of π-π stacking peak for 28b . It was evident that the incorporation of PC71BM prevents 28b from overcoming the kinetic barrier to crystallization, which resulted in a very poor BHJ blend and impeded achieving high PCEs. Because of the application prospect, 28a was used to study in the device engineering and the PCEs of 28a were further significantly improved. A. J. Heeger and co-authors utilized the device structure with Ba as the electron transporting layer, ITO/PEDOT:PSS/ 28a :PC71BM/Ba/Al, which resulted in high PCE of up to 8.57%

|
Download:
|
| Figure 4. Chemical structure of 26-35. | |
[45] . Moreover, using ZnO nanoparticles as electron transporting layer could further push up the PCE of up to 8.94% with a device configuration of ITO/PEDOT:PSS/28a:PC71BM/ZnO/Al [46]. H. Wu and co-authors used CH2Cl2 vapor treatment and tuned the device structure (ITO/PEDOT:PSS/28a:PC71BM/PFN/Al), the PCE of 28a could reach 8.31% [47]. By changing the Si atom in silolo[3, 2-b:4, 5- b']dithiophene of 28a to Ge, compound 29 also exhibited good performance with the PCE of 6.9% with a conventional device configuration [48] and 7.3% with a device configuration of ITO/ PEDOT:PSS/29:PC71BM/ZnO/Al [49]. A. K.-Y. Jen and co-authors [50] designed and synthesized three small-molecule donors, 30a 30b and 30c with different end groups. 30c with terthiophene showed the highest PCE of 6.54%, than those of 30b with bithiophene, 6.12%, and 30a with thieno[3, 2-bthiophene, 5.57%. J.A. Love and co-authors designed a silaindacenodithiophene- based small proximal molecule 31 with high PCE of 6.40% [51, 52]. Y. Li and co-authors [53] synthesized a mono-fluorinated proximal small molecule 32 that showed high PCE of 5.88% with a conventional device configuration. All these fluorine-substituted donor materials with a proximal configuration showed high PCEs among all organic small-molecule donor materials, which may be ascribed to strong intermolecular interaction originated from the relative strong and close π-π stacking. As a result, fiber-like morphology and suitable domain size can be formed when blended with PCBM. Besides, compound 33 with multiple fluorine substituents showed high thermal stability and hole mobility could retain 0.09 cm2 V-1 s-1 even under a high temperature of 310°C [54].
Because of deepened HOMO and LUMO energy levels, some of fluorine-substituted polymers exhibited ambipolar property in FETs. Similarly, the carrier mobility was also affected by the number of fluorine atom and the regiochemistry. Mono-fluorinat- ed regiorandom polymer 34a exhibited relative higher hole and electron mobilities, 0.21 cm2 V-1 s-1 and 0.42 cm2 V-1 s-1, than bi-fluorinated polymers 34b 0.10 cm2 V-1 s-1 and 0.30 cm2 V-1 s-1 [55]. Compared with mono-fluorinated regiorandom polymers 35a and bi-fluorinated polymers 35b regioregular polymer 35c exhibited the highest carrier mobilities. The carrier mobilities of 35a 35b and 35c were determined to be 0.4, 0.3, and 1.2 cm2 V-1 s-1, respectively [56].
4. Unsymmetrical thleno[3, 4-b]-thlophene moietiesThe application of thieno[3, 4-b-thiophene (TbT) moieties with significant quinoid-enhancing (Q) effect in the design of D-Q [57] conjugated polymers for OPV applications were firstly published by L. Yu and co-authors [4] in the year of 2010. By regioselectively synthesizing TbT-based oligomers (rr-OTbT), X. Zhu and coauthors [58] recently confirmed that the TbT moieties had a strong quinoidization effect that can regulate the optical bandgap for better performance in organic photovoltaics. rr-OTbTs are one of the newest families of oligothiophenes synthesized in the last decade. The D-Q strategy to achieve LBG-πolymers or small molecules was approved to be comparable to D-A strategy and can be utilized for the design of novel functional materials [59]. 36 (PTB7), One of the most well-known and widely used donor materials, was a typical D-Q polymer with high PCEs of up to 7.2% in the conventional device structure, ITO/PEDOT:PSS/36:PC71BM/ Ca/Al [60]. With the development of device engineering, H. Wu [61] and co-authors developed a novel electron transporting layer, PFN, to enhance the performance of 36. After optimization, the PCE of 36 with the device configuration of ITO/PFN/36:PC71BM/MoOx/ Ag, could reach as high as 9.3% with the Voc 0.75 V, Jsc 17.5 mA cm-2 and FF 70%, which were among the highest at that time. The promising applications of TbT units in organic electronics were intensively carried out since then, which resulted in a series of high-πerformance LBG-πolymers. Through fine tuning of the substituents on TbT moieties, J. Hou and co-authors [62] found polymer 37 exhibited relative high PCEs of up to 7.6% with high Jsc 17.5 mA cm-2. Besides, benzodithiophene (BDT), as another indispensable component applied in PTB7, also shows much space to regulate and control. Thus, 38 (PTB7-Th), has become one of the rare organic donor materials with high PCEs of up to 10% in singlejunction solar cells. Through the optimization of the inverted device (ITO/PFN/38:PC71BM/MoOx/Ag), H. Wu [63] and co-authors achieved the PCEs of up to 10.61% with the Voc, Jsc and FF of 0.83 V, 17.4 mA cm-2, and 73.8%, respectively. A. J. Heeger and co-authors [64] explored the practicability of applying 38 in homo-tandem solar cells and finally promoted the PCE to 11.3%. J. Hou and coauthors finely tuned the BDT moieties and designed polymers 39 (PBDT-TS1) [65] and 40 (PBT-TVT) [66]. Compared to 40, 8.13%, polymer 39 showed relative higher PCE, 9.47%. Compared with PTB7, two polymers had narrower bandgaps, deeper HOMO energy levels, and wider absorptions, which led to higher Voc and Jsc. D.-H. Hwang and co-authors [67] designed a two dimensional expanded BDT moiety and copolymerized with TbT moiety to give polymer 41. The PCE an d hole carrier mobility of 41 were 7.71% and 0.021 cm2 V-1 s-1, respectively. Besides, J. Hou and co-authors [68] inserted thiophene unit between BDT and TbT moieties. By fine tuning of the number of fluorine atom, polymer 42 with three fluorine per repeating unit exhibited the highest PCEs of up to 8.6% among those polymers with multiple fluorine atoms (Fig. 5).
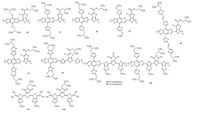
|
Download:
|
| Figure 5. Chemical structure of 36-44. | |
Unfortunately, those polymers with high performance were all regiorandom, which means that those materials still have promotion space if regioregular polymers could be synthesized and optimized. Thus, A.K.-Y. Jen and co-authors [69] synthesized three kinds of polymer 38 with different regiochemistry, 38', 38〃, and 38. They found that structural diversity strongly affects photoelectric property in both solid and solution states. The different arrangements and carrier mobilities helped elucidate the structure-πroperty relationships of high-πerformance polymers and provided new insights into rational design of superior optoelectronic materials. The performance of regiorandom 39 and regioregular 39' [70] were further explored by J. Hou and coauthors. 39' showed relative higher PCE, 10.2% than that of 39, 9.7% with higher FF, 70.6% than 68.6%, which was attributed to the stronger π-π stacking. Moreover, Y. Lee et al. [71] discussed the difference of regiorandom 37 and regioregular 37'. It is considered that 370 had effective intramolecular charge transfer, increased π-π interaction between polymer chains, which resulted in a high degree of crystallinity and thus higher PCEs, 7.8% as compared with 37, 6.6%. All these studies unambiguously indicated that the regiochemistry in TbT-based polymers has a great impact on their optical, electronic properties and thus the photovoltaic performance (Fig. 6).
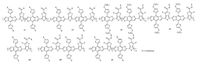
|
Download:
|
| Figure 6. Chemical structure of regioregular or regiorandom polymers 37–39. | |
There were two different orientations of S atoms, proximal or distal orientations. It is important to explore the impacts of sulfur orientations on the performance of organic electronics. X. Zhu and co-authors [72] designed and synthesized three LBG-πolymers, 43a 43b and 43c by regioselectively inserting TbT unit between BDT and thieno[3, 4-cpyrrole-4, 6-dione (TPD) units. The polymers 43a and 43c with proximal orientation blended with PCBM exhibited good phase separation and balanced hole/electron carrier mobilities. Thus, 43c exhibited high PCEs up to 7.5% with the promising Jsc up to 18.2mAcm-2 without any additional device treatments or additives. Besides, X. Zhu and co-authors [73] studied the effects of sulfur orientations in OFETs. Compound 44a and 44b were synthesized based on the regioselective functionalization of TbT moiety. 44b with the distal orientation exhibits obvious J-aggregation in solid state, good crystallinity, and "bricklayer" arrangement in crystal state, which in combination led to high electron mobilities of up to 3.0 cm2 V-1 s-1 under ambient conditions, which is among the highest for n-type organic semiconductors (Table 3).
|
|
Table 3 Optical, electrochemical, charge transporting and photovoltaic properties of 36-44. |
5. Conclusion and outlook
In this short review, we have summarized several kinds of unsymmetrical conjugated electron-deficiency moieties and their applications in high-πerformance OPVs and OFETs. Compared to homologous symmetrical moieties, because of the additional electron-withdrawing substitutions or quinoidization effect, conjugated backbones containing nitrogen- or fluorine-substituted moieties, and TbTs had relatively narrower bandgaps and wider absorptions from visible to near-infrared region, which led to better performance in most cases. For polymers, there are basically two types of configurations, regiorandom and regioregular, when introducing unsymmetrical conjugated moieties. By taking advantage of the different reactivity of H/Br on both sides, regioregular polymers can be synthesized, which usually have stronger intermolecular interaction, more ordered stacking, and thus higher PCEs in organic solar cells and higher carrier mobilities in organic field effect transistors. On the other hand, for small molecules, there exist three different orientations, proximal, distal, and syntropic, according to the symmetry axis. Through detailed investigations, we found that these compounds had the same molecular weight, similar absorption spectra, and similar HOMO and LUMO energy levels in solutions, but device performances might be disparate, which most likely comes from varied aggregation, crystallinity and morphology.
Up to date, the regiochemistry study indicates that the backbone configuration in polymers with unsymmetrical building blocks and the orientations in small molecules have great impact on their photoelectric properties and the corresponding photovoltaic and field-effect transistor performance. Organic optoelectronic materials based on unsymmetrical building blocks have been demonstrated to be important for organic electronics: Polymer 38 and small molecular 30a gave PCEs as high as 10.61% and 8.94%, respectively; Polymer 10b and small molecular 44b gave carrier mobilities as high as 23.7 cm2 V-1 s-1 for hole and 3.0 cm2 V-1 s-1 for electron, respectively. We believe that the investigation of the structure-πroperty relationship using unsymmetrical building blocks will be an extremely important research area, which needs to be considered for the design of new functional materials to achieve higher-πerformance OPVs and OFETs within a limited time.
Acknowledgments We thank the National Basic Research Program of China (973 Program, No. 2014CB643502), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB12010200), and the National Natural Science Foundation of China (No. 91333113) for financial support.| [1] | G. Li, R. Zhu, Y. Yang. Polymer solar cells. Nat. Photon. 6 (2012) 153–161. DOI:10.1038/nphoton.2012.11 |
| [2] | Q. Peng, K. Park, T. Lin, M. Durstock, L.M. Dai. Donor-π-acceptor conjugated copolymers for photovoltaic applications: tuning the open-circuit voltage by adjusting the donor/acceptor ratio. J. Phys. Chem. B 112 (2008) 2801–2808. DOI:10.1021/jp7105428 |
| [3] | J. Peet, J.Y. Kim, N.E. Coates, et al. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 6 (2007) 497–500. DOI:10.1038/nmat1928 |
| [4] | Y.Y. Liang, Z. Xu, J.B. Xia, et al. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 22 (2010) E135–E138. DOI:10.1002/adma.200903528 |
| [5] | Y. Diao, B.C.K. Tee, G. Giri, et al. Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains. Nat. Mater. 12 (2013) 665–671. DOI:10.1038/nmat3650 |
| [6] | R.D. McCullough. The chemistry of conducting polythiophenes. Adv. Mater. 10 (1998) 93–116. DOI:10.1002/(ISSN)1521-4095 |
| [7] | I. Osaka, R.D. McCullough. Advances in molecular design and synthesis of regioregular polythiophenes. Acc. Chem. Res. 41 (2008) 1202–1214. DOI:10.1021/ar800130s |
| [8] | N. Blouin, A. Michaud, D. Gendron, et al. Toward a rational design of poly(2, 7-Carbazole) derivatives for solar cells. J. Am. Chem. Soc. 130 (2008) 732–742. DOI:10.1021/ja0771989 |
| [9] | M.C. Scharber, D. Mühlbacher, M. Koppe, et al. Design rules for donors in bulkheterojunction solar cells-towards 10% energy-conversion efficiency. Adv. Mater. 18 (2006) 789–794. DOI:10.1002/(ISSN)1521-4095 |
| [10] | Y.M. Sun, G.C. Welch, W.L. Leong, et al. Solution-processed small-molecule solar cells with 6.7% efficiency. Nat. Mater. 11 (2012) 44–48. |
| [11] | C.J. Takacs, Y.M. Sun, G.C. Welch, et al. Solar cell efficiency, self-assembly, and dipole-dipole interactions of isomorphic narrow-band-gap molecules. J. Am. Chem. Soc. 134 (2012) 16597–16606. DOI:10.1021/ja3050713 |
| [12] | S. Steinberger, A. Mishra, E. Reinold, et al. Vacuum-processed small molecule solar cells based on terminal acceptor-substituted low-band gap oligothiophenes. Chem. Commun. 47 (2011) 1982–1984. DOI:10.1039/c0cc04541a |
| [13] | R.F. He, L. Yu, P. Cai, et al. Narrow-band-gap conjugated polymers based on 2,7-dioctyl-substituted dibenzo. Macromolecules 47 (2014) 2921–2928. DOI:10.1021/ma500333r |
| [14] | Y.Q. Wu, H.C. Chen, Y.S. Yang, et al. Comprehensive study of pyrido. J. Polym. Sci. Part A: Polym. Chem. 54 (2016) 1822–1833. DOI:10.1002/pola.28044 |
| [15] | H.X. Zhou, L.Q. Yang, S.C. Price, K.J. Knight, W. You. Enhanced photovoltaic performance of low-bandgap polymers with deep LUMO Levels. Angew. Chem. Int. Ed. 49 (2010) 7992–7995. DOI:10.1002/anie.v49:43 |
| [16] | Y. Sun, S.C. Chien, H.L. Yip, et al. High-mobility low-bandgap conjugated copolymers based on indacenodithiophene and thiadiazolo[3, 4-c]pyridine units for thin film transistor and photovoltaic applications. J. Mater. Chem. 21 (2011) 13247–13255. DOI:10.1039/c1jm11564b |
| [17] | W.T. Li, L. Yan, H.X. Zhou, W. You. A general approach toward electron deficient triazole units to construct conjugated polymers for solar cells. Chem. Mater. 27 (2015) 6470–6476. DOI:10.1021/acs.chemmater.5b03098 |
| [18] | L. Ying, B.B.Y. Hsu, H.M. Zhan, et al. Regioregular pyridal[2,1,3] thiadiazole π-conjugated copolymers. J. Am. Chem. Soc. 133 (2011) 18538–18541. DOI:10.1021/ja207543g |
| [19] | M. Wang, H.B. Wang, T. Yokoyama, et al. High open circuit voltage in regioregular narrow band gap polymer solar cells. J. Am. Chem. Soc. 136 (2014) 12576–12579. DOI:10.1021/ja506785w |
| [20] | W. Wen, L. Ying, B.B.Y. Hsu, et al. Regioregular pyridyl[2,1,3] thiadiazole-coindacenodithiophene conjugated polymers. Chem. Commun. 49 (2013) 7192–7194. DOI:10.1039/c3cc43229g |
| [21] | X.F. Liu, Y.M. Sun, L.A. Perez, et al. Narrow-band-gap conjugated chromophores with extended molecular lengths. J. Am. Chem. Soc. 134 (2012) 20609–20612. DOI:10.1021/ja310483w |
| [22] | Y. Kim, S. Cook, S.M. Tuladhar, et al. A strong regioregularity effect in selforganizing conjugated polymer films and high-efficiency polythiophene: fullerene solar cells. Nat. Mater 5 (2006) 197–203. DOI:10.1038/nmat1574 |
| [23] | C.H. Woo, B.C. Thompson, B.J. Kim, M.F. Toney, J.M.J. Fréchet. The influence of poly(3-hexylthiophene) regioregularity on fullerene-composite solar cell performance. J. Am. Chem. Soc. 130 (2008) 16324–16329. DOI:10.1021/ja806493n |
| [24] | L.A. Perez, P. Zalar, L. Ying, et al. Effect of backbone regioregularity on the structure and orientation of a donor-acceptor semiconducting copolymer. Macromolecules 47 (2014) 1403–1410. DOI:10.1021/ma4019679 |
| [25] | H.R. Tseng, H. Phan, C. Luo, et al. High-mobility field-effect transistors fabricated with macroscopic aligned semiconducting polymers. Adv. Mater. 26 (2014) 2993–2998. DOI:10.1002/adma.201305084 |
| [26] | C. Luo, A.K.K. Kyaw, L.A. Perez, et al. General strategy for self-assembly of highly oriented nanocrystalline semiconducting polymers with high mobility. Nano Lett. 14 (2014) 2764–2771. DOI:10.1021/nl500758w |
| [27] | A.C. Stuart, J.R. Tumbleston, H.X. Zhou, et al. Fluorine substituents reduce charge recombination and drive structure and morphology development in polymer solar cells. J. Am. Chem. Soc. 135 (2013) 1806–1815. DOI:10.1021/ja309289u |
| [28] | Z. Qiao, M. Wang, M.Z. Zhao, et al. Effect of fluorine substitution on the photovoltaic performance of poly (thiophene-quinoxaline) copolymers. Polym. Chem. 6 (2015) 8203–8213. DOI:10.1039/C5PY01193K |
| [29] | P. Verstappen, J. Kesters, W. Vanormelingen, et al. Fluorination as an effective tool to increase the open-circuit voltage and charge carrier mobility of organic solar cells based on poly(cyclopenta[2,1-b: 3, 4-b0] dithiophene-alt-quinoxaline) copolymers. J. Mater. Chem. A 3 (2015) 2960–2970. DOI:10.1039/C4TA06054G |
| [30] | P.Y. Yang, M.J. Yuan, D.F. Zeigler, et al. Influence of fluorine substituents on the film dielectric constant and open-circuit voltage in organic photovoltaics. J. Mater. Chem. C 2 (2014) 3278–3284. DOI:10.1039/C3TC32087A |
| [31] | J.W. Jo, J.W. Jung, E.H. Jung, et al. Fluorination on both D and A units in D-A type conjugated copolymers based on difluorobithiophene and benzothiadiazole for highly efficient polymer solar cells. Energy Environ. Sci. 8 (2015) 2427–2434. DOI:10.1039/C5EE00855G |
| [32] | S. Albrecht, S. Janietz, W. Schindler, et al. Fluorinated copolymer PCPDTBT with enhanced open-circuit voltage and reduced recombination for highly efficient polymer solar cells. J. Am. Chem. Soc. 134 (2012) 14932–14944. DOI:10.1021/ja305039j |
| [33] | Y.X. Li, J.Y. Zou, H.L. Yip, et al. Side-chain effect on cyclopentadithiophene/fluorobenzothiadiazole-based low band gap polymers and their applications for polymer solar cells. Macromolecules 46 (2013) 5497–5503. DOI:10.1021/ma4009302 |
| [34] | Q. Peng, X.J. Liu, D. Su, et al. Novel benzo[1,2-b:4, 5-b'] dithiophene-benzothiadiazole derivatives with variable side chains for high-performance solar cells. Adv. Mater. 23 (2011) 4554–4558. DOI:10.1002/adma.201101933 |
| [35] | G.W. Li, B.F. Zhao, C. Kang, et al. Side Chain Influence on the morphology and photovoltaic performance of 5-fluoro-6-alkyloxybenzothiadiazole and benzodithiophene based conjugated polymers. ACS Appl. Mater. Interfaces 7 (2015) 10710–10717. DOI:10.1021/acsami.5b00026 |
| [36] | G.W. Li, C. Kang, X. Gong, et al. 5-Alkyloxy-6-fluorobenzo[c][1,2,5] thiadiazoleand silafluorene-based D-A alternating conjugated polymers: synthesis and application in polymer photovoltaic cells. Macromolecules 47 (2014) 4645–4652. DOI:10.1021/ma500417r |
| [37] | J. Kim, M.H. Yun, G.H. Kim, et al. Synthesis of PCDTBT-based fluorinated polymers for high open-circuit voltage in organic photovoltaics: towards an understanding of relationships between polymer energy levels engineering and ideal morphology control. ACS Appl. Mater. Interfaces 6 (2014) 7523–7534. DOI:10.1021/am500891z |
| [38] | K. Li, Z.J. Li, K. Feng, et al. Development of large band-gap conjugated copolymers for efficient regular single and tandem organic solar cells. J. Am. Chem. Soc. 135 (2013) 13549–13557. DOI:10.1021/ja406220a |
| [39] | T.S. Qin, W. Zajaczkowski, W. Pisula, et al. Tailored donor-acceptor polymers with an A-D1-A-D2 structure: controlling intermolecular interactions to enable enhanced polymer photovoltaic devices. J. Am. Chem. Soc. 136 (2014) 6049–6055. DOI:10.1021/ja500935d |
| [40] | J.L. Wang, Q.R. Yin, J.S. Miao, et al. Rational design of small molecular donor for solution-processed organic photovoltaics with 8.1% efficiency and high fill factor via multiple fluorine substituents and thiophene bridge. Adv. Funct. Mater. 25 (2015) 3514–3523. DOI:10.1002/adfm.v25.23 |
| [41] | J.L. Wang, F. Xiao, J. Yan, et al. Difluorobenzothiadiazole-based small-molecule organic solar cells with 8.7% efficiency by tuning of π-conjugated spacers and solvent vapor annealing. Adv. Funct. Mater. 26 (2016) 1803–1812. DOI:10.1002/adfm.v26.11 |
| [42] | L. Yuan, Y.F. Zhao, J.Q. Zhang, et al. Oligomeric donor material for high-efficiency organic solar cells: breaking down a polymer. Adv. Mater. 27 (2015) 4229–4233. DOI:10.1002/adma.v27.28 |
| [43] | T.S. van der Poll, J.A. Love, T.Q. Nguyen, G.C. Bazan. Non-basic high-performance molecules for solution-processed organic solar cells. Adv. Mater. 24 (2012) 3646–3649. DOI:10.1002/adma.v24.27 |
| [44] | L.F. Lai, J.A. Love, A. Sharenko, et al. Topological considerations for the design of molecular donors with multiple absorbing units. J. Am. Chem. Soc. 136 (2014) 5591–5594. DOI:10.1021/ja501711m |
| [45] | V. Gupta, A.K.K. Kyaw, D.H. Wang, et al. Barium: an efficient cathode layer for bulk-heterojunction solar cells. Sci. Rep. 3 (2013) 1965. |
| [46] | A.K.K. Kyaw, D.H. Wang, D. Wynands, et al. Improved light harvesting and improved efficiency by insertion of an optical spacer (ZnO) in solution-processed small-molecule solar cells. Nano Lett. 13 (2013) 3796–3801. DOI:10.1021/nl401758g |
| [47] | J.S. Miao, H. Chen, F. Liu, et al. Efficiency enhancement in solution-processed organic small molecule: fullerene solar cells via solvent vapor annealing. Appl. Phys. Lett. 106 (2015) 183302. DOI:10.1063/1.4919707 |
| [48] | Y.M. Sun, J. Seifter, L.J. Huo, et al. High-performance solution-processed smallmolecule solar cells based on a dithienogermole-containing molecular donor. Adv. Energy Mater. 5 (2015) 1400987. DOI:10.1002/aenm.201400987 |
| [49] | M. Moon, B. Walker, J. Lee, et al. Dithienogermole-containing small-molecule solar cells with 7.3% efficiency: in-depth study on the effects of heteroatom substitution of Si with Ge. Adv. Energy Mater. 5 (2015) 1402044. DOI:10.1002/aenm.201402044 |
| [50] | J.J. Intemann, K. Yao, F.Z. Ding, et al. Enhanced performance of organic solar cells with increased end group dipole moment in indacenodithieno[3,2-b]thiophenebased molecules. Adv. Funct. Mater. 25 (2015) 4889–4897. DOI:10.1002/adfm.201501600 |
| [51] | J.A. Love, I. Nagao, Y. Huang, et al. Silaindacenodithiophene-based molecular donor: morphological features and use in the fabrication of compositionally tolerant, high-efficiency bulk heterojunction solar cells. J. Am. Chem. Soc. 136 (2014) 3597–3606. DOI:10.1021/ja412473p |
| [52] | J.A. Love, S.D. Collins, I. Nagao, et al. Interplay of solvent additive concentration and active layer thickness on the performance of small molecule solar cells. Adv. Mater. 26 (2014) 7308–7316. DOI:10.1002/adma.v26.43 |
| [53] | K. Wang, B. Guo, Z. Xu, et al. Solution-processable organic molecule for highperformance organic solar cells with low acceptor content. ACS Appl. Mater. Interfaces 7 (2015) 24686–24693. DOI:10.1021/acsami.5b07085 |
| [54] | X.F. Liu, B.B.Y. Hsu, Y.M. Sun, et al. High thermal stability solution-processable narrow-band gap molecular semiconductors. J. Am. Chem. Soc. 136 (2014) 16144–16147. DOI:10.1021/ja510088x |
| [55] | J. Lee, M. Jang, S.M. Lee, et al. Fluorinated benzothiadiazole (BT) groups as a powerful unit for high-performance electron-transporting polymers. ACS Appl. Mater. Interfaces 6 (2014) 20390–20399. DOI:10.1021/am505925w |
| [56] | M. Wang, M. Ford, H. Phan, et al. Fluorine substitution influence on benzo[2,1,3] thiadiazole based polymers for field-effect transistor applications. Chem. Commun. 52 (2016) 3207–3210. DOI:10.1039/C5CC10009G |
| [57] | L.T. Dou, J.B. You, Z.R. Hong, et al. 25th anniversary article: a decade of organic/polymeric photovoltaic research. Adv. Mater 25 (2013) 6642–6671. DOI:10.1002/adma.v25.46 |
| [58] | F. Liu, G.L. Espejo, S.H. Qiu, et al. Multifaceted regioregular oligo(thieno[3,4-b]thiophene)s enabled by tunable quinoidization and reduced energy band gap. J. Am. Chem. Soc. 137 (2015) 10357–10366. DOI:10.1021/jacs.5b05940 |
| [59] | Y.Y. Lina, L.P. Yu. A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 43 (2010) 1227–1236. DOI:10.1021/ar1000296 |
| [60] | H.J. Son, W. Wang, T. Xu, et al. Synthesis of fluorinated polythienothiophene-cobenzodithiophenes and effect of fluorination on the photovoltaic properties. J. Am. Chem. Soc. 133 (2011) 1885–1894. DOI:10.1021/ja108601g |
| [61] | Z.C. He, C.M. Zhong, S.J. Su, et al. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photon. 6 (2012) 591–595. |
| [62] | L.J. Huo, S.Q. Zhang, X. Guo, et al. Replacing alkoxy groups with alkylthienyl groups: a feasible approach to improve the properties of photovoltaic polymers. Angew. Chem. Int. Ed. 50 (2011) 9697–9702. DOI:10.1002/anie.201103313 |
| [63] | Z.C. He, B. Xiao, F. Liu, et al. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photon. 9 (2015) 174–179. DOI:10.1038/nphoton.2015.6 |
| [64] | H.Q. Zhou, Y. Zhang, C.K. Mai, et al. Polymer homo-tandem solar cells with best efficiency of 11.3%. Adv. Mater. 27 (2015) 1767–1773. DOI:10.1002/adma.201404220 |
| [65] | L. Ye, S.Q. Zhang, W.C. Zhao, H.F. Yao, J.H. Hou. Highly efficient 2D-conjugated benzodithiophene-based photovoltaic polymer with linear alkylthio side chain. Chem. Mater. 26 (2014) 3603–3605. DOI:10.1021/cm501513n |
| [66] | H.F. Yao, H. Zhang, L. Ye, et al. Molecular design and application of a photovoltaic polymer with improved optical properties and molecular energy levels. Macromolecules 48 (2015) 3493–3499. DOI:10.1021/acs.macromol.5b00649 |
| [67] | J.H. Kim, C.E. Song, B.S. Kim, et al. Thieno[3,2-b]thiophene-substituted benzo[1,2-b: 4, 5-b'] dithiophene as a promising building block for low bandgap semiconducting polymers for high-performance single and tandem organic photovoltaic cells. Chem. Mater. 26 (2014) 1234–1242. DOI:10.1021/cm4035903 |
| [68] | M.J. Zhang, X. Guo, S.Q. Zhang, J.H. Hou. Synergistic effect of fluorination on molecular energy level modulation in highly efficient photovoltaic polymers. Adv. Mater. 26 (2014) 1118–1123. DOI:10.1002/adma.201304427 |
| [69] | H.L. Zhong, C.Z. Li, J. Carpenter, H. Ade, A.K.Y. Jen. Influence of regio-and chemoselectivity on the properties of fluoro-substituted thienothiophene and benzodithiophene copolymers. J. Am. Chem. Soc. 137 (2015) 7616–7619. DOI:10.1021/jacs.5b04209 |
| [70] | H.F. Yao, W.C. Zhao, Z. Zheng, et al. PBDT-TSR: a highly efficient conjugated polymer for polymer solar cells with a regioregular structure. J. Mater. Chem. A 4 (2016) 1708–1713. DOI:10.1039/C5TA08614K |
| [71] | H. Kim, H. Lee, D. Seo, et al. Regioregular low bandgap polymer with controlled thieno[3,4-b]thiophene orientation for high-efficiency polymer solar cells. Chem. Mater. 27 (2015) 3102–3107. DOI:10.1021/acs.chemmater.5b00632 |
| [72] | C. Zhang, H. Li, J.Z. Wang, et al. Low-bandgap thieno[3,4-c]pyrrole-4, 6-dionepolymers for high-performance solar cells with significantly enhanced photocurrents. J. Mater. Chem. A 3 (2015) 11194–11198. DOI:10.1039/C5TA02376A |
| [73] | C. Zhang, Y.P. Zang, E. Gann, et al. Two-dimensional π-expanded quinoidal terthiophenes terminated with dicyanomethylenes as n-type semiconductors for high-performance organic thin-film transistors. J. Am. Chem. Soc. 136 (2014) 16176–16184. DOI:10.1021/ja510003y |
 2016, Vol. 27
2016, Vol. 27