Since Tang's innovative work in 1987 [1], organic light emitting diodes (OLEDs) have experienced nearly three-decades development and become one of the most promising technologies for flat- panel display and solid-state lightening sources due to the advantages of light weight, flexibility, and energy saving [2]. During the three-decade evolution of OLEDs, there are so many materials and devices that were reported. Although the diversity of materials and devices can be nearly infinite, their emission manners can be by far summarized as only three: (i) emission from the radiative transition of singlet excitons, corresponding to the fluorescent OLEDs [1]; (ii) emission from the radiative transition of triplet excitons, corresponding to the phosphorescent OLEDs [3]; (iii) emission from the radiative transition of singlet excitons converted from triplet excitons, corresponding to the OLEDs based on the mechanisms of thermally activated delayed fluorescence (TADF) [4], triplet-triplet annihilation (TTA) [5], hybridized local and charge-transfer (HLCT) emission [6] and triplet-polaron-interaction (TPI) induced upconversion [7]. It should be noted that all the light-emitting materials used in the OLEDs mentioned above are closed-shell molecules although their emission manners are different. Recently, a new kind of OLEDs using open-shell molecules such as neutral π-radicals as emitters wherein the emission comes from the radiative decay of doublet excitons was reported [8]. Because the transition back to the groud state of doublet excitons is spin-allowed, the transition problem of triplet excitons of fluorescent materials is thus circumvented.
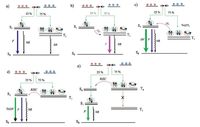
2. Summarization of all kinds of OLEDs classified by the emission mannersIt is known that 25% singlet excitons and 75% triplet excitons will be created when the injected hole and electron meet each other in the light-emitting region according to the simple spin statistics [9]. As shown in Fig. 1a, in OLED using traditional fluorescent materials as emitters, only singlet excitons can radiatively decay, and the triplets which account for 75% of the total excitons are wasted [9]. Thus the upper limit of internal quantum efficiency (IQE) which is defined as the ratio of the number of emitted photons to the number of injected charge carriers is 25%, given that the PL quantum yield of fluorescent materials is 100%.
|
Download:
|
| Figure 1. Summary of triplet and singlet transition process in OLEDs. Fluorescence process (a); phosphorescence (b); triplet–triplet annihilation (TTA) (c); thermally activated delayed fluorescence (TADF) (d); hybridized local and charge-transfer (HLCT) emission (e); F, P, DF, NR, ISC, RISC represent fluorescence (F), phosphorescence (P), delayed fluorescence (DF), non-radiation process (NR), intersystem conversion (ISC), reverse intersystem conversion (RISC). | |
To improve the IQE of OLEDs, efficient harvesting and utilizing the triplet excitons is always the most important issue in the following research of OLEDs. In 1998, the EL emission from triplet excited states of metal-to-ligand complexes made it possible to efficiently utilize triplet excitons via heavy metal effect [3a, 3b]. In OLEDs based on metal-to-ligand complexes (phosphorescent materials), 100% IQE can be achieved due to that the spin-orbit
coupling effect of heavy metals enhances the transition possibility from T1 to S0 (Fig. 1b). Thus phosphorescent OLEDs containing heavy metals can overcome the problem of IQE upper limit of the conventional fluorescent OLEDs. However, phosphorescent OLEDs have some disadvantages, e.g., room-temperature deep blue phosphorescent molecules are scarce, and the sky blue phosphorescent molecules always show instability during the vacuum evaporation and device operation [10]. Moreover, most of the useful heavy metals for phosphorescent materials are confined to iridium (Ir) and platinum (Pt), which are expensive and limited nature resources. As a result, production cost of the phosphorescent OLEDs is rather high.
In 1999, Cao et al.reported that the spin statistical limit of singlet formation ratio of 25% can be broken in the electroluminescence of some conjugated polymers [2a]. Shuai et al. proposed that the reverse intersystem crossing (RISC) can happen from triplet interchain polaron pair to singlet interchain polaron pair which are the precursors of triplet and singlet exciton, respectively, due to the strong interchain coupling [2b]. Other approaches to harvesting triplet excitons are TTA, TADF, HLCT and TPI induced upconversion that convert non-emissive triplet excitons to emissive singlet excitons. As shown in Fig. 1c, TTA is that two triplet excitons collide each other to form a ground state (S0) and a singlet exciton (S1) (2T1 > S1), which causes the π-type delayed fluorescence. Ideally, the upper limit of IQE of the devices via TTA process can be enhanced to 62.5% [11].
The first application of TADF in OLEDs was reported by Adachi et al. in 2009 [4a]. In TADF process, as shown in Fig. 1d, extremely small energy gap between Si and Ti (ΔEST) is required to benefit RISC from Ti to Si through thermal activation. In this way 75% nonemissive triplet excitons can be efficiently utilized, resulting in 100% IQE of TADF-based OLEDs. To design TADF molecules, spatial separated distribution between highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) is needed, which is matched by the donor-acceptor (D-A) type structure [4c, 12]. However, D-A architecture often leads to intramolecular charge transfer (ICT) property that is not beneficial to achieve blue TADF materials and high PL quantum yield. Another problem is that TADF-based OLEDs have serious efficiency roll off as operation voltage increases [13].
Another feasible method to harvest triplet excitons is HLCT emission (Fig. 1e). By using moderate donor (D) and accepter (A) groups and controlling the steric hindrance between D and A, a new state can be obtained in which charge transfer (CT) state and locally excited (LE) state simultaneously exist in a molecule. LE part has the large transition moment and CT part can enhance the S1 component through RISC between high-lying triplet and singlet excited states [6a, 6b, 14].
Recently, we reported a new possible way to harvest triplet excitons through TPI-induced upconversion [7]. Different from that TADF and HLCT benefit from the RISC processes at the lowest- and higher-level excited states, respectively, and the spin angular momentum is not conserved, TPI induced upconversion is a intermolecular process. It has two distinct characteristics: (i) TPI is a spin conserving process, i.e. the spin-flip does not need; (ii) TPI can occur circularly, that is TPI can continuously convert triplet to singlet, which may induce much higher singlet ratio in OLEDs.
It should be noted that all the emitters used in the OLEDs based on the mechanisms of phosphorescence, TTA, TADF, HLCT and TPI- induced upconversion are all closed-shell molecules. Last year, we demonstrated the possibility using neutral open-shell molecules as the emitter of OLEDs in which the emission comes from the doublet excitons [8].
3. Neutral π-radical-based OLEDsFor conventional luminescent materials (closed-shell molecules), their HOMO is filled with two electrons with opposite spin directions. If these molecules are excited, one electron leaps into the lowest unoccupied molecular orbital (LUMO) and the other electron leaves in the HOMO. The spin configurations of two electrons can be classified as singlet and triplet, respectively. According to Paul's exclusion principle, the transition of triplet excitons back to the ground state is spin-forbidden, only the transition of singlet excitons back to the ground state is spin- allowed and emits light.
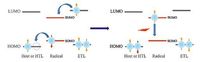
However, neutral conjugated radicals (open-shell molecules) have only one electron in the outer molecular orbital, i.e., the singly occupied molecular orbital (SOMO). The spin configuration of one electron is doublet (spin-up and spin-down). In the EL process of an OLED based on neutral conjugated radicals (as shown in Fig. 2), electron and holes are injected from cathode and anode respectively. Then holes are transmitted into the HOMO of the hole-transporting layer (HTL) or doping-host molecules, finally hop into the SOMO of the radical. And the SOMO of the radical becomes empty due to only one electron occupies the SOMO. Similarly, electrons are transmitted into the LUMO of the electrontransporting layer (ETL) or doping-host molecules, then hop into the singly unoccupied molecular orbital (SUMO) of the radical. Thus the doublet excitons at radical molecules are formed. In addition, the doublet excitons can also be created through the energy transfer from the host. The transition back of the electron in SUMO to SOMO will generate a photon. Theoretically, the transition of doublet excitons of neutral radicals is always spin- allowed due to the vacancy of SOMO.
|
Download:
|
| Figure 2. EL process of the open-shell organic molecules; HTL and ETL represent holetransporting layer and electron-transporting layer. | |
Up to date, the reported room-temperature stable emissive radicals are rare. However, rational molecular design not only endows the radicals stable enough in air and at room temperature but also makes them emissive. Most of the ambient-condition- emissive and stable radicals are concentrated on triphenylmethyl radical derivatives (for details please see Section 4).
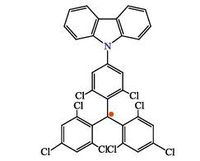
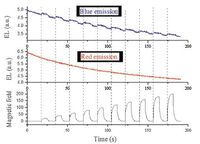
Aiming to confirm our idea, we chose a stable deeπ-redemissive radical (TTM-1Cz) whose molecular structure is given in Fig. 3 [15] as an emitter to successfully fabricate an OLED. Because TTM-1Cz compound is severely self-quenched in sold state, we dispersed it into the 4, 4'-N, N'-dicalbazolylbiphenyl (CBP) host. The configuration of the OLED is ITO/MoO3 (6 nm)/NPB (30 nm)/TTM- 1Cz: CBP (5 wt%, 40 nm)/TPBi (35 nm)/LiF (0.8 nm)/Al (100 nm). The device gives a deeπ-red emission at the lower voltages, whereas the blue component appears at the higher voltages. Maximum external quantum efficiency (EQE) of 2.4% was achieved, which is relatively high among the deep red OLEDs. To further identify whether the red emission originates from the doublet, magnetic field effect (MFE) testing was performed. In general, Zeeman Effect of the triplet is thought to be closely related with the MFE of OLEDs [16]. Thus, the existent magneto-electroluminescence (MEL) can verify the emission from singlet or triplet excitons (singlet and triplet always accompany each other). On the contrary, the non-existent MEL will confirm that the emission does not come from singlet or triplet excitons but from the doublet excitons. We used filters to separate the blue emission (from closed shell molecule) and red emission (from TTM-1Cz) and checked their MEL, respectively. As shown in Fig. 4, red emission shows no response to the magnetic field, whereas the blue emission has the obvious MEL. The result suggests that the red emission indeed comes from the doublet excitons of the radical molecule.
|
Download:
|
| Figure 3. Chemical structure of TTM-1Cz. | |
|
Download:
|
| Figure 4. The magnetic field effect measurements. | |
4. Triphenylmethyl-based radicals
The first organic free radical, triphenylmethyl radical, was discovered by Gomberg in 1900 [17]. Until now, the study of organic radicals has experienced more than a century and different kinds of radicals have been developed. Although organic radicals are generally believed to be short-lived and extremely active chemical substance, the long-lived radicals still can be obtained through proper molecular design, such as, organic nitroxides [18], (general formula R2NO ), nitronyl nitroxides (NNOs ) [19], and π-conjugated radicals [20]. Among the π-conjugated radicals, only triphenylmethyl derivatives, i.e., perchlorophenylmethyl (PTM) and tris(2, 4, 6-trichlorophenyl)methyl (TTM) derivative radicals are emissive and more stable. It was reported that the half-lives of the PTM radicals are more than 100 years [21]. Thus, we focused our attention on PTM and TTM series radicals as the emitters of OLEDs.
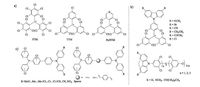
Triphenylmethyl radicals were found to hardly dissociate in solvent or solid state, and tend to dimerize a century ago [20]. The significant progress happened in 1970 when Ballester et al. successfully synthesized the first extremely stable and room- temperature-emissive PTM radical [22]. The PTM radical is monomeric even in the crystalline state [23]. The stability of the PTM radical is ascribed to essentially steric shielding effect of the tricovalent central carbon by three ortho and para-chloro- substituted phenyl rings. It is noteworthy that when ortho- and para-positions of the phenyl ring is substituted by some atoms (Cl, F, Br) or some substituent groups (OMe, OH, NO2), the stability is more increased and dimerization can also be avoided [23]. After Ballester's work on PTM radical, (Fig. 5a), numerous efforts have been focused on synthesizing the PTM and TTM radical derivatives and exploiting their physical and chemical properties [24].
|
Download:
|
| Figure 5. Moleculer structure of the radicals PTM, TTM and PyBTM (a); carbazole substituted TTM series radicals (b); PTM series chare transfer state radicals (c). | |
Since 1994, Julia and co-workers have been contributing their outstanding works on synthesizing and tuning the optical properties of PTM- and TTM-cored radical derivatives [25]. In 2006, Julia et al. introduced carbazole unit into TTM core with C-N coupling reaction in the presence of cesium carbonate and obtained a strong red-emissive carbazole-pendant radical, TTM- 1Cz [15, 26]. In the following year, they also reported series of emissive TTM-cored carbazole derivative radicals, as shown in Fig. 5b [27]. Lambert et al. have made another excellent series works on synthesizing and studying the intramolecular electron transfer PTM-cored radicals (Fig. 5c) in which PTM is linked to different substituted-amine donors by various spacers [28]. Recently Nishihara et al. reported a more stable radical, PyBTM (Fig. 5a). The photostability of PyBTM is over 115 times higher than TTM and the fluorescence quantum yield is up to 0.81 in EPA matrix (diethyl ether:isopentane:ethanol) at 77 K [29]. They also prepared a PyBTM-based metal complex radical. After PyBTM was cooperated with metal (Au) complex stability was more enhanced than PyBTM [30].
5. Conclusion and outlooksIn this review, we briefly introduced the working principles and existing problems of different emission manners of OLEDs. In the traditional fluorescent OLEDs, only 25% singlet excitons can be utilized, but in other type OLEDs, the exciton utilizing ratio approaches a unit or more than a half because of harvesting nonemissive triplet excitons. In particular, in radical-based OLEDs the transition problem of triplet excitons is circumvented because the emission comes from the radiative decay of doublet excitons which is spin-allowed. Nevertheless, there are some problems that need to be solved in radical-based OLEDs including: (i) solid state selfquenching should be overcome and PL quantum yield should be increased in term of the material designing; (ii) In order to obtain the full-color display, emission spectra of light-emitting radicals have to be tuned to green and blue region; (iii) the stability of light- emitting radicals is also crucial for devices; (iv) in term of the devices, the working principle of the radical-based OLEDs need to be further investigated, e.g., underlying energy transfer mechanism between closed-shell and open-shell molecules, relation between device structure and efficiency.
Although the study of radical-based OLEDs is staying on its young age, we believe it would give another optional choice in OLEDs development as the emergence of new kinds of light- emitting radicals.
Acknowledgments The authors are grateful for financial support from the Ministry of Science and Technology of China (No. 2015CB655003), National Natural Science Foundation of China (No. 61275036).| [1] | C.W. Tang, S. VanSlyke. Organic electroluminescent diodes. Appl. Phys. Lett. 51 (1987) 913–915. DOI:10.1063/1.98799 |
| [2] | (a) Y. Cao, I.D. Park, G. Yu, et al., Improved quantum efficiency for electroluminescence in semiconducting polymers, Nature 397(1999) 414-417; (b) Z. Shuai, D. Beljonne, R.J. Silbey, et al., Singlet and triplet exciton formation rates in conjugated polymer light-emitting diodes, Phys. Rev. Lett. 84(2000) 131-134; (c) Y.R. Sun,N.C. Giebink, H.Kanno,etal.,Managementof singlet andtriplet excitons for efficient white organic light-emitting devices, Nature 440(2006) 908-912; (d) Y.T. Tao, C.L. Yang, J.G. Qin, Organic host materials for phosphorescent organic light-emitting diodes, Chem. Soc. Rev. 40(2011) 2943-2970; (e) B.H. Zhang, G.P. Tan, C.S. Lam, et al., high-efficiency single emissive layer white organic light-emitting diodes based on solution-processed dendritic host and new orange-emitting iridium complex, Adv. Mater. 24(2012) 1873-1877; (f) M.R. Zhu, C.L. Yang, Blue fluorescent emitters: design tactics and applications in organic light-emitting diodes, Chem. Soc. Rev. 42(2013) 4963-4976; (g) T.H. Han, Y. Lee, M.R. Choi, et al., Extremely efficient flexible organic lightemitting diodes with modified graphene anode, Nat. Photonics 6(2012) 105-110; (h) M.A. Baldo, M.E. Thompson, S.R. Forrest, High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer, Nature 403(2000) 750-753; (i) J. Kido, M. Kimura, K. Nagai, Multilayer white light-emitting organic electroluminescent device, Science 267(1995) 1332-1334. |
| [3] | (a) M.A. Baldo, D.F. O'brien, Y. You, et al., Highly efficient phosphorescent emission from organic electroluminescent devices, Nature 395(1998) 151-154; (b) Y.G. Ma, H.Y. Zhang, J.C. Shen, et al., Electroluminescence from triplet metal-ligand charge-transfer excited state of transition metal complexes, Synth. Met. 94(1998) 245-248; (c) C. Adachi, M.A. Baldo, M.E. Thompson, et al., Nearly 100% internal phosphorescence efficiency in an organic light-emitting device, J. Appl. Phys. 90(2001) 5048-5051; (d) K. Li, G.S.M. Tong, Q.Y. Wan, et al., Highly phosphorescent platinum(II) emitters:photophysics, materials and biological applications, Chem. Sci. 7(2016) 1653-1673. |
| [4] | (a) A. Endo, M. Ogasawara, A. Takahashi, et al., Thermally activated delayed fluorescence from Sn4+-porphyrin complexes and their application to organic light emitting diodes a novel mechanism for electroluminescence, Adv. Mater. 21(2009) 4802-4806; (b) Q.S. Zhang, J. Li, K. Shizu, et al., Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes, J. Am. Chem. Soc. 134(2012) 14706-14709; (c) H. Uoyama, K. Goushi, K. Shizu, et al., Highly efficient organic light-emitting diodes from delayed fluorescence, Nature 492(2012) 234-238. |
| [5] | (a) J. Kido, Y. Iizumi, Fabrication of highly efficient organic electroluminescent devices, Appl. Phys. Lett. 73(1998); (b) C.J. Chiang, A. Kimyonok, M.K. Etherington, et al., Ultrahigh efficiency fluorescent single and bi-layer organic light emitting diodes: the key role of triplet fusion, Adv. Funct. Mater. 23(2013) 739-746; (c) B.H. Wallikewitz, D. Kabra, S. Gélinas, et al., Triplet dynamics in fluorescent polymer light-emitting diodes, Phys. Rev. B 85(2012) 045209. |
| [6] | (a) W.J. Li, D.D. Liu, F.Z. Shen, et al., A twisting donor-acceptor molecule with an intercrossed excited state for highly efficient, deep-blue electroluminescence, Adv. Funct. Mater. 22(2012) 2797-2803; (b) W.J. Li, Y.Y. Pan, R. Xiao, et al., Employing 100% excitons in OLEDs by utilizing a fluorescent molecule with hybridized local and charge-transfer excited state, Adv. Funct. Mater. 24(2014) 1609-1614; (c) L. Yao, S.T. Zhang, R. Wang, et al., Highly efficient near-infrared organic lightemitting diode based on a butterfly-shaped donor-acceptor chromophore with strong solid-state fluorescence and a large proportion of radiative excitons, Angew. Chem. 126(2014) 2151-2155. |
| [7] | A. Obolda, Q.M. Peng, C.Y. He, et al. Triplet-polaron-interaction-induced upconversion from triplet to singlet: a possible way to obtain highly efficient OLEDs. Adv. Mater. 28 (2016) 4740–4746. DOI:10.1002/adma.v28.23 |
| [8] | (a) F. Li, A kind of OLEDs based on the transition of doublet electron of neutral pi radicals, Chinese Patent ZL201410018393.9(01/2014). (b) QM. Peng, A. Obolda, M. Zhang, et al., Organic light-emitting diodes using a neutral π-radical as emitter: the emission from a doublet, Angew. Chem. Int. Ed. 54(2015) 7091-7095. |
| [9] | M. Pope, C.E. Swenberg. Electronic Processes in Organic Crystals and Polymers[M]. Oxford: Oxford University Press, 1999 . |
| [10] | (a) S.O. Jeon, K.S. Yook, C.W. Joo, et al., High-efficiency deep-blue-phosphorescent organic light-emitting diodes using a phosphine oxide and a phosphine sulfide high-triplet-energy host material with bipolar charge-transport properties, Adv. Mater. 22(2010) 1872-1876; (b) S. Schmidbauer, A. Hohenleutner, B. König, Chemical degradation in organic light-emitting devices: mechanisms and implications for the design of new materials, Adv. Mater. 25(2013) 2114-2129; (c) S.L. Gong, Y.H. Chen, C.L. Yang, et al., De Novo design of silicon-bridged molecule towards a bipolar host: all-phosphor white organic light-emitting devices exhibiting high efficiency and low efficiency roll-off, Adv. Mater. 22(2010) 5370-5373. |
| [11] | V. Jankus, E.W. Snedden, D.W. Bright, et al. Energy upconversion via triplet fusion in super yellow PPV films doped with palladium tetraphenyltetrabenzoporphyrin: a comprehensive investigation of exciton dynamics. Adv. Funct. Mater. 23 (2013) 384–393. DOI:10.1002/adfm.201201284 |
| [12] | (a) H. Wang, L. Xie, Q. Peng, et al., Novel thermally activated delayed fluorescence materials-thioxanthone derivatives and their applications for highly efficient OLEDs, Adv. Mater. 26(2014) 5198-5204; (b) Y. Tao, K. Yuan, T. Chen, et al., Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics, Adv. Mater. 26(2014) 7931-7958. |
| [13] | (a) K. Masui, H. Nakanotani, C. Adachi, Analysis of exciton annihilation in highefficiency sky-blue organic light-emitting diodes with thermally activated delayed fluorescence, Org. Electron. 14(2013) 2721-2726; (b) T. Komino, H. Nomura, T. Koyanagi, et al., Suppression of efficiency roll-off characteristics in thermally activated delayed fluorescence based organic lightemitting diodes using randomly oriented host molecules, Chem. Mater. 25(2013) 3038-3047. |
| [14] | (a) L. Yao, B. Yang, Y.G. Ma, Progress in next-generation organic electroluminescent materials: material design beyond exciton statistics, Sci. China Chem. 57(2014) 335-345; (b) Y.Y. Pan, W.J. Li, S.T. Zhang, et al., High yields of singlet excitons in organic electroluminescence through two paths of cold and hot excitons, Adv. Opt. Mater. 2(2014) 510-515. |
| [15] | V. Gamero, D. Velasco, S. Latorre, et al. [4-(N-Carbazolyl)-2,6-dichlorophenyl] bis (2,4,6-trichlorophenyl) methyl radical an efficient red light-emitting paramagnetic molecule. Tetrahedron Lett 47 (2006) 2305–2309. DOI:10.1016/j.tetlet.2006.02.022 |
| [16] | P. Chen, Z.H. Xiong, Q.M. Peng, et al. Magneto-electroluminescence as a tool to discern the origin of delayed fluorescence: reverse intersystem crossing or triplet-triplet annihilation?. Adv. Opt. Mater. 2 (2014) 142–148. DOI:10.1002/adom.201300422 |
| [17] | M. Gomberg. An Instance of trivalent carbon: triphenylmethyl. J. Am. Chem. Soc. 22 (1900) 757–771. DOI:10.1021/ja02049a006 |
| [18] | C.J. Hawker, A.W. Bosman, E. Harth. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 101 (2001) 3661–3688. DOI:10.1021/cr990119u |
| [19] | J. Joseph, B. Kalyanaraman, J.S. Hyde. Trapping of nitric oxide by nitronyl nitroxides: an electron spin resonance investigation. Biochem. Biophys. Res. Commun. 192 (1993) 926–934. DOI:10.1006/bbrc.1993.1504 |
| [20] | R.G. Hicks, Stable Radicals, Wiley Online Library, 2010. |
| [21] | M. Ballester. Inert free-radicals (IFR): a unique trivalent carbon species. Acc. Chem. Res. 18 (1985) 380–387. DOI:10.1021/ar00120a004 |
| [22] | (a) M. Ballester, J. Riera-Figueras, A. Rodríguez-Siurana, Synthesis and isolation of a perchlorotriphenylcarbonium salt, Tetrahedron Lett. 11(1970) 3615-3618; (b) M. Ballester, G. de la Fuente, Synthesis and isolation of a perchlorotriphenylcarbanion salt, Tetrahedron Lett. 11(1970) 4509-4510; (c) M. Ballester, J. Riera, J. Castañer, C. Badía, J.M. Monsó, Inert carbon free radicals. I. Perchlorodiphenylmethyl and Perchlorotriphenylmethyl radical series, J. Am. Chem. Soc. 93(1971) 2215-2225. |
| [23] | V.D. Sholle, E.G. Rozantsev. Advances in the chemistry of stable hydrocarbon radicals. Russ. Chem. Rev. 42 (1973) 1011–1019. DOI:10.1070/RC1973v042n12ABEH002781 |
| [24] | (a) J.M. Rawson, A. Alberola, A. Whalley, Thiazyl radicals: old materials for new molecular devices, J. Mater. Chem. 16(2006) 2560-2575; (b) T. Kurata, K. Koshika, F. Kato, et al., An unpaired electron-based hole-transporting molecule: triarylamine-combined nitroxide radicals, Chem. Commun. (2007) 2986-2988; (c) A.F. Marye, G. Elizabeth, C.C. Chia, Photochemistry of table free radicals: the photolysis of perchlorotriphenylmethyl radicals, J. Am. Chem. Soc. 109(1987) 7088-7094; (d) I. Ratera, C. Sporer, M.D. Ruiz, et al., Solvent tuning from normal to inverted marcus region of intramolecular electron transfer in ferrocene-based organic radicals, J. Am. Chem. Soc. 129(2007) 6117-6129; (e) V. Lloveras, J. Vidal-Gancedo, T.M. Figueira-Duarte, et al., Tunneling versus hopping in mixed-valence oligo-p-phenylenevinylene polychlorinated bis(triphenylmethyl) radical anions, J. Am. Chem. Soc. 133(2011) 5818-5833; (f) F. Vera, M. Mas-Torrent, J. Esquena, et al., Microstructured objects produced by the supramolecular hierarchical assembly of an organic free radical gathering hydrophobic-amphiphilic characteristics, Chem. Sci. 3(2012) 1958-1962; (g) J. Guasch, L. Grisanti, M. Souto, et al., Intra- and intermolecular charge transfer in aggregates of tetrathiafulvalene-triphenylmethyl radical derivatives in solution, J. Am. Chem. Soc. 135(2013) 6958-6967; (h) R. Frisenda, R. Gaudenzi, C. Franco, et al., Kondo effect in a neutral and stable all organic radical single molecule break junction, Nano Lett. 15(2015) 3109-3114; (i) S. Castellanos, F. López-Calahorra, E. Brillas, et al., All-organic discotic radical with a spin-carrying rigid-core showing intracolumnar interactions and multifunctional properties, Angew. Chem. 121(2009) 6638-6641. |
| [25] | J. Carilla, L. Fajarí, L. Juliá, et al. Two functionalized free radicals of the tris(2,4,6-trichlorophenyl)methyl radical series. Synthesis, stability and EPR analysis. Tetrahedron Lett. 35 (1994) 6529–6532. DOI:10.1016/S0040-4039(00)78264-5 |
| [26] | D. Velasco, S. Castellanos, M. López, et al. Red organic light-emitting radical adducts of carbazole and tris(2,4,6-trichlorotriphenyl)methyl radical that exhibit high thermal stability and electrochemical amphotericity. J. Org. Chem. 72 (2007) 7523–7532. DOI:10.1021/jo0708846 |
| [27] | S. Castellanos, D. Velasco, F. López-Calahorra, et al. Taking advantage of the radical character of tris(2,4,6-trichlorophenyl)methyl to synthesize new paramagnetic glassy molecular materials. J. Org. Chem. 73 (2008) 3759–3767. DOI:10.1021/jo702723k |
| [28] | (a) A. Heckmann, C. Lambert, M. Goebel, et al., Synthesis and photophysics of a neutral organic mixed-valence compound, Angew. Chem. Int. Ed. 43(2004) 5851-5856; (b) A. Heckmann, C. Lambert, Neutral organic mixed-valence compounds: synthesis and all-optical evaluation of electron-transfer parameters, J. Am. Chem. Soc. 129(2007) 5515-5527; (c) A. Heckmann, S. Dümmler, J. Pauli, et al., Highly fluorescent open-shell nir dyes: the time-dependence of back electron transfer in triarylamine-perchlorotriphenylmethyl radicals, J. Phys. Chem. C 113(2009) 20958-20966; (d) D.r. Reitzenstein, T. Quast, F. Kanal, et al., Synthesis and electron transfer characteristics of a neutral, low-band-gap, mixed-valence polyradical, Chem. Mater. 22(2010) 6641-6655; (e) A. Heckmann, C. Lambert, Organic mixed-valence compounds: a playground for electrons and holes, Angew. Chem. Int. Ed. 51(2012) 326-392. |
| [29] | Y. Hattori, T. Kusamoto, H. Nishihara. Luminescence, stability, and proton response of an open-shell (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl) methyl radical. Angew. Chem. Int. Ed. 53 (2014) 11845–11848. DOI:10.1002/anie.201407362 |
| [30] | Y. Hattori, T. Kusamoto, H. Nishihara. Enhanced luminescent properties of an open-shell (3,5-dichloro-4-pyridyl)-bis(2,4,6-trichlorophenyl)methyl radical by coordination to gold. Angew. Chem. Int. Ed. 54 (2015) 3731–3734. DOI:10.1002/anie.201411572 |
 2016, Vol. 27
2016, Vol. 27 


