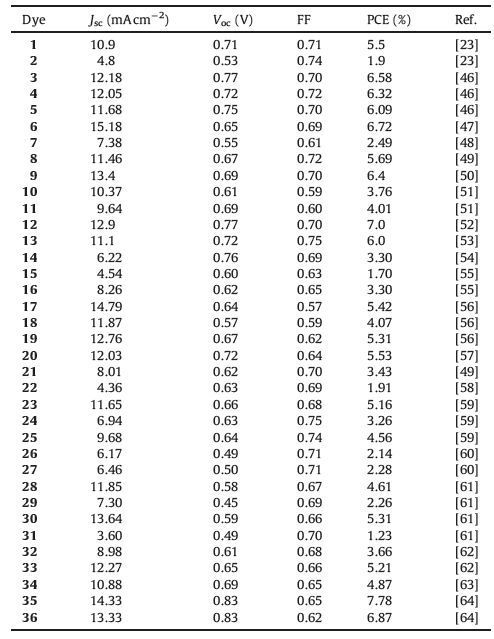
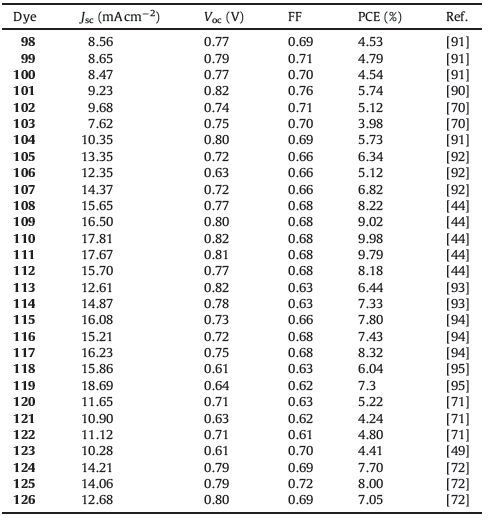
With increasing demand in energy consumption and environmental protection, many researchers have been focused on the development of new sustainable energy resources. Among several alternative sustainable energy sources, solar energy is regarded as one of the most promising alternatives to conventional fossil fuels owing to its abundance, sustainability, non-pollution and ease of access. Dye-sensitized solar cells (DSSCs) have been intensively studied since the pioneering work by Gra¨tzel and co-workers in 1991 [1]. A conventional DSSC typically contains four components (Fig. 1): a mesocrystalline oxide semiconductor deposited on a transparent conducting glass substrate, such as TiO2, anchored dye molecules, redox electrolyte and cathode (counter electrode) [2]. Upon irradiation, light is absorbed by the dye, which creates a high-energy state and results in electron injection from the photoexcited sensitizer into the conduction band (CB) of TiO2 semiconductor. The injected electron is collected by the conducting substrate and flow into the external circuit. Simultaneously, the oxidized sensitizer is subsequently reduced back to the ground state by electron transfer from redox mediators in the electrolyte solution (typically Ⅰ-/Ⅰ3 - and [Co(bpy)3]2+/3+). The oxidized ion in the electrolyte receives electron from the external circuit to complete the whole photoelectric chemical reaction cycle [1]. In these components of DSSCs, the sensitizer plays the most crucial role since it exerts a significant influence on power conversion efficiency (PCE), charge separation, light-harvesting as well as the device stability [3]. Actually, tremendous efforts have been devoted to develop new and highly efficient sensitizers through meticulous molecular engineering.
|
Download:
|
| Figure 1. Schematic representation of a DSSC | |
Up to now, the sensitizers can be divided into two general classes: metal-complex sensitizers and metal-free organic sensitizers. In addition, it must be pointed that, at present, nanosized light harvesters, especially methyl ammonium lead halide perovskites are gaining much attention [4-6]. However, there are two key issues for the application of perovskites based solar cell in industry, toxicity and device stability. Meanwhile, the perovskite materials are susceptible to the following factors: oxygen and moisture, UV light, solution process (solvents, solutes, additives) and temperature [7]. Compared with perovskite solar cells, the DSSCs have unique advantages, such as low production cost, excellent stability, non-toxic and easy of fabrication [8-10]. For the DSSCs without using co-dyes or co-adsorbents, the record high PCEs over 13% [11], 11.5% [12] and 12.5% [13], have been achievedfor zinc-porphyrin, ruthenium complex and metal-free organic sensitizers, respectively. It is obvious that the DSSCs based on metal-complex sensitizers exhibited slightly higher efficiency than those of metal-free organic sensitizers. However, the metalcomplex sensitizers have some problems such as limited resource, low molar extinction coefficient (ε) and high cost, which will limit their applications in DSSCs of large-scale [14-17]. To get rid of these problems, the focus has been shifted to metal-free organic sensitizers because of their low cost, easy synthesis, high e, as well as environmental friendly [18-21]. Furthermore, some studies have shown that the solid-state DSSCs based on metal-free organic sensitizers show better performance than ruthenium complexes because of higher molar absorption coefficients [22]. Overall, these results indicate that the commercial application of metal-free organic dyes in DSSCs is promising.
Metal-free organic dyes based on phenothiazine [23], triphenylamine [24-29], dithiafulvenyl [30-33], tetrahydroqui-noline [34, 35], carbazole [36-39], indoline [40-43] etc. have been developed and found to exhibit promising photovoltaic performances. Among the various species of metal-free organic dyes, the phenothiazine-based dyes hold a large proportion and have the unique advantages: (i) the non-planar butterfly conformation of phenothiazine in the ground state can impede the molecular aggregation and the formation of molecular excimers, which is favorable for achieving high photovoltage; (ii) the electron-rich sulfur and nitrogen heteroatom render phenothiazine a stronger donor than other amines, even better than triphenylamine, carbazole, tetrahydroquinoline and so on [23]. Notably, some researches revealed that the DSSCs based on phenothiazine dyes showed better photovoltaic performances than commercial N719 under identical fabrication and test conditions [44, 45]. In short, the feasibility of superior structure and electron-rich property of phenothiazine derivatives guarantees their bright future as dyes in DSSCs. In this paper, we intend to focus on recent developments in phenothiazine dyes and the relationship between device performance and the structure of phenothiazine dyes, especially focus on the small molecule phenothiazine dyes. We hope that this review can provide readers with a research direction through a systematic literature survey.
2. Small molecule dyes based on phenothiazine for DSSCsPhenothiazine has been attracted considerable research interests in organic dye as a novel electron donor owing to its excellent electron donating ability, rigid structure and large π conjugated system. Many efforts have been carried out to improve photovoltaic performances of phenothiazine-based DSSCs through use of molecular engineering on the phenothiazine reactive sites C(3), C(7) and N(10). Apart from above-mentioned reactive sites, attachment of an electron-donor unit on the C(2) or C(8) positions of phenothiazine were studied as well.
2.1. Alkyl (alkoxy) chain at N(10) of the phenothiazine periphery 2.1.1. Phenothiazine-based mono-anchoring dyesHagfeldt and Sun et al. were the first to investigate the sensitization behavior of organic dyes based on the phenothiazine chromophore in 2007 [23]. They reported two simple D-A phenothiazine dyes, containing an electron-rich phenothiazine unit, butyl carbon chains on the N(10) position and end-capped with electron-withdrawing cyanoacrylic acid (dye 1) or rhodanine- 3-acetic acid (dye 2) anchor group. Dye 1 containing a cyanoacrylic acid anchor group had much better performance than dye 2 containing a rhodanine-3-acetic acid group, which due to the lowest unoccupied molecular orbital (LUMO) of dye 1 had better orbital overlap with the CB of TiO2.
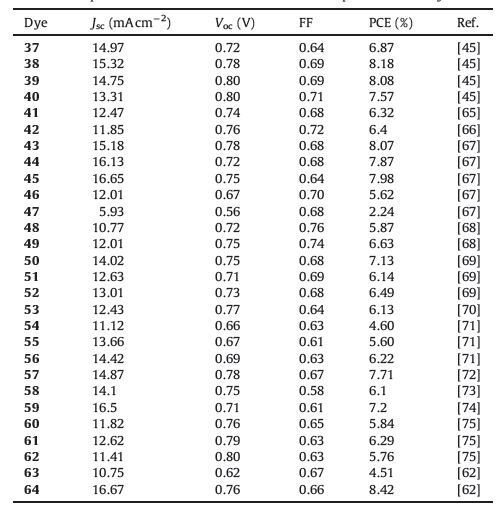
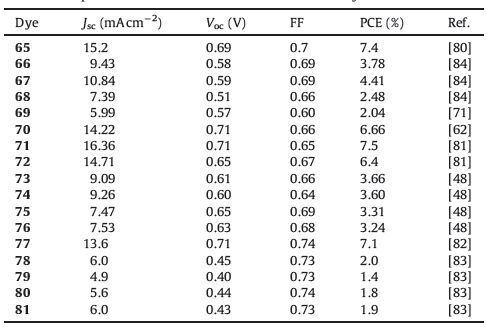
2.1.1.1. D-A dyes to D-p-A dyes.It is well known that a suitable π-conjugated spacer is required between the donor and the acceptor to easily realize electron transfer from donor to acceptor for improved efficiencies of DSSCs with D-π-A structure. For this, great efforts have been made to optimize molecular structures via incorporating a π-conjugated spacer between phenothiazine electron donor and the electron acceptor (Fig. 2), which are usually preferable to induce efficient intramolecular charge transfer and extend spectral response to low energy solar photons for their better electron delocalization over the whole molecules. In this section, we will discuss the use of an efficient p-conjugated spacer for D-p-A phenothiazine dyes. Fig. 3 shows the structures of these dyes (3-36), and the photovoltaic parameters are summarized in Table 1.
|
Download:
|
| Figure 2. Schematic representation of incorporating π-conjugated spacers into D–A dyes to construct D-π-A dyes. | |
|
Download:
|
| Figure 3. Molecular structures of the D-A (1-2) and D-π-A (3-36) phenothiazine dyes | |
|
|
Table 1 Photovoltaic parameters of the DSSCs based on D–A (1–2) and D-π-A (3–36) dyes. |
To study the effect of π-conjugated spacers on DSSCs performances, Kim and co-workers designed and synthesized three phenothiazine dyes by modifying the simple D-A dye 1, in which phenothiazine unit as electron donor, cyanoacrylic acid as the electron acceptor and different electron-rich linkers such as furan (dye 3), thiophene (dye 4) and 3, 4-ethylenedioxythiophene (EDOT) (dye 5) as the π-spacers [46]. They found that introduction of p-conjugated spacers and chenodeoxycholic acid (CDCA) could improve the short-circuit current density (Jsc) and open-circuit voltage (Voc) of the corresponding devices, resulting in impressively improved photovoltaic performance. Among them, the DSSC sensitized with dye 3 containing furan conjugated spacer gave the highest efficiency of 6.58% (Jsc = 12.18 mA cm-2, Voc = 0.77 V and fill factor (FF) = 0.70), exhibiting an improvement of 24% compared to the device sensitized with reference dye 1 under the same conditions. Further progresses in enhancement of efficiency were obtained by the introduction of ethane-EDOT π-spacer, the DSSC was fabricated employing dye 6 and showed a Jsc of 15.18 mA cm-2, a Voc of 0.65 V and a FF of 0.69, corresponding to an overall conversion efficiency of 6.72% under AM 1.5 irradiation (100mWcm-2) [47]. Similarly, Thomas et al. incorporated the bithiophene conjugated linker into the D-π-A phenothiazine dye 7 [48]. However, dye 7-based DSSC showed a lower efficiency of 1.63% (Jsc = 5.46 mA cm-2, Voc = 0.52 V and FF = 0.58). Furthermore, the PCE was improved to 2.49% when 10 mM CDCA was employed as co-adsorbent, which owing to the significant increase of Jsc and Voc.
Based on the above results, Kim et al. synthesized three phenothiazine-based dyes without or with different conjugated linkers and investigated the effect of π-conjugated bridges on the device performances [51]. The research results indicated that the introduction of π-bridges into the H-PTZ broadened the molecular absorption spectra and increased the ε values, which were highly desirable for the efficient light harvesting and large photocurrents in DSSCs. As a result, dyes 10 (PCE = 3.76%) and 11 (PCE = 4.01%), containing ethane-bithiophene and ethane-triphenylamine-thiophene p-conjugated bridges, have better performances than dye H-PTZ (PCE = 3.57%) without π-conjugated bridge. In 2013, Grätzel and co-workers reported a new metal-free dye 12 consisting Nbutyl- phenothiazine as a donor, didodecyl-cyclopentadithiophene (CPDT) unit as a p-conjugated spacer and cyanoacrylic acid as an acceptor to afford a PCE of 7.0%, with a Jsc of 12.9 mA cm-2, a Voc of 0.77 V and a FF of 0.70 [52].
Following the same trend, Sun and co-workers reported phenothiazine-based dyes featuring phenanthrenequinone derivative as the π-linker, cyanoacrylic acid as an acceptor and with butoxyltriphenylamine (JH201) or phenothiazine (dye 13) as electron donor [53]. They demonstrated that the molecule with phenothiazine as the electron donor had the broader absorption and higher Jsc, which due to phenothiazine having a stronger electron-donating ability than butoxytriphenylamine. The cell efficiencies were 4.5% and 6.0% for JH201 (Jsc = 8.5 mA cm-2, Voc = 0.70 V, FF = 0.76) and 13 (Jsc = 11.1 mA cm-2, Voc = 0.72 V, FF = 0.75), respectively. The better performance of dye 13 was attributed to the better light harvesting. Recently, our group also reported three novel organic dyes WY1, 14 and WY3 with the same p-conjunction linker (indole) and acceptor (cyanoacrylic acid) but different donor units (triphenylamine for WY1, N-hexylphenothiazine for 14 and N-hexylcarbazole for WY3) [54]. It was found that the optical and photovoltaic properties were mainly dependent on the electron donor. Accordingly, the solar cell performances based on these dyes were in the range from 2.09% to 3.30% depending on the electron donor. As a result, dye 14 with phenothiazine donor exhibited the best photovoltaic performance.
Baheti and coworkers reported two D-π-A metal-free organic dyes, 15 and 16, which contained fluorine-thiophene and fluorinebithiophene units in the conjugated spacers linking the phenothiazine donor and the cyanoacrylic acid acceptor [55]. Compared with dye 15 based on fluorine-thiophene π-linker, introduction of fluorine-bithiophene unit was found to red-shifted absorption spectrum and low-lying LUMO level, resulting in a better PCE. The PCEs were 2.96% and 3.30% for the cells based on dye 16 without and with 1 mmol/L CDCA, respectively. This promotion of PCE when using co-adsorbent can be explained by considering that CDCA with sterically demanding structure can inhibit unfavorable dye aggregation and facilitate electron injection.
In the study of D-π-A phenothiazine dyes, Cao et al. reported a new class of dyes, incorporating phenothiazine as donor, dithienopyrrolobenzothiadiazole (DTPBT) (dye 17), DTPBT-thiophene (dye 18) and thiophene-DTPBT (dye 19) as the π-bridges and cyanoacrylic acid as acceptor [56]. These dyes have broad absorption in the visible region and a very intense intramolecular charge transfer band (ε > 45000 L mol-1 cm-1). DTPBT is a planar fused segment consisting of both electron rich entity and electron deficient entity (benzothiazole), which causes strong intermolecular interactions and π-π stacking of the dyes. Therefore, CDCA is needed as the co-adsorbent during dye soaking of TiO2 for better cell performance. It was found that the cell based on dye 17 with a DTPBT spacer showed the highest PCE of 5.42% with 1 mmol/L CDCA, indicating that DTPBT was already an efficient π-bridge by itself, and it is no longer necessary to extend the conjugation system of the dye by increasing the π-bridge length. Meanwhile, the authors revealed that the linking position of the thiophene unit to the DTPBT unit could significantly influence the photovoltaic performances. The same research group further introduced a thiophene-benzotriazole-thiophene unit as the p-bridge between the phenothiazine donor and the cyanoacrylic acid acceptor to construct a new organic dye 20, and the DSSC based on dye 20 showed a PCE of 5.53% [57].
Considering the problem of S atom of DTPBT in the conjugated spacer has stronger interaction with Ⅰ2 and/or Ⅰ3 - ions than an oxygen atom and leads to more dark currents. In order to address this problem, Lin et al. introduced a rigid planar entity possessing both electron rich and electron deficient units dithieno[ 3', 2':3, 4;2'', 3'':5, 6]benzo[1,2-c]furazan (DTBF) between the phenothiazine donor and the cyanoacrylic acid acceptor to construct dye 22 [58]. The DSSC based on dye 22 showed inferior efficiency of 1.91%, which likely stemmed from several factors: (1) dye 22 is less effective in blocking the electrolytes from approaching the TiO2 surface; (2) dye 22 has high-lying highest occupied molecular orbital (HOMO) level, which slowdowns dye regeneration and leads to more facile charge recombination with the oxidized dye; (3) the cell of dye 22 has slower electron transport and/or slower electron injection.
In order to further improve the efficiency and explored the relationships of π-bridges and photovoltaic performances, Cao and co-workers designed and synthesized three organic dyes with diketopyrrolopyrrol (DPP) and phenyl units as an π-bridge, phenothiazine as the donor and cyanoacrylic acid as acceptor [59]. Dye 23 incorporated the donor segment directly to the DPP core, dyes 24 and 25 with a phenyl unit between the donor and DPP unit. Compared with dyes 24 and 25, dye 23 showed a better electron communication between the donor and acceptor, which allowing an efficient charge transfer process. Consequently, the DSSC based on dye 23 showed the best PCE of 5.16% among the three dyes without co-adsorbent. The results indicated that the position and number of phenyl unit significantly influenced the photovoltaic performances of the dyes. It is worth noting that DPP is a very planar framework which always readily causes a strong π-π intermolecular interaction. Although introducing hexyl group on both the DPP and the phenothiazine donor unit as a barrier, the dyes aggregation cannot be avoided completely. Therefore, the DSSCs based on DPP as π-bridges should add co-adsorbent during dye soaking to improve the efficiency.
In addition to the above-mentioned D-π-A dyes, many similar molecular structures have also been reported via changing the p-bridges. For example, Mao et al. reported the novel dyes 26, 27 [60], 28-31 [61] based on 2, 6-conjugated bodipy π-spacers. The derivatives of anthracene have been incorporated to the phenothiazine- based D-π-A dyes as π-linkers (32, 33) by Lin and coworkers [62]. In a similar trend, Thomas and co-works reported dye 34 featuring fluorene appended dithienopyrrole and thiophen as a linker, which showed a Jsc of 10.88 mA cm-2, a Voc of 0.69 V, and a FF of 0.65, corresponding to a PCE of 4.87% [63]. Chang et al. synthesized a series of organic dyes containing oligo-phenothiazine, in which the phenothiazine moiety functions both as an electron donor and as a π-bridge. The performance of the dimer system, i.e., 35, was better than the trimers 36 [64]. The high Voc values reached a level of >0.83 V, and the best conversion efficiency of 7.78% was obtained in dye 35 sensitized device with 10 mM deoxycholic acid (DCA).
Generally speaking, introduction of conjugated bridge between phenothiazine donor and acceptor is the most important strategy to improve the performances of DSSCs. However, large π-conjugation systems will lead to π-π aggregation and increase the possibility of electron transfer between molecules, thus reducing electron injection efficiency. In addition, large π-conjugation systems can also lead to photodegradation of dye molecules and reduce stability of the DSSCs. Therefore, the type, length and location of the p-conjugate spacers should be considered when designing and optimizing the new dye molecules.
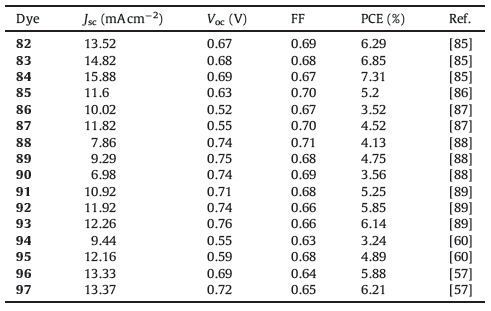
2.1.1.2. D-A dyes to D-D-A dyes.In addition, numerous attempts have been made to molecularly engineer phenothiazine dyes to broaden the absorption band and increase the ε. One successful strategy to achieve the above purposes is introducing an additional electron donor into the phenothiazine donor group at C(7) on the opposite side of the acceptor to form D-D-A structure (Fig. 4). Fig. 5 shows the structures of the dyes (37-64) in this category, and the photovoltaic parameters are summarized in Table 2.

|
Download:
|
| Figure 4. Schematic representation of incorporating additional electron donors into D–A dyes to construct D–D–A dyes. | |

|
Download:
|
| Figure 5. Chemical structures of the D–D–A phenothiazine dyes. | |
|
|
Table 2 Photovoltaic parameters of the DSSCs based on D–D–A phenothiazine dyes. |
Electron-rich alkoxy-phenyl groups have been incorporated to phenothiazine donor as an excellent additional electron donor. Hua and co-workers reported a series of simple phenothiazinebased dyes, in which a cyanoacrylic acid as acceptor, (4-hexyloxy)- phenyl group as an additional electron donor and an alkyl chain with different length (ethyl, 37; hexyl, 38; octyl, 39; dodecyl, 40) at N(10) of the phenothiazine periphery [45]. Under a standard 1.5G solar illumination, dye 38 based DSSC showed the highest PCE of 8.18%, which exceeded the reference dye N719 with an efficiency of 7.73% under identical fabrication conditions. In this study, they found that alkyl substituents with different chain length at the N(10) atom of phenothiazine could dramatically impact the Jsc and Voc, resulting in optimizing the performance through completely shielding the surface of TiO2 from the Ⅰ-/Ⅰ3- electrolyte and subsequently reducing the leakage of dark current. Similarly, a (4- octyloxy)-phenyl and 1, 3-bis-hexyloxy-benzene units were also introduced into the phenothiazine donor frame as the additional electron donors to construct dyes 41 and 42, which showed PCEs of 6.32% (Jsc = 12.47 mA cm-2, Voc = 0.74 V, FF = 0.68) and 6.4% (Jsc = 11.85 mA cm-2, Voc = 0.76 V, FF = 0.72), respectively [65, 66]. Further progresses in improvement of efficiency were obtained by the introduction of five-membered heteroaromatic (such as thiophene, EDOT) onto the phenothiazine donor as an additional electron donor. Hua and co-workers synthesized a series of new phenothiazine-based organic dyes (43-47) featuring thiophene, EDOT, their dimers and mixtures as the additional donor, and cyanoacrylic acid as the acceptor [67]. By tuning the number of the thiophene and/or EDOT units, they effectively tuned the HOMOLUMO level and absorption spectra. As a result, DSSC sensitized by dye 43 displayed the highest PCE of 8.07% (Jsc = 15.18 mA cm-2, Voc = 0.78 V, FF = 0.68), measured under simulated AM 1.5 sunlight in conjunction with Ⅰ-/Ⅰ3 - redox couple; the high PCE was attributed to fast dye regeneration dynamics and slow charge recombination kinetics.
As we know, tetrathiafulvene is an excellent electron-donating group and its derivatives as sensitizers have been applied to DSSCs [76-79]. By comparison, dithiafulvenyl can be regarded as a smaller version of the fulvene family. Dithiafulvenyl exhibits unique charge transport characteristics similar to tetrathiafulvene due to their coplanar molecular structures with strong π-π and S-S interactions. Recently, our group modified the simple phenothiazine organic dye (C6PTZ) by introducing excellent electron donor dithiafulvenyl unit with alkyl chains as additional donor to form dyes 48 and 49 [68]. It was found that the introduction of dithiafulvenyl unit with long alkyl chains could broaden the absorption spectra, increase the e and electron-donating ability, suppress dye aggregation, retard the charge recombination, resulting in improving the PCEs of DSSCs. Accordingly, the PCEs increased significantly from 4.16% to 5.87% (48) and 6.63% (49) compared to the reference dye C6PTZ.
Arylamine derivatives (carbazole, triphenylamine, indoline and their derivatives) are well known electron-rich compounds that are widely used in phenothiazine dyes as additional electron donor. Wang et al. developed three phenothiazine dyes based on different arylamine additional electron donors, i.e., 9-hexyl-9Hcarbazole (dye 50), bis(4-(2-phenylpropan-2-yl)phenyl)amine (dye 51) and N-phenyl-9, 9-dipropyl-N-(9, 9-dipropyl-9H-fluoren- 2-yl)-9H-fluoren-2-amine (dye 52), to investigate the electron donor influence on photovoltaic performances [69]. For comparison the simple amine donor free dye, dye WR7 was prepared as a reference. They found that the introduction of these additional electron donors extends the conjugation of the phenothiazine dyes, leading to red-shift their absorption spectra and enhance ε. Among these four dyes, dye 50 with 9-hexyl-9H-carbazole as additional electron donor showed the best photovoltaic performance: a Jsc of 14.02 mA cm-2, a Voc of 0.75 V and a FF of 0.68, corresponding to an overall conversion efficiency of 7.13% under standard global AM 1.5 solar light conditions. The individual fluorenyl unit has also been introduced into phenothiazine donor to construct the new dye 53 by Hua et al., which showed a relatively lower PCE of 6.13% [70].
The triphenylamine unit is well-known for its strong electrondonating ability and hole-transport properties. A large number of dyes based on triarylamine and phenothiazine donor have been developed, and most of them showed impressive PCEs in DSSCs. For example, Yang and co-workers reported a series of organic dyes (54-56) featuring cyanoacrylic acid was added at the C(3) position of the phenothiazine as electron acceptor, naphthalene based triarylamine moiety or naphthalene based triarylamine-thiophene was attached at the C(7) position as an additional electron donor and a variety of substituents, i.e., methyl, hexyl were added at the N(10) of phenothiazine [71]. The best performance was found in dye 56, in which a hexyl group was attached at the N(10) of phenothiazine and a naphthalene based triarylamine-thiophene at the C(7) position. The DSSC based on dye 56 showed a Jsc of 14.42 mA cm-2, a Voc of 0.69 V and a FF of 0.63, corresponding to a PCE of 6.22%. Similarly, Hua et al. reported dye 57 possessing triphenylamine unit as the additional donor in the phenothiazine based dye, the corresponding DSSC showed a PCE of 7.71% (Jsc = 14.87 mA cm-2, Voc = 0.78 V, FF = 0.67) [72]. Meanwhile, triphenylamine-ethane unit as the additional donor (dye 58) was reported by Kim et al., which showed a PCE of 6.1% [73].
Indoline as an important part of arylamine has also been combinated with phenothiazine dyes. Kumar and co-workers synthesized a phenothiazine dye 59 with indoline as the additional electron donor and cyanoacrylic acid moiety as acceptor [74]. Dye 59 sensitized cell gave a Jsc of 16.5 mA cm-2, a Voc of 0.71 V and a FF of 0.61, corresponding to a PCE of 7.2% with 5 mmol/L CDCA as co-adsorbent. Based on this molecular design strategy, other types of addition electron donors for phenothiazine dyes were also studied, such as styrene (60-62) [75] and anthracene (63-64) [62] derivatives, which obtained excellent photovoltaic performances.
2.1.1.3. D-D-A dyes to D-D-π-A dyes.On the basis of the D-D-A dyes (Fig. 5), a π-conjugation spacer was inserted in between phenothiazine donor and acceptor to form a new type of phenothiazine dyes (D-D-π-A) (Figs. 6 and 7). Table 3 summarized the photovoltaic performances of these dyes (65-81), which showed low PCEs on the whole, the dyes 65 [80], 71 [81] and 77 [82] were the only exception. Sun, Tian, Yang et al. reported a series of dyes based on this category with thiophene, benzene, benzothiadiazole, theirdimers, and mixtures as p-conjugation spacers (Fig. 7 and Table 4). In addition, Bae and co-workers reported a series of D-D-π-A sensitizers, featuring squaraine and thiophene derivatives as the π-linkers (dyes 78-81) [83]. It was found that these dyes displayed remarkable panchromatic responses in DSSC. However, the LUMO levels of the present squaraine-based sensitizers were slightly more negative than the CB edge of TiO2 photoanode, which led to low electron injection efficiency from the excited dyes to the CB of TiO2. As a result, the solar cells based on these squaraine dyes exhibited lower efficiencies from 1.4% to 2.0%.

|
Download:
|
| Figure 6. Schematic representation of incorporating p-conjugated spacers into D–D–A dyes to construct the D–D-π-A phenothiazine dyes. | |

|
Download:
|
| Figure 7. Chemical structures of D–D-π-A phenothiazine dyes | |
|
|
Table 3 Photovoltaic parameters of the DSSCs based on D–D-π-A dyes. |
|
|
Table 4 Photovoltaic parameters of the dyes based on di-anchoring phenothiazine dyes. |
2-1-2. Phenothiazine-based di-anchoring dyes
Compared to the mono-anchoring dyes, the di-anchoring dyes based on phenothiazine usually can gave stronger bonding with the TiO2 surface, led to red shift in absorption and enhance photocurrent generation, cause a high loading of the dye and promote higher electron extraction and injection rate. At present, there are two major categories of phenothiazine-based dianchoring dyes: A-π-D-π-A and (D-A)2 or (D-π-A)2 (Fig. 8). Both the two types of di-anchoring phenothiazine dyes have been studied for the past years. Up to now, A-π-D-π-A and (D-π-A)2 sensitizers have reached an overall conversion efficiencies of over 7.31% and 6.14%, respectively.
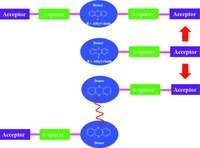
|
Download:
|
| Figure 8. The structural design of di-anchoring phenothiazine dyes | |
2.1.2.1. D-π-A dyes to A-π-D-π-A dyes.
The configuration of A-π- D-π-A phenothiazine dyes (82-87) are shown in Fig. 9 and the photovoltaic parameters are summarized in Table 4, in which phenothiazine with alkyl chain as donor, cyanoacrylic acid as acceptor and thiophene, furan, bodipy and their derivatives as π-conjugation spacers. Lin and co-workers employed phenothiazine with 2-ethylhexyl as donor, cyanoacrylic acid as double acceptors and thiophene (dye 82), 3-hexylthiophene (dye 83), 4-hexyl-2, 2'- bithiophene (dye 84) as the various p spacers to construct a series of A-π-D-π-A phenothiazine dyes [85]. They found that the hexyl chains incorporated into the thiophene of dye 83 and bithiophene of dye 84 help to suppress dye aggregation and there is no advantage gained by the addition of co-adsorbent. Meanwhile, the dyes with two anchors have more efficient interfacial charge generation and transport compared with their congeners with only single anchor. Consequently, the best PCE (7.31%) was achieved for dye 84, which reached approximately 99% of N719 (7.38%) fabricated and measured under similar conditions.
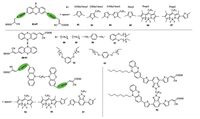
|
Download:
|
| Figure 9. Molecular structures of di-anchoring phenothiazine dyes | |
In like manner, dye 85 was reported by Yang et al., which was similar to dye 82 but with hexyl instead of 2-ethylhexyl [86]. This research revealed that the organic dye with the double electron acceptors exhibited a better light absorption at long wavelength and an effective electron extraction pathway from the electron donor to the TiO2 surface, leading to an improved Jsc. An overall PCE of 5.2% was achieved for dye 85 sensitized cell with a Jsc of 11.6 mA cm-2, a Voc of 0.63 V and a FF of 0.70.
The bodipy unit has also been incorporated to A-π-D-π-A dyes as p-conjugation spacers by Mao and co-workers [87]. They designed and synthesized two novel A-π-D-π-A dyes by modifying the reference single anchor dye (UY1), in which a rigid alkylfunctionalized phenothiazine core as the donor, two cyanoacrylic acid units as the electron acceptors, and different electron-rich spacers such as bodipy (dye 86) and bodipy-furan (dye 87) as the π-linkers. The results showed that both dyes 86 (Jsc = 10.02 mA cm-2, Voc = 0.52 V, FF = 0.67 and PCE = 3.52%) and 87 (Jsc = 11.82 mA cm-2, Voc = 0.55 V, FF = 0.70 and PCE = 4.52%) with double anchoring units showed an enhanced light absorption coefficient and provided an improved Jsc and Voc in DSSCs in comparison to dye UY1 (Jsc = 7.70 mA cm-2 Voc = 0.51 V, FF = 0.67 and PCE = 2.65%) with a single acceptor.
2.1.2.2. D-A dyes to (D-A)2 or D-π-A dyes to (D-π-A)2 dyes.The (D- A)2 and (D-π-A)2 dyes (Fig. 9 and Table 4) contain two separate D- A and D-π-A segments, respectively, which usually are connected with a non-conjugated alkyl linkage by N(10) of the phenothiazine. Cao and co-workers reported a series of novel (D-A)2 dyes with a phenothiazine unit as electron donor, cyanoacrylic acid unit as electron acceptor and butyl (dye 88), hexyl (dye 89) chains and 1, 4- bis(methyl)benzene (dye 90) as the connective units to contact the two phenothiazine donors [88]. The PCEs based on these dyes were in the range from 3.56% to 4.75% depending on the connective unit, which were effectively enhanced compared to the corresponding single D-A phenothiazine dye (2.91%). In order to further study the effect of the connective unit on optical and photovoltaic performances of dyes, the same group designed and synthesized three (D-A)2 organic dyes (dyes 91-93) with ortho-, meta- and para-positional linkages for DSSCs [89]. It was found that the UV- vis absorption, dye loading and photovoltaic performances were all affected by the diverse connective units. The solar cell based on dye 93 exhibited the highest PCE of 6.14% (Jsc = 12.26 mA cm-2, Voc = 0.76 V, FF = 0.66). This mainly originates from the paraposition linkage can significantly improve the light-harvesting ability and suppress the charge recombination, and thus enhancing the Jsc and the Voc.
Following the above pioneering study, Mao and co-workers reported the (D-π-A)2 dyes, in which phenothiazine as donor, cyanoacrylic acid as acceptor, boradiazaindacene (BODIPY) (dye 94) and BODIPY-thiophene (dye 95) as the p-conjugated bridges [60].The two separate D-π-A segments were connected with a hexyl. In addition, the corresponding single D-π-A branched dye UY2, was synthesized for a comparison. The structure-property relationship showed that the PCEs of DSSCs increased both by the introduction of double D-π-A branches and by the addition of thiophene as p-conjugated bridge. Hence, DSSCs using dye 95 gave the best photovoltaic performance with a Jsc of 12.16 mA cm-2, a Voc of 0.59 V and a FF of 0.68, corresponding to a PCE of 4.89%, which was increased by about 129% compared with using single D-π-A branched UY2 (PCE = 2.14%) under similar conditions.
Huang and co-workers synthesized two novel (D-π-A)2 organic dyes and investigated the influence of the linkage location in (D-π-A)2 dyes on the performances of DSSCs [57]. The dyes contained phenothiazine as a donor, thiophene-benzotriazole unit as the p bridge, cyanoacrylic acid as the electron-acceptor and hexylene chain as the connective unit which was linked in the benzotriazole p-bridge part (dye 96) and in the phenothiazine donor part (dye 97), respectively. The results showed that the location of the connective unit has a small effect on the physical and electrochemical properties of the dyes. However, when the dyes are applied in DSSCs, an obvious decline of Jsc and Voc was found by moving the connective unit from the donor part to the pbridge part. As a result, the DSSC based on dye 97 with the connective unit in the donor obtained a PCE of 6.21%, which was higher than that (5.88%) of the DSSC based on dye 96 with the connective unit in the π-bridge. Dye 96-based device exhibited a lower efficiency owing to its serious aggregation and short electron lifetime. The results indicated that the connective unit location of the dyes had a big effect on the performances of the DSSCs.
2.2. Bulky substituent at N(10) of the phenothiazine peripheryAll the above dyes having a common characteristic is that contain alkyl (alkoxy) chains on N(10) of the phenothiazine. In this section, we will discuss the use of bulky substituent instead of alkyl chain at N(10) of the phenothiazine to contrast the bulky dyes. The bulky substituents usually contain the derivatives of the benzene, carbazole, fluorine and triphenylamine. The structures of dyes (98-126) in this category were showed in Fig. 10, and the corresponding photovoltaic data were summarized in Table 5. Some research results indicated that the introduction of a bulky substituent to the phenothiazine nitrogen atom could increase the steric hindrance, reduce dye aggregation and suppress the electron recombination between the electrons injected on the TiO2 and the I3 in the electrolyte, resulting in impressively improved Voc, especially for di-anchoring phenothiazine dyes. The PCEs of these dyes are lying between 3.98% and 9.98%. Itwas worth noting that when 4-alkoxyphenyl groups were incorporated to N-10 and C-7 of phenothiazine unit, the corresponding PCEs could become very high [44]. Dye 110 sensitized cell showed a PCE of 9.98%, which represented the highest value reported to date for metal-free phenothiazine-based DSSCs. Its Jsc was extremely high and its Voc reached the theoretical maximum. Meanwhile, the same high Voc of 0.82 V was achieved for dye 101 sensitized cell based on phenothiazine with N-methoxyphenyl and ethylthiol groups [90]. However, the Jsc value (9.23 mA cm-2) was relatively low, which due to the loading amount on the TiO2 surfacewas very limited.
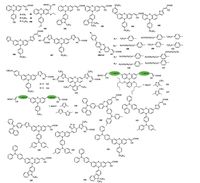
|
Download:
|
| Figure 10. Molecular structures of dyes based on bulky substituents at N(10) of phenothiazine | |
|
|
Table 5 Photovoltaic parameters of the DSSCs based on bulky substituents at N(10) of phenothiazine. |
In addition to the above-mentioned dyes, other type dyes (127- 156) contain phenothiazine unit have also been studied and achieved impressive photovoltaic performances. Only two examples were presented here, the molecular structures and photovoltaic parameters of these dyes were shown in Fig. 11 and Table 6 in detail. For example, Jia and co-workers reported a series of dyes (127-131) based on phenothiazine and triphenylamine, and the best performance up to 6.79% for organic dye 131 sensitized cell, in which two phenothiazine groups, as additional electron donors, were introduced into a triphenylamine, a furan group as conjugated linker, cyanoacrylic acid as acceptor [96]. Moreover, it was worth noting that two efficient ruthenium sensitizers with a phenothiazine modified bipyridine as an ancillary ligand, coded 151 and 152, have been developed as dyes by She and co-workers [97]. Under AM1.5G irradiation (100mWcm-2), dyes 151 and 152 sensitized DSSCs showed impressive PCEs up to 10.4% and 10.2%, respectively, which exceeded that of N719 (9.9%) under the same conditions.

|
Download:
|
| Figure 11. Molecular structures of other type phenothiazine dyes | |
|
|
Table 6 Photovoltaic parameters of the DSSCs based on the other type dyes. |
3. Polymer dyes based on phenothiazine for DSSCs
Polymer dyes constitute another class of dyes in DSSCs. However, polymer dyes have been rarely investigated due to the poor solubility, complicated synthesis and lower efficiency. The molecular structures of polymer phenothiazine dyes (157-164) are shown in Fig. 12. Zhong and co-workers developed three polymeric metal complexes dyes (157-159), which used phenothiazine appended with octyl as donor, C=C bond as π-bridge and thiophene-phenanthroline metal complexes as acceptor [113]. DSSC based on dye 157 showed the highest PCE of 1.57% (Jsc = 4.12 mA cm-2, Voc = 0.62 V and FF = 61.7%) under simulate AM 1.5G solar irradiation. Meanwhile, the polymeric metal-free dyes based on phenothiazine also were reported. For example, Pan et al. reported a set of new polymer dyes containing phenothiazine and triphenylamine donor, cyanoacrylic acid acceptor, thiophene (dye 160), alternating with either EDOT (dye 161) or EDOTthiophene (dye 162) π-bridges [114]. The DSSCs with these polymer dyes reached PCEs of 4.7%, 3.7% and 4.1% for dyes 160, 161 and 162, respectively. The authors attributed the difference of the PCEs to energy levels and absorption spectra of the dyes. As far as we know, the PCE of 4.7% is the highest value reported to date for DSSCs based on polymer dyes. In a similar way, the same group also reported another two polymer dyes based on phenothiazine, in which contain a phenothiazine chromophore, a 9, 9-dioctylfluorene (dye 163) or 9, 9-dioctyl-carbazole (dye 164) group as the donor unit in the main chain and a cyanoacrylic acid as acceptor in the side chain, linked by a thiophene unit [115]. The DSSCs based on dyes 163 and 164 display PCEs of 3.0% and 3.5%, respectively.
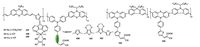
|
Download:
|
| Figure 12. Molecular structures of the polymer phenothiazine dyes | |
4. Phenothiazine dyes for co-sensitized DSSCs
Apart from development of novel organic dyes, co-sensitization is another practical approach to enhance the DSSCs performances through a combination of two or more dyes, possessing different spectral coverage, sensitized on semiconductor films together, which extends the light-harvesting ability so as to increase the Jsc. Phenothiazine-based dyes have been used as co-sensitizers with zinc-porphyrin, ruthenium complex and squaraine dyes for highly efficient DSSCs (Fig. 13).
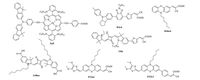
|
Download:
|
| Figure 13. Molecular structures of the co-sensitizers | |
The co-sensitization of zinc-porphyrin dye (ZnP) and phenothiazine dye 38 (Fig. 5) via a stepwise approach was reported by Chang and co-workers, the co-sensitized DSSC exhibited an improved PCE of 10.1% with respect to that of the individual DSSC sensitized with dye 38 (PCE = 8.2%) or ZnP (PCE = 7.4%), which is mainly attributed to the phenothiazine-based dye in porphyrin-sensitized TiO2 surface plays dual roles: (i) to retard the charge recombination between electrons in CB of TiO2 and the Ⅰ3 - in the electrolyte; (ii) to enhance the spectral response [116]. Meanwhile, the same group also reported the co-sensitization of dye 38 with the black dye N749 to realize the panchromatic light harvesting and improve the photovoltaic performances [117].
Co-sensitization based on phenothiazine zinc-porphyrin dye and a metal-free dye has been reported as well. Xie et al. reported the co-sensitization of dye 156 (Fig. 11) with WS-5 [112]. The dyes 156 and WS-5 co-sensitized device showed significant improvement of both Jsc and Voc values, with a conversion efficiency of 11.5%, which is improved by 47.4% when compared with the individual dye 156-sensitized device. To the best of our knowledge, the PCE of 11.5% represented the highest PCE value reported to date for phenothiazine-based dyes for co-sensitization.
The squaraine dye is a very promising candidate for cosensitized DSSCs to extend and enhance the sunlight harvesting in the near-infrared region due to its extremely high ε in the longwavelength region. Kim et al. mixed phenothiazine dye M-Red with the squaraine dye S-Blue for co-sensitizered DSSC [118]. It was found that the mixed dye solution had ability to reduce the aggregation of S-Blue dye adsorbed on TiO2 surface, leading to an enhancement of Jsc. This research obtained a Jsc of 7.69 mA cm-2, a Voc of 0.62 V and a FF of 0.66, corresponding to an overall PCE of 3.17%, which was higher than that of cells based on the individual dyes. They further achieved an efficiency of 4.23% based on a similar method by co-sensitization of phenothiazine dye 89 (Fig. 9) and the squaraine dye S-Blue. Hua et al. also reported a cosensitization method using the phenothiazine dye 125 (Fig. 10) together with the squaraine dye YR6 as sensitizers due to their complementary absorption properties [72]. It is worth mentioning that the IPCE spectra of 125/YR6 co-sensitized DSSC showed an impressive panchromatic response from 300 to 750 nm. In this report, the PCEs of only dyes 125 and YR6 are 8.00% and 2.16%, respectively. Under the same fabrication and test conditions, the co-sensitized DSSC showed a PCE of 9.84%, which was the highest reported efficiency ever reported for the squaraine dye-based cosensitized DSSCs.
Recently, our group used a similar strategy to achieve highly efficient DSSCs by co-sensitization of dithiafulvenyl-phenothiazine based organic dyes (PTZ-2 and PTZ-3) with N719 [119]. It is worth noting that the dyes of PTZ-2 and PTZ-3 have high ε and strong absorption, which can compensate for that of N719 in the low wavelength region, especially in the region of ~300-500 nm. As a result, when dyes PTZ-2 and PTZ-3 co-sensitized with N719, the PCEs were improved to 8.12% and 8.01%, exhibiting an improvement of 16.50% and 14.92%, respectively, compared to the device sensitized with N719 (PCE = 6.97%) alone under the same conditions.
5. ConclusionsIn conclusion, phenothiazine-based organic sensitizers have attracted increasing attention, and large improvements in the structural design and DSSC performance have been achieved. In this paper, we mainly reviewed the recent development of small molecule phenothiazine dyes, with an emphasis on molecular design strategies, the correlation between molecular structures and the photovoltaic performances. In addition, polymer phenothiazine dyes and phenothiazine dyes for co-sensitization have also been discussed briefly. It is worth noting that the DSSCs based on metalfree small molecule sensitizer, polymer sensitizer and co-sensitizer of phenothiazine have gained conversion efficiencies up to 9.98%, 4.7% and 11.5%, respectively. Although considerable progress has been made by designing phenothiazine dyes, further enhancing PCE and maximizing light harvesting as well as resolving long-term stability issues still are challenging for the research of DSSCs. We hope this review can provide an outlook for the further development of new phenothiazine-dyes with better performances.
Acknowledgment We are grateful to the National Natural Science Foundation of China (Nos. 21572030, 21272033, 21402023) and Fundamental Research Funds for the Central Universities (No. ZYGX2014J026) for financial support.| [1] | B. O'Regan, M. Grätzel. A low-cost, high-efficiency solar cell based on dyesensitized colloidal TiO2 films. Nature 353 (1991) 737–740. DOI:10.1038/353737a0 |
| [2] | M. Grätzel. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 42 (2009) 1788–1798. DOI:10.1021/ar900141y |
| [3] | A. Hagfeldt, G. Boschloo, L.C. Sun, L. Kloo, H. Pettersson. Dye-sensitized solar cells. Chem. Rev. 110 (2010) 6595–6663. DOI:10.1021/cr900356p |
| [4] | A. Kojima, K. Teshima, Y. Shirai, et al. Organometal halide perovskites as visiblelight sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131 (2009) 6050–6051. DOI:10.1021/ja809598r |
| [5] | H.S. Kim, C.R. Lee, J.H. Im, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2 (2012) 591. |
| [6] | H.P. Zhou, Q. Chen, G. Li, et al. Interface engineering of highly efficient perovskite solar cells. Science 345 (2014) 542–546. DOI:10.1126/science.1254050 |
| [7] | G.D. Niu, X.D. Guo, L.D. Wang. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 3 (2015) 8970–8980. DOI:10.1039/C4TA04994B |
| [8] | S.F. Zhang, X.D. Yang, Y. Numata, et al. Highly efficient dye-sensitized solar cells: progress and future challenges. Energy Environ. Sci. 6 (2013) 1443–1464. DOI:10.1039/c3ee24453a |
| [9] | A. Mishra, M.K.R. Fischer, P. Bauerle. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48 (2009) 2474–2499. DOI:10.1002/anie.v48:14 |
| [10] | A. Hagfeldt, M. Gräetzel. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 95 (1995) 49–68. DOI:10.1021/cr00033a003 |
| [11] | S. Mathew, A. Yella, P. Gao, et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6 (2014) 242–247. DOI:10.1038/nchem.1861 |
| [12] | C.Y. Chen, M. Wang, J.Y. Li, et al. Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 3 (2009) 3103–3109. DOI:10.1021/nn900756s |
| [13] | Z.Y. Yao, M. Zhang, H. Wu, et al. Donor/acceptor indenoperylene dye for highly efficient organic dye-sensitized solar cells. J. Am. Chem. Soc. 137 (2015) 3799–3802. DOI:10.1021/jacs.5b01537 |
| [14] | I. Stengel, N. Pootrakulchote, R.R. Dykeman, et al. Click-functionalized Ru(II) complexes for dye-sensitized solar cells. Adv. Energy Mater. 2 (2012) 1004–1012. DOI:10.1002/aenm.v2.8 |
| [15] | C.Y. Chen, N. Pootrakulchote, T.H. Hung, et al. Ruthenium sensitizer with thienothiophene-linked carbazole antennas in conjunction with liquid electrolytes for dye-sensitized solar cells. J. Phys. Chem. C 115 (2011) 20043–20050. DOI:10.1021/jp206312g |
| [16] | S.M. Feldt, E.A. Gibson, E. Gabrielsson, et al. Design of organic dyes and cobalt polypyridine redox mediators for high-efficiency dye-sensitized solar cells. J. Am. Chem. Soc. 132 (2010) 16714–16724. DOI:10.1021/ja1088869 |
| [17] | C.Y. Chen, J.G. Chen, S.J. Wu, et al. Multifunctionalized ruthenium-based supersensitizers for highly efficient dye-sensitized solar cells. Angew. Chem. Int. Ed. 47 (2008) 7342–7345. DOI:10.1002/anie.v47:38 |
| [18] | Y.S. Yen, H.H. Chou, Y.C. Chen, et al. Recent developments in molecule-based organic materials for dye-sensitized solar cells. J. Mater. Chem. 22 (2012) 8734–8747. DOI:10.1039/c2jm30362k |
| [19] | T.W. Hamann, R.A. Jensen, A.B.F. Martinson, et al. Advancing beyond current generation dye-sensitized solar cells. Energy Environ. Sci. 1 (2008) 66–78. DOI:10.1039/b809672d |
| [20] | Z.J. Ning, Y. Fu, H. Tian. Improvement of dye-sensitized solar cells: what we know and what we need to know. Energy Environ. Sci. 3 (2010) 1170–1181. DOI:10.1039/c003841e |
| [21] | M. Zhang, Y.L. Wang, M.F. Xu, et al. Design of high-efficiency organic dyes for titania solar cells based on the chromophoric core of cyclopentadithiophenebenzothiadiazole. Energy Environ. Sci. 6 (2013) 2944–2949. DOI:10.1039/c3ee42331j |
| [22] | B. Xu, E. Sheibani, P. Liu, et al. Carbazole-based hole-transport materials for efficient solid-state dye-sensitized solar cells and perovskite solar cells. Adv. Mater. 26 (2014) 6629–6634. DOI:10.1002/adma.v26.38 |
| [23] | H.N. Tian, X.C. Yang, R.K. Chen, et al. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun (2007) 3741–3743. |
| [24] | D. Joly, L. Pellejà, S. Narbey, et al. Metal-free organic sensitizers with narrow absorption in the visible for solar cells exceeding 10% efficiency. Energy Environ. Sci. 8 (2015) 2010–2018. DOI:10.1039/C5EE00444F |
| [25] | N. Cai, Y.L. Wang, M.F. Xu, et al. Engineering of push-pull thiophene dyes to enhance light absorption and modulate charge recombination in mesoscopic solar cells. Adv. Funct. Mater. 23 (2013) 1846–1854. DOI:10.1002/adfm.v23.14 |
| [26] | Z.F. Chai, M. Wu, M.M. Fang, et al. Similar or totally different: the adjustment of the twist conformation through minor structural modification, and dramatically improved performance for dye-sensitized solar cell. Adv. Energy Mater. 5 (2015) 1500846. DOI:10.1002/aenm.201500846 |
| [27] | A.Scrascia, L.DeMarco, S.Laricchia, etal.. Fluorine-thiophene-substitutedorganic dyes for dye sensitized solar cells. J. Mater. Chem. A 1 (2013) 11909–11921. DOI:10.1039/c3ta10423k |
| [28] | H.Y. Li, Y.Z. Yang, Y.Q. Hou, et al. Organic sensitizers featuring 9, 10-diarylsubstituted anthracene unit. ACS Sustain. Chem. Eng. 2 (2014) 1776–1784. DOI:10.1021/sc500234a |
| [29] | Q.Q. Li, J. Shi, H.Y. Li, et al. Novel pyrrole-based dyes for dye-sensitized solar cells: from rod-shape to "H" type. J. Mater. Chem. 22 (2012) 6689–6696. DOI:10.1039/c2jm30200d |
| [30] | Z.Q. Wan, C.Y. Jia, Y.D. Duan, et al. Novel organic sensitizers containing dithiafulvenyl units as additional donors for efficient dye-sensitized solar cells. RSC Adv. 4 (2014) 34896–34903. DOI:10.1039/C4RA04782F |
| [31] | P.A. Bouit, M. Marszalek, R. Humphry-Baker, et al. Donor-p-acceptors containing the 10-(1, 3-dithiol-2-ylidene)anthracene unit for dye-sensitized solar cells. Chem. Eur. J. 18 (2012) 11621–11629. DOI:10.1002/chem.201201022 |
| [32] | K.P. Guo, K.Y. Yan, X.Q. Lu, et al. Dithiafulvenyl unit as a new donor for highefficiency dye-sensitized solar cells: synthesis and demonstration of a family of metal-free organic sensitizers. Org. Lett. 14 (2012) 2214–2217. DOI:10.1021/ol300477b |
| [33] | Z.Q. Wan, C.Y. Jia, Y.D. Duan, et al. Novel organic dye employing dithiafulvenylsubstituted arylamine hybrid donor unit for dye-sensitized solar cells. Org. Electron. 14 (2013) 2132–2138. DOI:10.1016/j.orgel.2013.05.011 |
| [34] | R.K. Chen, X.C. Yang, H.N. Tian, et al. Effect of tetrahydroquinoline dyes structure on the performance of organic dye-sensitized solar cells. Chem. Mater. 19 (2007) 4007–4015. DOI:10.1021/cm070617g |
| [35] | Y. Hao, X.C. Yang, J.Y. Cong, et al. Engineering of highly efficient tetrahydroquinoline sensitizers for dye-sensitized solar cells. Tetrahedron 68 (2012) 552–558. DOI:10.1016/j.tet.2011.11.004 |
| [36] | N. Koumura, Z.S. Wang, S. Mori, et al. Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J. Am. Chem. Soc. 128 (2006) 14256–14257. DOI:10.1021/ja0645640 |
| [37] | Y.Q. Wang, B. Chen, W.J. Wu, et al. Efficient solar cells sensitized by porphyrins with an extended conjugation framework and a carbazole donor: from molecular design to cosensitization. Angew. Chem. Int. Ed. 53 (2014) 10779–10783. DOI:10.1002/anie.201406190 |
| [38] | A. Venkateswararao, K.R.J. Thomas, C.T. Li, et al. Functional tuning of organic dyes containing 2, 7-carbazole and other electron-rich segments in the conjugation pathway. RSC Adv. 5 (2015) 17953–17966. DOI:10.1039/C4RA15234D |
| [39] | A. Venkateswararao, K.R.J. Thomas, C.P. Lee, et al. Organic dyes containing carbazole as donor and p-linker: optical, electrochemical, and photovoltaic properties. ACS Appl. Mater. Interfaces 6 (2014) 2528–2539. DOI:10.1021/am404948w |
| [40] | B. Liu, W.Q. Li, B. Wang, et al. Influence of different anchoring groups in indoline dyes for dye-sensitized solar cells: electron injection, impedance and charge recombination. J. Power Sources 234 (2013) 139–146. DOI:10.1016/j.jpowsour.2013.01.152 |
| [41] | G. Li, M. Liang, H. Wang, et al. Significant enhancement of open-circuit voltage in indoline-based dye-sensitized solar cells via retarding charge recombination. Chem. Mater. 25 (2013) 1713–1722. DOI:10.1021/cm400196w |
| [42] | S. Higashijima, Y. Inoue, H. Miura, et al. Organic dyes containing fluorenesubstituted indoline core for zinc oxide dye-sensitized solar cell. RSC Adv. 2 (2012) 2721–2724. DOI:10.1039/c2ra01358d |
| [43] | J.B. Yang, P. Ganesan, J. Teuscher, et al. Influence of the donor size in D-p-A organic dyes for dye-sensitized solar cells. J. Am. Chem. Soc. 136 (2014) 5722–5730. DOI:10.1021/ja500280r |
| [44] | R.Y. Lin, F.L. Wu, C.T. Li, et al. High-performance aqueous/organic dye-sensitized solar cells based on sensitizers containing triethylene oxide methyl ether. ChemSusChem 8 (2015) 2503–2513. DOI:10.1002/cssc.201500589 |
| [45] | Y. Hua, S. Chang, D.D. Huang, et al. Significant improvement of dye-sensitized solar cell performance using simple phenothiazine-based dyes. Chem. Mater. 25 (2013) 2146–2153. DOI:10.1021/cm400800h |
| [46] | S.H. Kim, H.W. Kim, C. Sakong, et al. Effect of five-membered heteroaromatic linkers to the performance of phenothiazine-based dye-sensitized solar cells. Org. Lett. 13 (2011) 5784–5787. DOI:10.1021/ol2023517 |
| [47] | M.H. Tsao, T.Y. Wu, H.P. Wang, et al. An efficient metal-free sensitizer for dyesensitized solar cells. Mater. Lett. 65 (2011) 583–586. DOI:10.1016/j.matlet.2010.10.072 |
| [48] | G.B. Bodedla, K.R.J. Thomas, C.T. Li, K.C. Ho. Functional tuning of phenothiazinebased dyes by a benzimidazole auxiliary chromophore: an account of optical and photovoltaic studies. RSC Adv. 4 (2014) 53588–53601. DOI:10.1039/C4RA09300C |
| [49] | X.X. Liu, J. Long, G. Wang, et al. Effect of structural modification on the performances of phenothiazine-dye sensitized solar cells. Dyes Pigm. 121 (2015) 118–127. DOI:10.1016/j.dyepig.2015.05.012 |
| [50] | H.N. Tian, X.C. Yang, J.Y. Cong, et al. Effect of different electron donating groups on the performance of dye-sensitized solar cells. Dyes Pigm. 84 (2010) 62–68. DOI:10.1016/j.dyepig.2009.06.014 |
| [51] | K.H. Kim, S.M. Lee, M.H. Seo, et al. Syntheses of organic dyes based on phenothiazine as photosensitizers and effects of their π-conjugated bridges on the photovoltaic performances of dye-sensitized solar cells. Macromol. Res. 20 (2012) 128–137. DOI:10.1007/s13233-012-0017-2 |
| [52] | M. Marszalek, S. Nagane, A. Ichake, et al. Structural variations of D-π-A dyes influence on the photovoltaic performance of dye-sensitized solar cells. RSC Adv. 3 (2013) 7921–7924. DOI:10.1039/c3ra22249g |
| [53] | J.H. Zhao, X.C. Yang, M. Cheng, S.F. Li, L.C. Sun. New organic dyes with a phenanthrenequinone derivative as the p-conjugated bridge for dye-sensitized solar cells. J. Phys. Chem. C 117 (2013) 12936–12941. DOI:10.1021/jp400011w |
| [54] | Y. Wang, Z.Q. Wan, C.Y. Jia, et al. Indole-based organic dyes with different electron donors for dye-sensitized solar cells. Synth. Met. 211 (2016) 40–48. DOI:10.1016/j.synthmet.2015.10.024 |
| [55] | A. Baheti, K.R.J. Thomas, C.T. Li, et al. Fluorene-based sensitizers with a phenothiazine donor: effect of mode of donor tethering on the performance of dyesensitized solar cells. ACS Appl. Mater. Interfaces 7 (2015) 2249–2262. DOI:10.1021/am506149q |
| [56] | Z.S. Huang, H.L. Feng, X.F. Zang, et al. Dithienopyrrolobenzothiadiazole-based organic dyes for efficient dye-sensitized solar cells. J. Mater. Chem. A 2 (2014) 15365–15376. DOI:10.1039/C4TA02639J |
| [57] | Z.S. Huang, C. Cai, X.F. Zang, et al. Effect of the linkage location in double branched organic dyes on the photovoltaic performance of DSSCs. J. Mater. Chem. A 3 (2015) 1333–1344. DOI:10.1039/C4TA05652C |
| [58] | J.S. Ni, J.H. You, W.I. Hung, et al. Organic dyes incorporating the dithieno. ACS Appl. Mater. Interfaces 6 (2014) 22612–22621. DOI:10.1021/am5067145 |
| [59] | X.F. Zang, Z.S. Huang, H.L. Wu, et al. Molecular design of the diketopyrrolopyrrole-based dyes with varied donor units for efficient dye-sensitized solar cells. J. Power Sources 271 (2014) 455–464. DOI:10.1016/j.jpowsour.2014.08.030 |
| [60] | M. Mao, X.L. Zhang, X.Q. Fang, et al. Highly efficient light-harvesting boradiazaindacene sensitizers for dye-sensitized solar cells featuring phenothiazine donor antenna. J. Power Sources 268 (2014) 965–976. DOI:10.1016/j.jpowsour.2014.05.079 |
| [61] | M. Mao, X.L. Zhang, X.Q. Fang, et al. 2, 6-Conjugated bodipy sensitizers for highperformance dye-sensitized solar cells. Org. Electron 15 (2014) 2079–2090. DOI:10.1016/j.orgel.2014.05.024 |
| [62] | R.Y.Y. Lin, T.M. Chuang, F.L. Wu, et al. Anthracene/phenothiazine π-conjugated sensitizers for dye-sensitized solar cells using redox mediator in organic and water-based solvents. ChemSusChem 8 (2015) 105–113. DOI:10.1002/cssc.201403016 |
| [63] | S. Kumar, K.R.J. Thomas, C.T. Li, et al. Synthesis and photovoltaic properties of organic dyes containing N-fluoren-2-yl dithieno. Org. Electron. 26 (2015) 109–116. DOI:10.1016/j.orgel.2015.07.019 |
| [64] | Y.J. Chang, P.T. Chou, Y.Z. Lin, et al. Organic dyes containing oligo-phenothiazine for dye-sensitized solar cells. J. Mater. Chem. 22 (2012) 21704–21712. DOI:10.1039/c2jm35556f |
| [65] | Z. Iqbal, W.Q.Wu, H. Zhang, et al. Impact of hydroxy and octyloxy substituents of phenothiazine based dyes on the photovoltaic performance. Dyes Pigm. 99 (2013) 299–307. DOI:10.1016/j.dyepig.2013.05.032 |
| [66] | M. Cheng, X.C. Yang, C. Chen, et al. Effect of the acceptor on the performance of dye-sensitized solar cells. Phys. Chem. Chem. Phys. 15 (2013) 17452–17459. DOI:10.1039/c3cp52314d |
| [67] | Y. Hua, S. Chang, J. He, et al. Molecular engineering of simple phenothiazinebased dyes to modulate dye aggregation, charge recombination, and dye regeneration in highly efficient dye-sensitized solar cells. Chem. Eur. J. 20 (2014) 6300–6308. DOI:10.1002/chem.201304897 |
| [68] | Z.Q. Wan, C.Y. Jia, Y. Wang, et al. Significant improvement of phenothiazine organic dye-sensitized solar cell performance using dithiafulvenyl unit as additional donor. Org. Electron. 27 (2015) 107–113. DOI:10.1016/j.orgel.2015.09.009 |
| [69] | S.B. Wang, H.R. Wang, J.C. Guo, et al. Influence of the terminal electron donor in D-D-π-A phenothiazine dyes for dye-sensitized solar cells. Dyes Pigm. 109 (2014) 96–104. DOI:10.1016/j.dyepig.2014.05.015 |
| [70] | Y. Hua, S. Chang, H.D. Wang, et al. New phenothiazine-based dyes for efficient dye-sensitized solar cells: positioning effect of a donor group on the cell performance. J. Power Sources 243 (2013) 253–259. DOI:10.1016/j.jpowsour.2013.05.157 |
| [71] | C.J. Yang, Y.J. Chang, M. Watanabe, et al. Phenothiazine derivatives as organic sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. 22 (2012) 4040–4049. DOI:10.1039/c2jm13961h |
| [72] | Y. Hua, L.T.L. Lee, C.S. Zhang, et al. Co-sensitization of 3D bulky phenothiazinecored photosensitizers with planar squaraine dyes for efficient dye-sensitized solar cells. J. Mater. Chem. A 3 (2015) 13848–13855. DOI:10.1039/C5TA01665G |
| [73] | M.J. Kim, Y.J. Yu, J.H. Kim, et al. Tuning of spacer groups in organic dyes for efficient inhibition of charge recombination in dye-sensitized solar cells. Dyes Pigm. 95 (2012) 134–141. DOI:10.1016/j.dyepig.2012.04.002 |
| [74] | C.V. Kumar, D. Raptis, E.N. Koukaras, et al. Study of an indoline-phenothiazine based organic dye for dye-sensitized solar cells. Theoretical calculations and experimental data. Org. Electron. 25 (2015) 66–73. DOI:10.1016/j.orgel.2015.06.009 |
| [75] | C.J. Chen, J.Y. Liao, Z.G. Chi, et al. Effect of polyphenyl-substituted ethylene endcapped groups in metal-free organic dyes on performance ofdye-sensitized solar cells. RSC Adv. 2 (2012) 7788–7797. DOI:10.1039/c2ra20819a |
| [76] | A. Amacher, C.Y. Yi, J.B. Yang, et al. A quinoxaline-fused tetrathiafulvalene-based sensitizer for efficient dye-sensitized solar cell. Chem. Commun 50 (2014) 6540–6542. DOI:10.1039/C4CC02696A |
| [77] | S. Wenger, P.A. Bouit, Q.L. Chen, et al. Efficient electron transfer and sensitizer regeneration in stable π-extended tetrathiafulvalene-sensitized solar cells. J. Am. Chem. Soc. 132 (2010) 5164–5169. DOI:10.1021/ja909291h |
| [78] | C.A. Echeverry, M.Á. Herranz, A. Ortiz, et al. Rhodanine-3-acetic acid and pextended tetrathiafulvalene (exTTF) based systems for dye-sensitized solar cells. New J. Chem. 38 (2014) 5801–5807. DOI:10.1039/C4NJ01261E |
| [79] | Y. Geng, F. Pop, C.Y. Yi, et al. Electronic tuning effects via π-linkers in tetrathiafulvalene-based dyes. New J. Chem. 38 (2014) 3269–3274. DOI:10.1039/c4nj00428k |
| [80] | M. Marszalek, S. Nagane, A. Ichake, et al. Tuning spectral properties of phenothiazine based donor-p-acceptor dyes for efficient dye-sensitized solar cells. J. Mater. Chem. 22 (2012) 889–894. DOI:10.1039/C1JM14024H |
| [81] | X. Yang, J. Zhao, L. Wang, et al. Phenothiazine derivatives-based D-p-A and D-A-p-A organic dyes for dye-sensitized solar cells. RSC Adv. 4 (2014) 24377–24383. DOI:10.1039/c4ra01858c |
| [82] | M. Cheng, X.C. Yang, F.G. Zhang, et al. Tuning the HOMO and LUMO energy levels of organic dyes with N-carboxomethylpyridinium as acceptor to optimize the efficiency of dye-sensitized solar cells. J. Phys. Chem. C 117 (2013) 9076–9083. |
| [83] | S.H. Bae, K.D. Seo, W.S. Choi, et al. Near-IR organic sensitizers containing squaraine and phenothiazine units for dye-sensitized solar cells. Dyes Pigm. 113 (2015) 18–26. DOI:10.1016/j.dyepig.2014.07.031 |
| [84] | W.J. Wu, J.B. Yang, J.L. Hua, et al. Efficient and stable dye-sensitized solar cells based on phenothiazine sensitizers with thiophene units. J. Mater. Chem. 20 (2010) 1772–1779. DOI:10.1039/b918282a |
| [85] | W.I. Hung, Y.Y. Liao, C.Y. Hsu, et al. High-performance dye-sensitized solar cells based on phenothiazine dyes containing double anchors and thiophene spacers. Chem. Asian J. 9 (2014) 357–366. DOI:10.1002/asia.201301228 |
| [86] | Y.S. Yang, H.D. Kim, J.H. Ryu, et al. Effects of anchoring groups in multianchoring organic dyes with thiophene bridge for dye-sensitized solar cells. Synth. Met. 161 (2011) 850–855. DOI:10.1016/j.synthmet.2011.02.012 |
| [87] | M. Mao, X.L. Zhang, L. Cao, et al. Design of bodipy based organic dyes for highefficient dye-sensitized solar cells employing double electron acceptors. Dyes Pigm. 117 (2015) 28–36. DOI:10.1016/j.dyepig.2015.02.001 |
| [88] | D.R. Cao, J.A. Peng, Y.P. Hong, et al. Enhanced performance of the dye-sensitized solar cells with phenothiazine-based dyes containing double D-A branches. Org. Lett. 13 (2011) 1610–1613. DOI:10.1021/ol2000167 |
| [89] | X.F. Zang, T.L. Zhang, Z.S. Huang, et al. Impact of the position isomer of the linkage in the double D-A branch-based organic dyes on the photovoltaic performance. Dyes Pigm. 104 (2014) 89–96. DOI:10.1016/j.dyepig.2013.12.028 |
| [90] | S.H. Kim, C. Sakong, J.B. Chang, et al. The effect of N-substitution and ethylthio substitution on the performance of phenothiazine donors in dye-sensitized solar cells. Dyes Pigm. 97 (2013) 262–271. DOI:10.1016/j.dyepig.2012.12.007 |
| [91] | Z. Iqbal, W.Q. Wu, D.B. Kuang, et al. Phenothiazine-based dyes with bilateral extension of π-conjugation for efficient dye-sensitized solar cells. Dyes Pigm. 96 (2013) 722–731. DOI:10.1016/j.dyepig.2012.11.010 |
| [92] | Z. Iqbal, W.Q. Wu, H. Zhang, et al. Influence of spatial arrangements of π-spacer and acceptor of phenothiazine based dyes on the performance of dye-sensitized solar cells. Org. Electron. 14 (2013) 2662–2672. DOI:10.1016/j.orgel.2013.07.007 |
| [93] | Z. Iqbal, W.Q. Wu, Z.S. Huang, et al. Trilateral π-conjugation extensions of phenothiazine-based dyes enhance the photovoltaic performance of the dyesensitized solar cells. Dyes Pigm. 124 (2016) 63–71. DOI:10.1016/j.dyepig.2015.09.001 |
| [94] | W.I. Hung, Y.Y. Liao, T.H. Lee, et al. Eugenic metal-free sensitizers with double anchors for high performance dye-sensitized solar cells. Chem. Commun. 51 (2015) 2152–2155. DOI:10.1039/C4CC09294E |
| [95] | H.J. Jo, J.E. Nam, D.H. Kim, et al. A comparison of the electronic and photovoltaic properties of novel twin-anchoring organic dyes containing varying lengths of π-bridges in dye-sensitized solar cells. Dyes Pigm 102 (2014) 285–292. DOI:10.1016/j.dyepig.2013.10.032 |
| [96] | Z.Q. Wan, C.Y. Jia, Y.D. Duan, et al. Phenothiazine-triphenylamine based organic dyes containing various conjugated linkers for efficient dye-sensitized solar cells. J. Mater. Chem. 22 (2012) 25140–25147. DOI:10.1039/c2jm34682f |
| [97] | Z.J. She, Y.Y. Cheng, L.Q. Zhang, et al. Novel ruthenium sensitizers with a phenothiazine conjugated bipyridyl ligand for high-efficiency dye-sensitized solar cells. ACS Appl. Mater. Interfaces 7 (2015) 27831–27837. DOI:10.1021/acsami.5b09160 |
| [98] | Z.Q. Wan, C.Y. Jia, Y.D. Duan, et al. Effects of different acceptors in phenothiazine-triphenylamine dyes on the optical, electrochemical, and photovoltaic properties. Dyes Pigm. 94 (2012) 150–155. DOI:10.1016/j.dyepig.2011.12.009 |
| [99] | K.D. Seo, I.T. Choi, H.K. Kim. D-π-A organic dyes with various bulky amine-typed donor moieties for dye-sensitized solar cells employing the cobalt electrolyte. Org. Electron 25 (2015) 1–5. DOI:10.1016/j.orgel.2015.06.011 |
| [100] | J.H. Jia, K.Y. Cao, P.C. Xue, et al. Y-shaped dyes based on triphenylamine for efficient dye-sensitized solar cells. Tetrahedron 68 (2012) 3626–3632. DOI:10.1016/j.tet.2012.02.077 |
| [101] | X.X. Dai, H.L. Feng, W.J. Chen, et al. Synthesis and photovoltaic performance of asymmetric di-anchoring organic dyes. Dyes Pigm. 122 (2015) 13–21. DOI:10.1016/j.dyepig.2015.06.004 |
| [102] | Z.B. Xie, A. Midya, K.P. Loh, et al. Highly efficient dye-sensitized solar cells using phenothiazine derivative organic dyes. Prog. Photovolt.: Res. Appl. 18 (2010) 573–581. DOI:10.1002/pip.v18:8 |
| [103] | C.Y. Jung, C.J. Song, W. Yao, et al. Synthesis and performance of new quinoxalinebased dyes for dye sensitized solar cell. Dyes Pigm. 121 (2015) 204–210. DOI:10.1016/j.dyepig.2015.05.019 |
| [104] | C.J. Chen, J.Y. Liao, Z.G. Chi, et al. Metal-free organic dyes derived from triphenylethylene for dye-sensitized solar cells: tuning of the performance by phenothiazine and carbazole. J. Mater. Chem. 22 (2012) 8994–9005. DOI:10.1039/c2jm30254c |
| [105] | M.J. Cho, S.S. Park, Y.S. Yang, et al. Molecular design of donor-acceptor-type cruciform dyes for efficient dyes-sensitized solar cells. Synth. Met. 160 (2010) 1754–1760. DOI:10.1016/j.synthmet.2010.06.013 |
| [106] | T.N. Duan, K. Fan, C. Zhong, et al. Synthesis and photovoltaic property of new kind of organic dyes containing 2, 2'-bithiophene unit with three electrondonors. J. Photochem. Photobiol. A: Chem. 278 (2014) 39–45. DOI:10.1016/j.jphotochem.2013.12.019 |
| [107] | X.X. Dai, H.L. Feng, Z.S. Huang, et al. Synthesis of phenothiazine-based dianchoring dyes containing fluorene linker and their photovoltaic performance. Dyes Pigm. 114 (2015) 47–54. DOI:10.1016/j.dyepig.2014.10.016 |
| [108] | G. Marotta, M.A. Reddy, S.P. Singh, et al. Novel carbazole-phenothiazine dyads for dye-sensitized solar cells: a combined experimental and theoretical study. ACS Appl. Mater. Interfaces 5 (2013) 9635–9647. DOI:10.1021/am402675q |
| [109] | K.S.V. Gupta, J. Zhang, G. Marotta, et al. Effect of the anchoring group in the performance of carbazole-phenothiazine dyads for dye-sensitized solar cells. Dyes Pigm. 113 (2015) 536–545. DOI:10.1016/j.dyepig.2014.09.032 |
| [110] | B.C. Jeon, M.S. Kim, M.J. Cho, et al. Effect of solvent on dye-adsorption process and photovoltaic properties of dendritic organic dye on TiO2 electrode of dyesensitized solar cells. Synth. Met. 188 (2014) 130–135. DOI:10.1016/j.synthmet.2013.12.006 |
| [111] | M. Chandrasekharam, G. Rajkumar, C.S. Rao, et al. Phenothiazine conjugated bipyridine as ancillary ligand in Ru(II)-complexes for application in dye sensitized solar cell. Synth. Met. 161 (2011) 1469–1476. DOI:10.1016/j.synthmet.2011.04.001 |
| [112] | Y.S. Xie, Y.Y. Tang, W.J. Wu, et al. Porphyrin cosensitization for a photovoltaic efficiency of 11.5%: a record for non-ruthenium solar cells based on iodine electrolyte. J. Am. Chem. Soc. 137 (2015) 14055–14058. DOI:10.1021/jacs.5b09665 |
| [113] | Q.F. Xie, J. Zhou, J.M. Hu, et al. Synthesis and photovoltaic properties of branched chain polymeric metal complexes containing phenothiazine and thiophene derivative for dye-sensitized solar cells. J. Chem. Sci. 127 (2015) 395–403. DOI:10.1007/s12039-015-0790-5 |
| [114] | G. Wang, Y.Y. Wu, W.H. Ding, et al. Photovoltaic performance of long-chain poly(triphenylamine-phenothiazine) dyes with a tunable π-bridge for dyesensitized solar cells. J. Mater. Chem. A 3 (2015) 14217–14227. DOI:10.1039/C5TA03425F |
| [115] | H.J. Tan, C.Y. Pan, G. Wang, et al. Synthesis and characterization of conjugated polymers with main-chain donors and pendent acceptors for dye-sensitized solar cells. RSC Adv. 3 (2013) 16612–16618. DOI:10.1039/c3ra42161a |
| [116] | S. Chang, H.D. Wang, Y. Hua, et al. Conformational engineering of co-sensitizers to retard back charge transfer for high-efficiency dye-sensitized solar cells. J. Mater. Chem. A 1 (2013) 11553–11558. DOI:10.1039/c3ta12714a |
| [117] | S. Chang, K.Y. Wong, X.D. Xiao, et al. Effective improvement of the photovoltaic performance of black dye sensitized quasi-solid-state solar cells. RSC Adv. 4 (2014) 31759–31763. DOI:10.1039/C4RA04017A |
| [118] | Y.R.Kim, H.S. Yang, K.S.Ahn, et al. Enhancedperformanceof dye co-sensitizedsolar cells by panchromatic light harvesting. J. Korean Phys. Soc. 64 (2014) 904–909. DOI:10.3938/jkps.64.904 |
| [119] | J.S. Luo, Z.Q. Wan, C.Y. Jia, et al. Co-sensitization of dithiafulvenyl-phenothiazine based organic dyes with N719 for efficient dye-sensitized solar cells. Electrochim. Acta 211 (2016) 364–374. DOI:10.1016/j.electacta.2016.05.175 |
 2016, Vol. 27
2016, Vol. 27