Energy and environment issues are becoming more and more critical in modern society, thus clean and effective energy sources like sunlight and wind are most urgent than ever before. As a promising candidate for solving this energy problem, organometal halide perovskite solar cell (PSC) is now garnering more and more attention and being widely studied due to its remarkable photovoltaic performance and attractive potential for low-cost production [1-6]. In general, perovskite compounds typically consist of three sections with the formula of ABX3 in which the A represnts the organic cations like CH3NH3 + (MA) [7, 8] or HC(NH2)2 + (FA) [9, 10]; the B is typically divalent metal ions like Pb2+ and Sn2+; and the X usually represents the halogen anions (Cl-, Br- or I-) in which the X can be one halogen atom or two different halogen atoms mixed together through certain ratio (Fig. 1) [11-14]. Due to the merits of intense absorption in almost whole visible region, much long excitons diffusion length and ambipolar chargetransporting property with high carrier conductivity, the power conversion efficiency (PCE) of PSC has witnessed rapid increase from 3.8% to 20.1% in past few years [15-19]. Besides the ultrahigh PCE, the relatively low production cost of PSCs has also attracted many scientists and researchers expecially those from the industrial area who aim to make this kind of technology into commercialization. What's more, another striking advantage of PSC is the band gap of perovskite materials can also be easily tuned via altering the halogen. For example, by partialy or completely relacing the iodide in commonly used CH3NH3PbI3 with bromide or chorine, absorption edge of perovskite semiconductors can conveniently move from 800 nm to orange region or even to blue region [19].

|
Download:
|
| Figure 1. Crystal structures of cubic perovskite with generic chemical formula ABX3. Reprinted with permission. Copyright 2014, Nature Publishing Group. (b) Scheme diagram of device structure (ITO/TiOx/Perovskite/HTMs/Ag). Reprinted with permission. Copyright 2016, Willy Publishing Group. (c) The structure of the first hole-transporting material, spiro-OMeTAD, for use in PSCs. | |
In the view of structure, the perovskite solar cell can be classified as the mesoporous and planar type. Compared with the mesoporous structure, planar PSCs in which perovskite layer is sandwiched between flat hole and electron transporting layer show predominant advantages like versatility to perform the optimization and great potential in flexible applications to avoid high temperature [20]. Depending on whether the bottom electrode (indium tin oxide (ITO) or fluorine-doped tin oxide (FTO)) acts as a cathode or an anode, planar perovskite solar cells can be sorted into n-i-p (conventional) or p-i-n (inverted) structure, where n- and p-represent n-type and p-type chargetransporting material respectively and i represents the active perovskite layer.
Perovskite materials firstly emerged as light absorbers in dyes sensitized solar cells (DSSCs) with the liquid electrolyte were introduced by Miyasaka and co-workers in 2009 [21]. At that stage, the PCEs of both triiodide and tribromide perovskite were merely around 4%. In 2011, Park and co-workers further pushed the PCE to around 6.5% also with the liquid electrolyte at AM 1.5G illumination by modifying the surface of TiO2 and optimizing the deposition process of perovskite materials [22]. Nevertheless, due to existence of liquid electrolyte which can easily dissolve the perovskite material and deteriorate the stability, the perspective of PSC was greatly impeded for a while. In 2012, a big breakthrough was made both by Snaith et al. and Grä tzel et al. who independently applied a solid small molecule hole-transporting material (HTM) i.e. 2, 2', 7, 7'-tetrakis(N, N-di-pmethoxyphenyl- amine) 9, 9'-spiro-bifluorene (spiro-OMeTAD) in PSCs to replace the liquid electrolyte and achieved a PCE of 7.6% and 9.7%, respectively [7, 8]. Since then, much effort has been devoted on improving PSCs in the aspects of stability, PCEs, interfacial optimization, techniques to make the perovskite film smoother and more uniform and optimization of HTMs to ensure effective charge dissociation [23-26]. Among them, the intense research on HTMs is of great importance due to its key role in ensuring ohmic contact of perovskite layer and the back metal electrode, suppressing the charge recombination and transfer holes from the active perovskite material to the corresponding electrode and so on. Considering the significant role of HTMs in PSCs, in this review, we will extensively expound the multiple functions of HTMs, followed by the recent development of various types of HTMs (especially on p-conjugated small molecules) and then the design rules for highly efficient HTMs in planar type PSCs. In the last, the direction toward further development of HTMs will also be discussed.
2. Energy level tuning function of HTMsAs an interfacial material between the active perovskite layer and the metal electrode, energy levels of HTMs play vital roles to simultaneously enhance charge decombination and transportation and determine the open circuit voltage (Voc) [27]. Fig. 2a displayed the energy diagram of PSCs. Generally, the Voc of PSC is mainly governed by difference between the highest occupied molecular orbital (HOMO) of the HTM and the lowest occupied molecular orbital (LUMO) of the perovskite semiconductor [28]. To achieve higher Voc, the ohmic contact with the electrode is desirable, since the Schottky barrier formed at the electrode can cause the Voc loss and lead to lower Voc [29]. Hence, appropriate HOMO to align the perovskite layer and the metal electrode is required to minimize the barrier thus achieve higher Voc. Besides to minimize the barrier loss and yield high Voc, appropriate HOMO can also promote the holes transportation and reduce the probability of charge recombination. Meanwhile, there are reports about the shallower LUMO of HTMs which can also show some positive effects in blocking the hole-electron pair recombination [30, 31]. Considering the key functions of HOMO level and aiming to delicately tune energy level of HTMs, Meng and co-workers once developed two effective HTMs based on N, N, N', N'-tetraphenyl-benzidine (TPB) core structure [23]. Due to the superb hole mobility of the TPB building block and convenience to modification, the authors utilized TPB as building block and appended 3, 4-ethylenedioxythiophene (EDOT) and N-ethylcarbazole (NEC) as the side group to tune the HOMO level of target conjugative molecules. As the results showed in Fig. 2, the two HTMs based on TPB core show suitable HOMO levels and both materials exhibit deeper HOMO than the spiro-OMeTAD. As expected, when utilizing these two molecules as HTMs, the Voc of both materials is higher than that of spiro-OMeTAD. Nevertheless, given that the driving force to transport holes is needed, the HOMO of HTM is usually shallower than valence band of perovskite materials. Thus, there is a limit for the maximum Voc theoretically.
|
Download:
|
| Figure 2. (a) Molecular structures of HTMs; (b) the energy level diagram of the corresponding materials used in perovskite solar cells. Reprinted with permission. Copyright 2014, ROYAL SOCIETY OF CHEMISTRY Publishing Group. | |
3. Improving charge carrier mobility of HTMs
The first solid small conjugative HTM i.e. spiro-OMeTAD aimed to replace the liquid electrolyte and solve the encapsulation and stability issues [7, 8]. For small molecule spiro-OMeTAD, it has significant advantage in thermal stability because of its rigid spiro structure. Nevertheless, low hole mobility as well as fussy synthetic and purification process have greatly increased fabrication cost and impeded its further commercialization process [32-34]. On the other hand, fill factor (FF) of PSC is mainly affected by the resistance and conductivity of HTMs. In order to improve the FF, many efforts have been focused on improving the hole mobility and conductivity of spiro-OMeTAD, or replacing it. In small π-functionalized molecules, recently, Jung Son et al. developed a novel HTM based on 2, 2 paracyclophane core (PCP-TPA) [35]. Starting from commercially available material, PCP-TPA can be prepared facilely through several steps. Besides, considering rigid, cylindrical and 3-D structure of the target molecules, efficient and dense packing pattern can be predicted which is conductive to the charge hopping between neighboring molecules (Fig. 3). Noticeably, the PCE based on PCP-TPA is much higher than that of spiro-OMeTAD with 17.6% vs. 15.4%. Aiming to improve chargetransporting mobility and developed dopant-free HTMs, Sun and co-workers designed and synthesized an A-D-A type small molecule based on a rigid benzo-dithiophene building block [36]. By utilizing phenoxazine as linker and 3-ethylrhodanine as terminal group, a dopant-freeHTM(M1) was implemented in PSCs. Due to intense absorption in visible region, large conjugate length and efficient π-π stacking; an impressive PCE of around 13% was delivered which was comparable to that of doped spiro-OMeTAD (12.4%).
|
Download:
|
| Figure 3. J vs. V plots of the polymer films. The solid lines are fits of the data points.Reprinted with permission. Copyright 2015, ROYAL SOCIETY OF CHEMISTRY Publishing Group. | |
4. Dopant-free HTMs to enhance stability
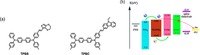
As aforementioned, dopant-free HTMs mean the HTMs do not need any dopants to enhance mobility, which is a fascinating property for HTMs research. Before starting this part, it is should be noted that spiro-OMeTAD actually has relative low hole conductivity in pristine and it commonly needs chemical P-type dopants like lithium-bis(trifluoromethanesulfonyl)imide and 4-tert-butylpyridine (TBP) whose deliquescent property is detrimental to the stability [37]. Thus, it is desirable to develop dopant-free HTMs to enhance stability and promote the process of commercialization of PSCs. Without dopants, the fabrication steps can be simplified and the cost can also be reduced. Very recently, Jiang et al. developed an novel small molecule HTM SAF-OMe. [27] It is believed to combine the advantages of two star HTMs in small molecule and conducting polymers. When utilized as dopant-free HTM in a specific device, an high PCE of 12.39% was achieved, while spiro-OMeTAD only gave PCE around 5%. Comparing the main difference between all the parameters involved, it was clearly to find out that the FF which reflecting ability of conductivity accounted for this advance (Fig. 4b). As expected, hole mobility of SAF-OMe was greatly improved because of more active spiro-core for hole transport. Furthermore, when utilizing this material with doped form, an encouraging PCE of 16.73% was also affored. Noticeably, the stability based on this HTM was also improved compared with the reference one with spiro-OMeTAD and the authors tenatively acribed this to the larger contact angle which could effectivly expel moisture from the perovskite film (Fig. 4c and d).

|
Download:
|
| Figure 4. (a) Scheme diagram of device structure (ITO/TiOx/Perovskite/HTMs/Ag). (b) The hole mobility measurement of SAF-OMe and spiro-OMeTAD from the space charge limitation of current (SCLC) J-V characteristics obtained in the dark for hole-only devices. (c) Contact angle of SAF-OMe. (d) Contact angle of spiro-OMeTAD. Reprinted with permission. Copyright 2015, Willy Publishing Group | |
Han et al. once reported a dopant-free HTM with tetrathiafulvalene derivative (TTF-1) [37]. In merits of the suitable HOMO level, efficient π-π stacking and intense S-S interactions between adjacent molecules, a record PCE around 11% was achieved. Besides high PCE, stability based on TTF-1 is also two times higher than that of spiro-OMeTAD which provided a design to for efficient HTMs. In order to improve the hole mobility of core structure, Zakeeruddin et al. once developed a HTM (named spiro-s) by replacing the spiro-bifluorene core with spiro-dithiophene [38]. Through this way, the hole mobility was much increased. The novel HTM yielded high PCE of 13% which was comparable to the doped spiro-OMeTAD (~15%). However, when further incorporating P-type dopants or additives in HTM, PCE was a little lower than the dopant-free one. Besides, light-soaking effect which has proven to be an efficient way to enhance performance also observed with this material as HTM.
5. Recent progress on π-functionalized small molecule HTMsGiven the important role of HTMs, now research on this field is developing rapidly and numerous novel HTMs which can enhance PCE and/or stability as well as reduce the fabrication cost in aspects of organic π-functionalized small molecules. We are attracted in how these π-functionalized units are combined to construct materials. In order to account for these new developments, we focus in this review on the chemical structures of various HTMs,
5.1. Spiro structure based small HTMsThe first HTM spiro-OMeTAD was constructed by the spirobifluorene core, which enlightened a wide range of HTMs with different spiro structures. The hole conductor contains a spirocenter with a tetrahedral atom to link two aromatic moieties, which was initially introduced in order to improve the glassforming properties and prevent crystallization of the organic material. Previous report found that molecules with a symmetric globular structure, large molecular weight, and small intermolecular cohesion result in a high stability of the amorphous state. That is an important reason why spiro-type HTMs are so prevalent up to date.
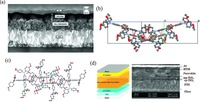
The arylamine side chains of spiro-OMeTAD have p-OMe substituents, which can be tailored by position engineering in organic synthesis. Seol et al. prepared pp-, pm-, and po-spiro- OMeTAD derivatives that were applied as HTMs in MAPbI3 perovskite based solar cells. They find this is an effective way to improve the material performance without changing the spirocore. The highest PCE of 16.7% was achieved by po-spiro-OMeTAD, and it was supposed that the increased FF due to its low series resistance (Rs) and high shunt resistance (Rsh) accounted for this result [39]. The core of spiro-OMeTAD is 9, 9'-spiro-bifluorene, which can also be changed. The spiro-bipropylenedioxythiophene block can replace it to form a new spiro-type HTM named PST1 [40]. And the SEM image which shows film morphology is displayed in Fig. 5a. By fully investigating single crystal structure of PST1, multiple CH/π and π-π intermolecular contacts had been observed. Due to the existence of these conjugative forces, packing structure of target molecule can be enhanced. For the first time, single crystal structure of spiro-OMeTAD was also investigated for comparison (Fig. 5b and c). When applying PST1 as HTM in PSCs, an encouraging PCE of 13.44% was achieved. Surprisingly, when used as HTM without adding cobalt dopant, PSC can also work efficiently and a comparable PCE of 12.74% was achieved. Besides the spiro-s discussed above [40], Tu et al. synthesized a novel HTM (SCPDT-BiT) by using thiophene derivative as spiro-core unit [41]. Instead using the diphenylamine as terminal group, thiophene with alkyl group was used to enhance the solubility and tune HOMO level. When applying as HTM, promising PCE of 10.4% was afforded.
|
Download:
|
| Figure 5. (a) Cross-section SEM image of the device with PST1 as HTM. (b) Dashed red lines illustrate CH/π hydrogen bonds and π-π short contacts observed in crystal of PST1 (ca. 2.8 A and 3.25 A). (c) Dashed red lines illustrate π-π short contacts (ca. 3.39 A and 3.09 A). (d) Device structure and cross-section SEM image of the device. Reprinted with permission. Copyright 2016, ROYAL SOCIETY OF CHEMISTRY Publishing Group. | |
Chen and co-workers once combined advantages of triphenylamine and spiro structure and developed three novel HTMs (CW3, CW4 and CW5) [42]. By changing the bulk alkyl group at the paraposition of nitrogen atom in the triphenylamine unit, the morphology of HTM as well as the hole mobility can be finely tuned (Fig. 5d showed SEM and device diagram). As results showed, when size the alkyl group is moderate, both film morphology and conductivity are suitable to achieve high efficiency. When utilizing two additives without further cobalt dopant, a champion PCE of 16.56% was achieved by using CW4 as HTM.
The spiro block is not always constructed as central group, Jin and co-workers reported two small molecule HTMs using cabarzole derivative as core structure and two 9, 9'-spiro-bifluorene as the terminal group [43]. In order to enhance the solubility of target molecules and expand conjugation length, alkyl-fluorene was inserted between these two functionalized units. From the point of design concept, a contrary way was utilized compared to spiro-OMeTAD which used 9, 9'-spiro-bifluorene as core and diphenylamine as ending group (CzPAF-SBF and CzPAF-SBFN). When utilized as HTMs, an record PCE of 17.21% was achieved. When further investigating stability based on these two HTMs in PSCs, results were also very desirable with CzPAF-SBF decreased only around 30% at the end of test time (500 h), while spiro- OMeTAD based device showed much reduced PCE near to 60%. Contact angle tests showed that as compared with spiro-OMeTAD (65.1°), CzPAF-SBF (95.2°) exhibited much larger contact angle which means it can expel moisture more efficiently. All the relative molecules are sketched in Fig. 6.
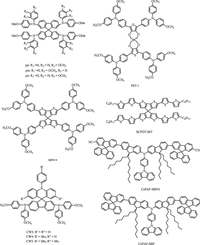
|
Download:
|
| Figure 6. HTMs with spiro structures | |
5.2. Starburst structure based small HTMs
The amorphous glassy state is particularly desirable in active materials, primarily because the amorphous character of the resulting thin films prevents any grain boundary which is believed to deteriorate the device. Small organic molecules can also form stable amorphous glasses above room temperature if their molecular structures are properly designed. Many compounds that readily form amorphous glasses are π-electron starburst molecules, with more than three branches stretching into different directions. For example, oligothiophenes are very crystalline in nature; however, oligothiophenes end-capped by a triarylamine group easily form amorphous states. In this way, numerous HTMs with star-shape are designed and synthesized.
DMFA-FA and DMFA-TPA are two star-type triphenylamine based HTMs developed by Ko and co-workers [44]. When introducing bis-dimethylfluoreny to triphenylamine or fused triphenylamine, the capacity of good film can be maintained due to the existence of bulky nonplanar property of bi-dimethyfluorenyl group. When using the fused triphenylamine as HTM, an encouraging PCE of 14.21% was achieved. Besides, stability results showed that the durability of PSC based on this HTM was also comparable to that of spiro-OMeTAD. To simplify synthetic routes, they further developed two HTMs by utilizing varing numbers of triphenylamine derivative as decorating unit and linked by double bond (Ethene DTPA and TTPA) [45]. A champion PCE of 12.77% was achieved when using starburst Ethene TTPA as HTM, which was comparable of that of spiro-OMeTAD (13.28%).
Besides, Kim et al. recently reported three novel star-shaped HTMs with hexyloxyl diphenylamine-modified carbazole functional unit as ending group (SGT-409, SGT-410 and SGT-411) [46]. When varying the core structure from 1, 4-phenyl, 4, 4'-biphenyl or 1, 3, 5-trisphenylbenzene, the effective π-π stacking can be finely tuned thus the hole conductivity changed. When utilizing 1, 3, 5- trisphenylbenzene based conjugated small molecule as HTM, an impressive PCE of 13.0% was afforded.
As discussed above, extended π-conjugation functional units are useful for transferring carriers. In this field, Nazeeruddin and co-workers successfully synthesized a series of trizatruxene-based HTMs [47]. Starting from relatively cheap materials and adopting facile synthetic procedure, HTMs (KR122, KR131, KR133 and KR145) with suitable energy level to align perovskite and high conductivity was developed. When applied as HTMs in PSCs, an high PCE around 18% was successfully delivered with KR131.
Without the appending side groups, triazatruxene core can also act as good HTM because of its co-planar property to enhance π-π stacking and hole mobility. Yang and co-workers once utilized the triazatruxene derivative as HTM (TPDI) and carbon cathode to fabricate high efficiency and stable device in PSCs. [47] In this research, the effects of the dopants on stability were also investigated. As can be seen from the results, the stability of PSC with doped TPDI showed reduced PCE with time going on, while the PCE of pristine film almost maintained constant. Besides, the PCEs with doped and pristine TPDI were both higher than 13%. This research provides us an effective way to achieve both high and stable PSCs.
Recently, Grätzel and co-workers developed a promising HTM based on linear carbazole unit [48]. By appending two methylmodified carbazole to the ortho-position of benzene and decorating four side groups to the 3, 6 positions of carbazole, the researchers prepared a highly efficient HTM (V886). As compared with spiro-OMeTAD, a lower lateral while a little higher vertical mobility was observed for V886. Considering the amorphous film of V886, it was legitimate to link horizontal conductivity with vertical conductivity. However, in PSC type device structure, the vertical conductivity was a more predominant parameter due to its vertical transportation. As expected, PCE based on V886 was as high as 16.91% which was comparable to that of spiro-OMeTAD.
Nanographenes are typical P-type molecules which have been thoroughly investigated in last few years. In generally, nanographenes have strong electron-donating property and large coplanar structure which are suitable for use as charge tranportation materials in PSCs. In this field, Zheng and co-workers firstly used thiolated nanographene derivative (TSHBC) as HTM in PSCs [49]. Interestingly, due to the existence of sulfur (S) atom in thiolated derivate, Pb-S bond was formed between perovskite and TSHBC HTM. This extra bond was conductive to the hole extraction even hole mobility of TSHBC was very low (~4.0×10-15 cm2 V-1 s-1). In order to enhance hole mobility and overall performance, highly conductive graphene sheets was utilized. As expected, when doped with graphene sheets, both Voc and Jsc were improved and a champion PCE of 14.0% was successfully achieved. Another great advantage of TSHBC or thiolated derivatives is its hydrophobic nature which is desirable to improve stability. Herein, TSHBC based material exhibited much larger contact angle (~107°) than spiro- OMeTAD (~70°), thus much better stability for TSHBC was expected. All the relative molecules are shown in Fig. 7.
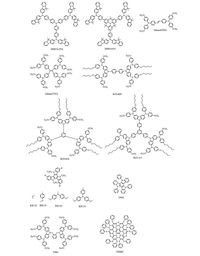
|
Download:
|
| Figure 7. HTMs with starburst structures | |
5.3. Other types of small HTMs
Besides spiro or starburst structures, there are other linking ways to design HTMs. The first one is constructing a π-conjugated backbone then appending electron-donating group at the end to modulating HOMO levels, another one is utilizing donor-acceptor alternating structures, which is commonly used in organic photovoltaic active materials. Both methods lead to enlarged conjugated backbone, enabling the resultant molecules to absorb visible light. But consider the much strong light-absorption of perovskite active layer, this minor drawback might be ignored.
In view of this, Grimsdale and co-workers developed a simple HTM just by appending two triphenylamine derivatives to the 2, 5 position of furan (F101) [50]. For efficient HTM, suitable HOMO to transfer holes and high conductivity to minimize series resistance are desirable. In this work, the oxygen atom in furan can tune the HOMO level and the triphenylamine part can enhance hole mobility. From theoretical simulation results, a bigger reorganization energy of F101 was observed, while the hole mobility was larger than that of spiro-OMeTAD which was not reasonable due to the larger reorganization energy. When further investigating the stacking model of F101, a more organized face-to-face stacking model was found which is conductive to the hopping-mechanism of organic molecules. When worked as HTM, a good PCE of around 13% was achieved.
Murata and co-workers once developed a two-dimensionally expanded p-system with azulene as core structure [51]. Azulene which consists of a five- and a seven-membered ring is a nonbenzenoid aromatic hydrocarbon. Via decorating with oxygen- bridged triarylamines as quasiplanar scaffolds, target molecules can exhibit much delocalized p-conjugation and thus high carrier mobility (AZU-1, AZU-2, AZU-3 and AZU-4). When varying numbers of modificants, hole mobility and energy level can be finely tuned. By optimizing ratio of dopants and additives, morphology and other parameters, an impressive PCE of 16.5% was finally achieved. This work highlights the great potential of extended π-conjugated HTMs in PSCs.
Perylenediimide which has extend conjugation length, deep HOMO to assure high Voc is commonly used in OPV application. Besides, as we mentioned above, when varying halogen atom of perovskite material, the band-gap can be finely tuned. On the other hand, CH3NH3PbBr3 which has a band-gap about 1.5 eV is of great interest due to its potential use as top cell in tandem device to achieve high Voc and excellent PCE. Given advantages of both PDI core structure and bromide perovskite material, Hodes and coworkers once developed an PDI based HTM (Th-PDI) which was modified with alkyl-thiophene unit to further enhance solubility and hole mobility [52]. As expected, a champion PCE of 5.6% was delivered. Besides, stability based on this HTM was also desirable with <20% drop off after storage of 37 days at 25%-30% relative humidity.
For spiro-OMeTAD, another big issue for its further use is the fussy purification process. In order to reduce synthetic cost and simplify purification process of HTM, Docampo and co-workers developed a low cost azomethine-based HTM (EDOT-OMeTPA) [53]. Without using expensive transition metal catalysts and inert atmosphere, a green synthetic route - Schiff base condensation reaction was introduced to develop a simple azomethine-based HTM with water being the only by-product. Besides, when utilized as HTM, PCE exceeding 11% was reached. These results provide a promising way to develop environment-friendly HTM for further commercialization of PSCs.
Small conjugated D(onar)-A(cceptor) type molecules that have increased crystallinity and appropriate energy level are ideal as HTMs and have been widely investigated in organic electronic devices. However, for HTMs in PSCs, they have been rarely reported. So Yang and co-workers once introduced one D-A type small molecule (DOR3T-TBDT) as dopant-free HTM [54]. Due to the good packing structure and super hole mobility and conductivity, a record PCE of 14.9% was achieved which was even higher than that of doped spiro-OMeTAD (14%). This result highlighted the potential of D-A type small molecule dopant-free HTM.
As discussed above, D-A type molecule can show great advantages when acts as charge transportation materials. In this regard, Mishra and co-workers developed two S, N-heteropentacene A-D-A type conjugated molecules [55]. Due to the extended and rigid conjugation length of the central S, N-based fused aromatic compound and strong dipole interaction of donor and acceptor, the hole mobility of target molecules can be greatly improved (SN-1 and SN-2). Besides, the suitable HOMO assured cascade transfer of holes and ohmic-contact which can guarantee favorable FF and Voc. Based on these merits, a favorable PCE of 11.4% was reached.
For now, main task for further commercialized PCE is the stability. In this regard, Nazeeruddin and co-workers did a great job in improving the stability [56]. Besides, predicting stability of PSCs is still a big problem due to lack of corresponding research. In this study, a protocol which was according to the conditions of measurement was also proposed to forecast the lifetime of PSCs. By utilizing the silicon based core and triphenylamine derivative as side chain, two stable HTMs (PEH-1 and PEH-2) were synthesized and implemented in PSCs. When utilizing the advocating mechanism in this work, reference spiro-OMeTAD showed a half-lifetime around 1 K h, which is much shorter than that of PEH-2 (6 K h). In this research, the authors ascribed the much enhanced stability to the superior thermal stability of silolothiophene and confirmed the HTMs are playing vital role for better stability. All the relative molecules are sketched in Fig. 8. And all the pertinent parameters for perovskite solar cells with different HTMs are summarized in Table 1.
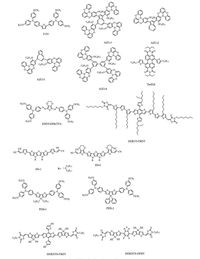
|
Download:
|
| Figure 8. HTMs other than spiro or starburst structures | |
|
|
Table 1 Device parameters for perovskite solar cells with different HTMs |
6. Conclusion and outlook
In this review, we have extensively summarized the latest progress in small molecule HTMs from their functionalization point of view. Given numerous great merits especially chemical versatility to endow varying functionalizations, small π-conjugated molecules are gradually playing important role in HTMs.
As the PCE of PSCs has reached to a certificate of 20.1%, further development of PSC will involve around commercialization process from aspects of flexible device and up-scale application in daily life especially its great potential as decorations like used on the surface of the windows [20]. Nevertheless, stability of PSC is still a big issue and should not be neglected. As previous work reported, P-type dopants and additives which are usually used to enhance hole mobility, morphology and Voc are detrimental to the stability due to their moisture-sensitive property. Besides, due to the superfast mobility (~66 cm2 V-1 S-1) of commonly used perovskite material (MAPbI3) [57], thicker HTMs (around 200 nm) are usually necessary to assure large shunting paths. However, thicker film and low hole mobility of generally HTMs can easily cause minimized series resistance thus reduced FF. In this regard, developing high conductive HTMs with extended π-conjugation length are ideal. Besides, from point of further commercialization, facile synthetic and purification routes with relatively cheap starting materials are also required.
As for the design rules of HTMs, recently, D-A type molecule with rigid structure is attracting increasing attention. Due to good co-planar property and long conjugated chain, D-A type materials usually show intense π-π stacking as well as high hole mobility and conductivity. For example, Yang and co-workers recently developed two dopant-free HTMs (DERDTS-TBDT and DORDTSDFBT) for further understanding the relationship between the molecular structure and device performance [58]. With dithienosilole (DTS) and 3-alkyl rodanine as electron-donating and withdrawing unit, core structure which used to tune energy level was varied from electron donating-type to withdrawing type. Noticeably, a encouraging PCE of 16.2% was achieved without dopants or additives. This work evidently proved a good HTM cannot simply be understood by only using electron-rich blocks. This design method provided us an efficient way to develop more dopant-free HTMs by reasonably combing both donor and acceptor units.
Although PSCs have reached extremely high PCE and achieved lots of great achievements, there is still much room for further improvement and a long way to go. For HTMs, it is also just a beginning and still needs chemists to design betterHTMs. Aiming to resolve all the existed problems, we believe, the booming progress of π-functionalized small molecules is not only inexpensible, but also a powerful tool to further propel the progress of all organic optoelectronics.
Acknowledgments We thank to the financial support from the National Natural Science Foundation of China (Nos. 21572152 and 61575136). This project is also funded by Collaborative Innovation Center (CIC) of Suzhou Nano Science and Technology, and by the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD).| [1] | L. Etgar, P. Gao, Z. Xue, et al. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 134 (2012) 17396–17399. DOI:10.1021/ja307789s |
| [2] | M.Z. Liu, M.B. Johnston, H.J. Snaith. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501 (2013) 395–398. DOI:10.1038/nature12509 |
| [3] | P. Gao, M. Grätzel, M.K. Nazeeruddin. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 7 (2014) 2448–2463. DOI:10.1039/C4EE00942H |
| [4] | M.A. Green, A. Ho-Baillie, H.J. Snaith. The emergence of perovskite solar cells. Nat. Photonics 8 (2014) 506–514. DOI:10.1038/nphoton.2014.134 |
| [5] | P. Qin, S. Paek, M.I. Dar, et al. Perovskite solar cells with 12.8% efficiency by using conjugated quinolizino acridine based hole transporting material. J. Am. Chem. Soc. 136 (2014) 8516–8519. DOI:10.1021/ja503272q |
| [6] | M. Grätzel. The light and shade of perovskite solar cells. Nat. Mater. 13 (2014) 838–842. DOI:10.1038/nmat4065 |
| [7] | H.S. Kim, C.R. Lee, J.H. Im, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2 (2012) 591. |
| [8] | M.M. Lee, J. Teuscher, T. Miyasaka, T.N. Murakami, H.J. Snaith. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338 (2012) 643–647. DOI:10.1126/science.1228604 |
| [9] | G.E. Eperon, S.D. Stranks, C. Menelaou, et al. Formamidinium lead trihalide: a broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 7 (2014) 982–988. DOI:10.1039/c3ee43822h |
| [10] | J.W. Lee, D.J. Seol, A.N. Cho, N.G. Park. High-efficiency perovskite solar cells based on the black polymorph of HC(NH2)2PbI3. Adv. Mater. 26 (2014) 4991–4998. DOI:10.1002/adma.201401137 |
| [11] | F. Hao, C.C. Stoumpos, D.H. Cao, R.P.H. Chang, M.G. Kanatzidis. Lead-free solidstate organic-inorganic halide perovskite solar cells. Nat. Photonics 8 (2014) 489–494. DOI:10.1038/nphoton.2014.82 |
| [12] | F. Hao, C.C. Stoumpos, R.P.H. Chang, M.G. Kanatzidis. Anomalous band gap behavior in mixed Sn and Pb perovskites enables broadening of absorption spectrum in solar cells. J. Am. Chem. Soc. 136 (2014) 8094–8099. DOI:10.1021/ja5033259 |
| [13] | N.K. Noel, S.D. Stranks, A. Abate, et al. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 7 (2014) 3061–3068. DOI:10.1039/C4EE01076K |
| [14] | J.H. Heo, D.H. Song, S.H. Im. Planar CH3NH3PbBr3 hybrid solar cells with 10.4% power conversion efficiency, fabricated by controlled crystallization in the spincoating process. Adv. Mater. 26 (2014) 8179–8183. DOI:10.1002/adma.201403140 |
| [15] | J. Burschka, N. Pellet, S.J. Moon, et al. Sequential deposition as a route to highperformance perovskite-sensitized solar cells. Nature 499 (2013) 316–319. DOI:10.1038/nature12340 |
| [16] | W. Nie, H. Tsai, R. Asadpour, et al. Solar cells. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 347 (2015) 522–525. DOI:10.1126/science.aaa0472 |
| [17] | H.P. Zhou, Q. Chen, G. Li, et al. Interface engineering of highly efficient perovskite solar cells. Science 345 (2014) 542–546. DOI:10.1126/science.1254050 |
| [18] | G. Hodes. Perovskite-based solar cells. Science 342 (2013) 317–318. DOI:10.1126/science.1245473 |
| [19] | P. Qin, S. Tanaka, S. Ito, et al. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 5 (2014) 3834. |
| [20] | Y. Wang, S. Bai, L. Cheng, et al. High-efficiency flexible solar cells based on organometal halide perovskites. Adv. Mater. 28 (2016) 4532–4540. DOI:10.1002/adma.v28.22 |
| [21] | A. Kojima, K. Teshima, Y. Shirai, T. Miyasaka. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131 (2009) 6050–6051. DOI:10.1021/ja809598r |
| [22] | J.H. Im, C.R. Lee, J.W. Lee, S.W. Park, N.G. Park. 6.5% efficient perovskite quantumdot-sensitized solar cell. Nanoscale 3 (2011) 4088–4093. DOI:10.1039/c1nr10867k |
| [23] | Y.K. Song, S.T. Lv, X.C. Liu, et al. Energy level tuning of TPB-based hole-transporting materials for highly efficient perovskite solar cells. Chem. Commun. 50 (2014) 15239–15242. DOI:10.1039/C4CC06493C |
| [24] | J.W. Jung, C.C. Chueh, A.K.Y. Jen. High-performance semitransparent perovskite solar cells with 10% power conversion efficiency and 25% average visible transmittance based on transparent CuSCN as the hole-transporting material. Adv. Energy Mater. 5 (2015) 1500486. DOI:10.1002/aenm.201500486 |
| [25] | G.A. Sepalage, S. Meyer, A. Pascoe, et al. Copper (Ⅰ) iodide as hole-conductor in planar perovskite solar cells: probing the origin of J-V hysteresis. Adv. Funct. Mater. 25 (2015) 5650–5651. DOI:10.1002/adfm.201502541 |
| [26] | H.R. Li, K.W. Fu, A. Hagfeldt, et al. A Simple 3, 4-ethylenedioxythiophene based hole-transporting material for perovskite solar cells. Angew. Chem. Int. Ed 53 (2014) 4085–4088. DOI:10.1002/anie.201310877 |
| [27] | Y.K. Wang, Z.C. Yuan, G.Z. Shi, et al. Dopant-free spiro-triphenylamine/fluorene as hole-transporting material for perovskite solar cells with enhanced efficiency and stability. Adv. Funct. Mater. 26 (2016) 1375–1381. DOI:10.1002/adfm.v26.9 |
| [28] | K.G. Lim, H.B. Kim, J. Jeong, et al. Boosting the power conversion efficiency of perovskite solar cells using self-organized polymeric hole extraction layers with high work function. Adv. Mater. 26 (2014) 6461–6466. DOI:10.1002/adma.201401775 |
| [29] | C.C. Chueh, C.Z. Li, A.K.Y. Jen. Recent progress and perspective in solutionprocessed Interfacial materials for efficient and stable polymer and organometal perovskite solar cells. Energy Environ. Sci. 8 (2015) 1160–1189. DOI:10.1039/C4EE03824J |
| [30] | Z.J. Ning, Y. Fu, H. Tian. Improvement of dye-sensitized solar cells: what we know and what we need to know. Energy Environ. Sci. 3 (2010) 1170–1181. DOI:10.1039/c003841e |
| [31] | N.J. Jeon, J. Lee, J.H. Noh, et al. Efficient inorganic-organic hybrid perovskite solar cells based on pyrene arylamine derivatives as hole-transporting materials. J. Am. Chem. Soc. 135 (2013) 19087–19090. DOI:10.1021/ja410659k |
| [32] | M.K. Wang, J.Y. Liu, N.L. Cevey-Ha, et al. High efficiency solid-state sensitized heterojunction photovoltaic device. Nano Today 5 (2010) 169–174. DOI:10.1016/j.nantod.2010.04.001 |
| [33] | B. Cai, Y.D. Xing, Z. Yang, W.H. Zhang, J.S. Qiu. High performance hybrid solar cells sensitized by organolead halide perovskites. Energy Environ. Sci. 6 (2013) 1480–1485. DOI:10.1039/c3ee40343b |
| [34] | J.J. Wang, S.R. Wang, X.G. Li, et al. Novel hole transporting materials with a linear π-conjugated structure for highly efficient perovskite solar cells. Chem. Commun. 50 (2014) 5829–5832. DOI:10.1039/c4cc01637h |
| [35] | S. Park, J.H. Heo, C.H. Cheon, et al. A[2, 2] paracyclophane triarylamine-based hole-transporting material for high performance perovskite solar cells. J. Mater. Chem. A 3 (2015) 24215–24220. DOI:10.1039/C5TA08417B |
| [36] | M. Cheng, B. Xu, C. Chen, et al. Phenoxazine-based small molecule material for efficient perovskite solar cells and bulk heterojunction organic solar cells. Adv. Energy Mater. 5 (2015) 1401720. DOI:10.1002/aenm.201401720 |
| [37] | J. Liu, Y.Z. Wu, C.J. Qin, et al. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci 7 (2014) 2963–2967. DOI:10.1039/C4EE01589D |
| [38] | M. Franckevičius, A. Mishra, F. Kreuzer, et al. A dopant-free spirobi[cyclopenta[2, 1-b: 3, 4-b'] dithiophene] based hole-transport material for efficient perovskite solar cells. Mater. Horiz 2 (2015) 613–618. DOI:10.1039/C5MH00154D |
| [39] | N.J. Jeon, H.G. Lee, Y.C. Kim, et al. o-Methoxy substituents in spiro-OMeTAD for efficient inorganic-organic hybrid perovskite solar cells. J. Am. Chem. So 136 (2014) 7837–7840. DOI:10.1021/ja502824c |
| [40] | P. Ganesan, K.W. Fu, P. Gao, et al. A simple spiro-type hole transporting material for efficient perovskite solar cells. Energy Environ. Sci 8 (2015) 1986–1991. DOI:10.1039/C4EE03773A |
| [41] | S.Y. Ma, H. Zhang, N. Zhao, et al. Spiro-thiophene derivatives as hole-transport materials for perovskite solar cells. J. Mater. Chem. A 3 (2015) 12139–12144. DOI:10.1039/C5TA01155H |
| [42] | M.H. Li, C.W. Hsu, P.S. Shen, et al. Novel spiro-based hole transporting materials for efficient perovskite solar cells. Chem. Commun. 51 (2015) 15518–15521. DOI:10.1039/C5CC04405G |
| [43] | S.S. Reddy, K. Gunasekar, J.H. Heo, et al. Highly efficient organic hole transporting materials for perovskite and organic solar cells with long-term stability. Adv. Mater. 28 (2016) 686–693. DOI:10.1002/adma.201503729 |
| [44] | H. Choi, J.W. Cho, M.S. Kang, J. Ko. Stable and efficient hole transporting materials with a dimethylfluorenylamino moiety for perovskite solar cells. Chem. Commun. 51 (2015) 9305–9308. DOI:10.1039/C5CC01471A |
| [45] | H. Choi, K. Do, S. Park, J.S. Yu, J. Ko. Efficient hole transporting materials with two or four N, N-Di(4-methoxyphenyl)aminophenyl arms on an ethene unit for perovskite solar cells. Chem. Eur. J. 21 (2015) 15919–15923. DOI:10.1002/chem.201502741 |
| [46] | M.S. Kang, S.D. Sung, I.T. Choi, et al. Novel carbazole-based hole-transporting materials with star-shaped chemical structures for perovskite-sensitized solar cells. ACS Appl. Mater. Interfaces 7 (2015) 22213–22217. DOI:10.1021/acsami.5b04662 |
| [47] | P. Gratia, A. Magomedov, T. Malinauskas, et al. A methoxydiphenylamine-substituted carbazole twin derivative: an efficient hole-transporting material for perovskite solar cells. Angew. Chem. Int. Ed 54 (2015) 11409–11413. DOI:10.1002/anie.201504666 |
| [48] | F.G. Zhang, X.C. Yang, M. Cheng, et al. Engineering of hole-selective contact for low temperature-processed carbon counter electrode-based perovskite solar cells. J. Mater. Chem. A 3 (2015) 24272–24280. DOI:10.1039/C5TA07507F |
| [49] | J. Cao, Y.M. Liu, X.J. Jing, et al. Well-defined thiolated nanographene as holetransporting material for efficient and stable perovskite solar cells. J. Am. Chem. Soc. 137 (2015) 10914–10917. DOI:10.1021/jacs.5b06493 |
| [50] | A. Krishna, D. Sabba, J. Yin, et al. Facile synthesis of a furan-arylamine holetransporting material for high-efficiency, mesoscopic perovskite solar cells. Chem. Eur. J. 21 (2015) 15113–15117. DOI:10.1002/chem.201503099 |
| [51] | H. Nishimura, N. Ishida, A. Shimazaki, et al. Hole-transporting materials with a two-dimensionally expanded π-system around an azulene core for efficient perovskite solar cells. J. Am. Chem. Soc. 137 (2015) 15656–15659. DOI:10.1021/jacs.5b11008 |
| [52] | J. Das, R.B.K. Siram, D. Cahen, B. Rybtchinski, G. Hodes. Thiophene-modified perylenediimide as hole transporting material in hybrid lead bromide perovskite solar cells. J. Mater. Chem. A 3 (2015) 20305–20312. DOI:10.1039/C5TA04828A |
| [53] | M.L. Petrus, T. Bein, T.J. Dingemans, P. Docampo. A low cost azomethine-based hole transporting material for perovskite photovoltaics. J. Mater. Chem. A3 (2015) 12159–12162. |
| [54] | Y.S. Liu, Q. Chen, H.S. Duan, et al. A dopant-free organic hole transport material for efficient planar heterojunction perovskite solar cells. J. Mater. Chem. A 3 (2015) 11940–11947. DOI:10.1039/C5TA02502H |
| [55] | C. Steck, M. Franckevicius, S.M. Zakeeruddin, et al. A-D-A-type S, N-heteropentacene-based hole transport materials for dopant-free perovskite solar cells. J. Mater. Chem 3 (2015) 17738–17746. DOI:10.1039/C5TA03865K |
| [56] | A. Abate, S. Paek, F. Giordano, et al. Silolothiophene-linked triphenylamines as stable holetransporting materials for high efficiency perovskite solar cells. Energy Environ. Sci. 8 (2015) 2946–2953. DOI:10.1039/C5EE02014J |
| [57] | C.C. Stoumpos, C.D. Malliakas, M.G. Kanatzidis. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52 (2013) 9019–9038. DOI:10.1021/ic401215x |
| [58] | Y. Liu, Z. Hong, Q. Chen, et al. Perovskite solar cells employing dopant-free organic hole transport materials with tunable energy levels. Adv. Mater. 28 (2016) 440–446. DOI:10.1002/adma.v28.3 |
 2016, Vol. 27
2016, Vol. 27