b Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
π-Conjugated donor-acceptor type (D-A) copolymers are an important class of organic semiconductors and have attracted great attentions due to their applications in organic field effect transistors (OFETs) [1-3] and organic photovoltaics (OPVs) [4-6]. Currently, most of the reported high mobility OFETs and high power conversion efficiency (PCE) OPVs are based on D-A copolymers. To develop new type of acceptor unit is vital and one of the challenging research topic for new type high performance D-A copolymers. As an ideal acceptor unit for D-A copolymers, it should possess some merits: low LUMO energy level, planar structure, good solubility, easy synthesis, easy modification and so on. Among the commonly used acceptor units in D-A copolymers, thieno[3, 4-c]pyrrole-4, 6-dione (TPD) has all the merits mentioned above. TPD unit was firstly synthesized in 1998 [7] and used as a building block for low band gap copolymers. However, it did not attract the attentions of material chemists until 2010 Recently, great progress has been made on TPD-based copolymers, a PCE above 8% and a hole mobility larger than 1.0 cm2 V-1 s-1 were reported, suggesting the applications of TPDbased copolymers in organic optoelectronics. Till now, the reviews of TPD-based copolymers are very rare. The last review was reported by Leclerc [8] in 2013 which mainly focused on the synthetic methods of TPD unit and summarized the application of TPD copolymers in OPVs before the year of 2013. In this review, we summarized the recent results of TPD-containingD-A copolymers in OFET and OPVs. The TPD copolymers reported in the review are classifiedby theirmolecular structurewiththe aimtounderstandthe correlation between molecular structures and devices performance.
2. Mono-TPD based D-A copolymers 2.1. TPD-based D-A copolymers with oligothiophenes and thieno[3, 2-b]thiophene as donor unitOligothiophenes and thieno[3, 2-b]thiophene are the common donor units in D-A copolymers. In this part, we summarized the TPD-based copolymers containing oligothiophenes and/or thieno[2, 3-b]thiophenes as donor units. Fig. 1 shows the chemical structures of the copolymers discussed in this part.

|
Download:
|
| Figure 1. Chemical structures of mono-TPD based D–A copolymers with thiophene units as donors | |
In 2010, Wei et al. [9] prepared copolymer P1 with the dodecyl substituted 2, 20-bithiophene as electron-donating unit. P1 exhibited high crystallinity, high thermal stability and a low-lying HOMO energy level (-5.56 eV). The solar cells incorporating the P1/PCBM blend at a weight ratio of 1:1.5 showed an open-circuit voltage (Voc) of 0.95 V and a PCE of 4.7%. Later, Imahori et al. [10] synthesized copolymers P2-P5 in which the TPD moieties were substituted with different kinds of alkyl chains. The estimatedoptical band gaps of P2-P5 were in the range of 1.8-2.1 eV, and their HOMO energy levels were from -5.4 to -5.7 eV. The solar cells based on P2 and PCBM showed the highest performance with a PCE of 0.75%, a Voc of 0.71 V, a short circuit current density (Jsc) of 2.29 mA cm-2 and a fill factor (FF) of 0.46.
Li et al. [11] synthesized TPD-based copolymers P6-P8. P6-P8 had the same conjugation backbones but with different alkyl chain orientations. Though the orientation of alkyl chains had nearly no effect on the HOMO-LUMO energy levels of P6-P8, it strongly affected the molecular packing structures of P6-P8 in films and thus led to the dramatic changes of the transistor performance. The hole mobility was 0.15 cm2 V-1 s-1 for P6 and 1.1×10-3 cm2 V-1 s-1 for P7. P8 based devices exhibited the highest performance with a mobility of 1.29 cm2 V-1 s-1, three orders of magnitudes higher than that of P7. Moreover P6-P8 displayed excellent environmental stability and humidity stability, the transistors showed nearly no decay even they were stored under ambient for two months with humidity larger than 60%.
Marks et al. [12] prepared a series of copolymers P9-P16. Experimental results showed the number of the thiophene units in oligothiophene building blocks and the alkyl chains on oligothiophenes had a significant effect on the OFET electrical properties. P10 with monothiophene as donor unit exhibited ambipolar behavior under vaccum with a hole mobility of 10-4 cm2 V-1 S-1 and an electron mobility of 10-3 cm2 V-1 S-1. And P11-P15 which incorporated bithiophene, terthiophene and tetrathiophene units displayed p-type performance with hole mobility about 0.1 cm2 V-1 S-1. Among all the polymers, P12 showed the highest transistor performance with a hole mobility of 0.6 cm2 V-1 S-1. Further they [13] fabricated polymer solar cells by blending P12a with N2200 and investigated the effect of number-average molecular weight (Mn) on the film morphology and the photovoltaic property. They reported the variation of Mn affected both the intrachain and interchain interactions of the polymers and ultimately the degree of phase separation and morphology evolution. The increase of the Mn of polymers afforded higher Jsc, but lower FF. By carefully tuning the Mn of both donor and acceptor polymers, a PCE of 3.22% was observed, two times higher than the devices based on polymers with non-optimal Mns.
Wang et al. [14] synthesized a series of TPD based random copolymers P17-P20. They found the optical band gaps of the polymers could be tuned from 1.66 eV to 1.78 eV with the change of the contents of TPD unit. The corresponding solar cells based on the blend of P17-P20 and PC71BM (w/w, 1:2) showed molecular energy level dependent photovoltaic properties. Among them, the P19-based device exhibited the highest PCE of 0.86%.
Kim et al. [15] reported two TPD-based copolymers P21 and P22 in which thiophene-vinylene-thiophene (TVT) and selenophene- vinylene-selenophene (SVS) were used as donor unit respectively. Both polymers showed narrow band gaps (1.72 eV for P21 and 1.58 eV for P22), good coplanarity and high hole mobility. The P21/ PC71BM based device showed a PCE of 4.87% and the P22/PC71BMbased device exhibited a higher PCE of 5.74%. The higher PCE of P22 was ascribed to its stronger interchain aggregations and enhanced carrier mobility. Later on, by changing the alkyl chain on the Natom of TPD unit, Won et al. [16] synthesized polymer P23, and a PCE up to 7.16% was observed with blending P23 and PC71BM. This value was about 20% higher than the corresponding copolymers without vinylene groups.
Tan et al. [17] investigated the linker effect of TPD based copolymers. They synthesized three copolymers P24-P26, in which 2, 2'-bithiophene, TVT and thiophene-ethylene-thiophene were used as donor unit respectively. Experimental results showed P26 had the lowest LUMO energy level and P25 exhibited the narrowest band gap. The thin film transistors of P24 and P25 exhibited typical p-type characteristics with hole mobility of 2.2×10-3 cm2 V-1 s-1 and 0.01 cm2 V-1 s-1 respectively. And P26 based thin film transistors showed ambipolar behavior with a hole mobility of 1.2×10-6 cm2 V-1 s-1 and an electron mobility of 0.6×10-6 cm2 V-1 s-1.
2.2. TPD-based D-A copolymers with benzo[1, 2-b:4, 5-b']dithiophene (BDT) as donor unitComparing with oligothiophenes, BDT unit has more planar structure and stronger intermolecular interactions. D-A type copolymers containing TPD and DBT units are one of the most important class of polymer semiconductors for OPVs. Most the reported high PCE devices are based on these polymers. The chemical structures of the copolymers discussed in this part are shown in Fig. 2.

|
Download:
|
| Figure 2. Chemical structures of TPD-based D–A copolymers with benzo[1, 2-b:4, 5-b']dithiophene unit as donor. | |
In 2010, Leclerc et al. [18] reported the low band gap copolymer Q1. The thin films of Q1 showed broad absorption in the range of 350-685 nm. The Q1/PC71BM based solar cells exhibited a high PCE of 5.5% with a Voc of 0.85 V, a Jsc of 9.81 mA cm-2 and a FF of 0.66 in air. Nearly at the same time, Jen [19], Fréchet [20] and Zhang [21] also reported the synthesis and photovoltaic properties of Q1 and its analogues Q2-Q5. And a higher PCE of 6.8%with a Voc of 0.85 V, a Jsc of 11.5 mA cm-2 and a FF of 0.68 was observed for Q1 by Fre´ chet group. Later Leclerc et al. [22] synthesized a series of new type of copolymers Q6-Q13 containing TPD, BDT and thiophene units in 2011. They claimed the length of the alkyl chain on TPD units and thiophene units affected the PCE of devices. However, these new copolymers containing thiophene units displayed lower PCE than that of Q1, and the highest PCE was 3.9% which was observed for Q9 based devices. In 2012, Leclerc et al. [23] reinvestigated the photovoltaic property of Q1 by using high boiling-point solvents 1-chioronaphthalene (CN) and 1, 8-diiodooctane (DIO) as co-additives. CN was used to suppress the formation of large polymer domains and DIO acted as PCBM crystallization controller. By carefully tuning the ratio of CN and DIO, a PCE of 7.1% and a FF up to 70% were achieved.
Beaujuge et al. [24] investigated the effect of alkyl chains on the photovoltaic properties of copolymers Q14-Q18. They found the replacement of branched side chains with linear ones in the BDT motifs would induced a critical change in polymer self-assembly and backbone orientation in thin films, and this change was unfavorable for PCE. Moreover, it would improve the PCE of copolymers by controlling the number of aliphatic carbons in the linear N-alkyl substituent of TPD motifs. Among them, Q17 showed the highest PCE of 8.5%.
Wei et al. [25] replaced the alkyl substituted BDT unit with the two-dimensional 5-alkyl-hiophene-2yl-substituted BDT unit and synthesized copolymers Q19 and Q20. The optical energy band gap was 1.85 eV for Q19 and 1.86 eV for Q20. Q20 films displayed higher hole mobility (6.1×10-3 cm2 V-1 s-1) than Q19 films (1.0×10-3 cm2 V-1 s-1). And the PCEs of Q19 and Q20 with blending PCBM could reach 3.87% and 6.08%, respectively. The distinct PCEs of Q19 and Q20 demonstrated the great effect of alkyl-thiophene spacer on device performance. Later, Zhu et al. [26] prepared copolymers Q21-Q23 in which 2-alkylthieno[3,4-b]thiophene was used to replace the 3-alkylthiophene of Q20. The optical band gaps of Q21-Q23 were in the range of 1.46-1.56 eV, lower than that of Q18. Among them, Q23 displayed the highest PCE of 7.50% after blending with PCBM.
2.3. TPD-based D-A copolymers with other typep-conjugated, fusedring units as donor unitWith the aim to further lower the HOMO-LUMO band gap and tune the packing structures as well as morphology of thin films, TPD-based D-A polymers with other type of π-conjugated, fusedring building blocks have been synthesized and investigated. Fig. 3 shows the chemical structures of these polymers discussed in this part.
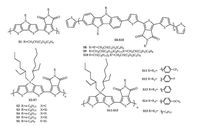
|
Download:
|
| Figure 3. Chemical structures of TPD-based D–A copolymers with other type π-conjugated fused-ring units as donors. | |
In 2011, Shi et al. [27] synthesized copolymer S1, consisting of dithieno[3,2-b:2',3'-d]pyrrole (DTP) as donor unit and TPD as acceptor unit. Though S1 had low molecular weight (MW: 5.3 kDa), it showed narrow optical band gap of 1.62 eV and low HOMO energy level of -5.09 eV. The solar cells based on the blend of S1/PCBM displayed a PCE of 1.9%, a Voc of 0.7 V and a Jsc of 6.97 mA cm-2.
By using cyclopenta[2, 1-b:3, 4-b'] dithiophene, dithieno [3, 2- b:2', 3'-d]siole (DTS) and DTP as donors and TPD as acceptor, Jen et al. [28] synthesized copolymers S2, S3 and S4. The number average molecular weight of S2, S3 and S4 were 16.0, 13.6 and 10.6 kDa respectively. The HOMO energy level and optical band gap were -5.43/1.67 eV for S2, -5.44/1.70 eV for S3 and -5.16/1.59 eV for S4. Among them, S2 showed the highest hole mobility of 1.5×10-3 cm2 V-1 s-1 and PCE of 3.74%, while the hole mobilities and PCEs of S3 and S4 were 6.0×10-4 cm2 V-1 s-1/2.13% and 3.9×10-4 cm2 V-1 s-1/1.69%, respectively.
In the same year, Lu et al. [29] synthesized the same copolymer S3. The number average molecular weight of the reported S3 was 28 kDa. Its HOMO energy level and optical band gap were reported as -5.57 and 1.73 eV respectively. The optimized OPV devices of S3 exhibited a PCE of 7.3% with a Voc of 0.88 V, a Jsc of 12.2 mA cm-2 and a FF of 0.68. Later, they [30] investigated the effect of the molecular weight and alkyl chain length of TPD unit on the photovoltaic properties of S3, S5 and S6. They found that higher molecular weight copolymers displayed higher hole mobility and hence induced higher PCE. The alkyl chain length of the TPD unit not only affected the solubility of the copolymers but also had strong impact on the packing structure and morphology of films. By optimizing the molecular weight of copolymer, the length of alkyl chain on TPD and the device fabrication process, a PCE of 7.5% was observed for S3-based solar cell.
Reynolds et al. [31] incorporated dithienogermole (DTG) unit into TPD-based copolymer and prepared polymer S7. S7 showed a longer wavelength absorption and a deeper HOMO energy level comparing with the DTS analogous S3. The invert structure photovoltaic devices of the blend of S7 and PCBM exhibited a high PCE of 7.3% while that of S3 displayed a PCE of 6.8%. The higher PCE of S7 was ascribed to its improved Jsc and FF, though its Voc is slightly lower than that of S3.
Owczarczyk et al. [32] synthesized copolymers S8-S10 in which 5, 10-dihyroindolo[3, 2-b]indoles (DINI) were used as electron donating unit. The size and the shape of the alkyl chains substituted on the N atom of DINI unit significantly affected the optical and electronic properties of the copolymers. The HOMO energy level and optical band gap were -5.1 eV/1.99 eV for S8, -5.2 eV/1.97 eV for S9 and -5.3 eV/2.04 eV for S10. Among them S10 displayed the highest PCE of 0.98% with a Voc of 0.797 V, a Jsc of 2.2 mA cm-2 and a FF of 0.59.
In 2014, Ikai et al. [33] synthesized a series of TPD and DTS based copolymers S11-15. They found that the HOMO and LUMO energy levels of S11-S15 could be tuned by the phenyl substituents on DTS unit.TheHOMOandLUMOenergy levels of the polymerswere about 0.6 eV lowered by replacing the methoxyl group with trifluoromethyl group on the phenyl substituent. However, no solar cell or transistor performance were reported for these copolymers
3. Bi-TPD based D-A copolymersComparing with mono-TPD based copolymers, the reported bi- TPD based D-A copolymers are rare. The introduction of bi-TPD units into D-A copolymers can not only further lower the HOMO and LUMO energy levels of polymers, thus increase their environmental stability, but also tune their intermolecular interactions. Moreover, the low LUMO energy levels of bi-TPD based copolymers suggest their potential applications in n-type and/or ambipolar transistors. Fig. 4 shows the chemical structures of the copolymers discussed in this part.
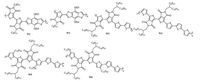
|
Download:
|
| Figure 4. Chemical structures of Bi-TPD based D-A copolymers | |
In 2011, Leclerc et al. [34] synthesized bi-TPD unit. Single crystal results showed TPD adopted an anticoplanar conformation with strong noncovalent interactions between the oxygen atoms and the sulfur atoms in Bi-TPD unit. Besides that, bi-TPD unit could further decrease the LUMO energy level and the band gap of thecopolymers. They polymerized bi-TPD unit and BDT unit and obtained copolymer W1. The LUMO energy level and optical band gap of W1 was -3.94 eV and 1.67 eV, lower than that of corresponding W2. However, no photovoltaic property was reported for W1 copolymer.
Recently Li et al. [35] synthesized the copolymers W3-W6 and systematically investigated the effect of alkyl chain density and orientation on the transistor performance. The HOMO energy level was -5.80 eV for W3, -5.52 eV for W4, -5.49 eV for W5 and -5.49 eV for W6. They found the alkyl chain density strongly influenced the packing structure of polymers in films, and higher alkyl chain densitywas favorable tothe face-on packingstructure. All these polymers showed p-channel behavior with holemobility up to 1.4 cm2 V-1 s-1. Interestingly, the packing structure of W5 could converted fromface-on structure to edge-on structurewith thermal annealing, andthe transistor performance basedonface-onstructure films was close to that based on edge-on structure films, suggesting face-on packing structures also facilitated charge carrier transport.
4. ConclusionIn this review, the recent progress of TPD-based copolymers in OPV and OFETs are summarized. The reported PCE of TPD containing copolymers was higher than 8% and the mobility of these copolymers was over 1.0 cm2 V-1 s-1, demonstrating the applications of TPD-based copolymers in organic optoelectronic devices. Though great progress has been made, comparing with the deeply investigated diketopyrrolopyrrole (DPP)-based and NDIbased copolymers, more efforts must be taken to TPD-based copolymers. To further improve the performance of TPD based copolymers, two main paths should be considered. One is to design and syntheses new type of copolymers. The bi-TPD based copolymers should be paid more attentions. And considering the small size of TPD units, the introduction of larger π-conjugated fused-ring donor units will be helpful to improve the performance of TPD-based copolymers. The other way is to optimize the device fabrication process through high boiling point solvent and additives. We strongly believe great progress on TPD-based copolymers will be achieved in the near future.
Acknowledgments This work was supported by the National Natural Science Foundation of China (Nos. 21190031, 51303201, 21474128).| [1] | Z. Cai, Y. Guo, S. Yang, et al. New donor-acceptor-donor molecules with pechmann dye as the core moiety for solution-processed good-performance organic field-effect transistors. Chem. Mater. 25 (2013) 471–478. DOI:10.1021/cm303793g |
| [2] | S.S. Dharmapurikar, A. Arulkashmir, C. Das, et al. Enhanced hole carrier transport due to increased intermolecular contacts in small molecule based field effect transistors. ACS Appl. Mater. Interfaces 5 (2013) 7086–7093. DOI:10.1021/am401379a |
| [3] | S. Xu, N. Ai, J. Heng, N. Zhao. Extended isoindigo core: synthesis and applications as solution-processable n-OFET materials in ambient conditions. RSC Adv. 5 (2015) 8340–8344. DOI:10.1039/C4RA14072A |
| [4] | X. Ren, S. Jiang, M. Cha, et al. Thiophene-bridged double D-π-A dye for efficient dye-sensitized solar cell. Chem. Mater. 24 (2012) 3493–3499. DOI:10.1021/cm302250y |
| [5] | X. He, B. Gao, T.C. Hauger, et al. Donor-acceptor small molecules for organic photovoltaics: single-atom substitution (Se or S). ACS Appl. Mater. Interfaces 7 (2015) 8188–8199. DOI:10.1021/acsami.5b01063 |
| [6] | A. Mishra, M.K. Fischer, P. Bauerle. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48 (2009) 2474–2499. DOI:10.1002/anie.v48:14 |
| [7] | Q. Zhang, J.M. Tour. Alternating donor/acceptor repeat units in polythiophenes intramolecular charge transfer for reducing band gaps in fully substituted conjugated polymers. J. Am. Chem. Soc. 120 (1998) 5356–5362. |
| [8] | A. Pron, P. Berrouard, M. Leclerc. Thieno. Macromol. Chem. Phys. 214 (2013) 7–16. DOI:10.1002/macp.201200549 |
| [9] | M. Yuan, M. Chiu, S. Liu, et al. A thieno[3, 4-c]pyrrole-4, 6-dione-based donor-acceptor polymer exhibiting high crystallinity for photovoltaic applications. Macromolecule 43 (2010) 6936–6938. DOI:10.1021/ma101523a |
| [10] | T. Umeyama, M. Oodoi, O. Yoshikawa, et al. Synthesis and photovoltaic properties of thiophene-imide-fused thiophene alternating copolymers with different alkyl side chains. J. Mater. Chem. 21 (2011) 12454–12461. DOI:10.1039/c1jm11531f |
| [11] | Q. Wu, M. Wang, X. Qiao, et al. Thieno. Macromolecules 46 (2013) 3887–3894. DOI:10.1021/ma400544s |
| [12] | X. Guo, R.P. Ortiz, Y. Zheng, et al. Thieno. J. Am. Chem. Soc. 133 (2011) 13685–13697. DOI:10.1021/ja205398u |
| [13] | N. Zhou, A.S. Dudnik, T.I.N.G. Li, et al. All-polymer solar cell performance optimized via systematic molecular weight tuning of both donor and acceptor polymers. J. Am. Chem. Soc. 138 (2016) 1240–1251. DOI:10.1021/jacs.5b10735 |
| [14] | X. Wang, C. Gao, K. Wang, et al. Synthesis and electronic energy-level regulation of imide-fused poly(thienoylene vinylene) derivatives. Polym. Chem. 51 (2013) 4975–4982. DOI:10.1002/pola.v51.23 |
| [15] | Y.R. Cheon, Y.J. Kim, J. Ha, et al. TPD-based copolymers with strong interchain aggregation and high hole mobility for efficient bulk heterojunction solar cells. Macromolecule 47 (2014) 8570–8577. DOI:10.1021/ma501888z |
| [16] | J.W. Jung, T.P. Russell, W.H. Jo. Highly crystalline low band gap polymer based on thieno. ACS Appl. Mater. Interfaces 7 (2015) 13666–13674. DOI:10.1021/acsami.5b03446 |
| [17] | W. Qing, Z. Liu, S. Yang, et al. Modulating carrier transfer ability-linker effect on thieno. RSC Adv. 5 (2015) 55619–55624. DOI:10.1039/C5RA08723F |
| [18] | Y. Zou, A. Najari, P. Berrouard, et al. A thieno[3, 4-c]pyrrole-4, 6-dione-based copolymer for efficient solar cells. J. Am. Chem. So 132 (2010) 5330–5331. DOI:10.1021/ja101888b |
| [19] | Y. Zhang, S.K. Hau, H. Yip, et al. Efficient polymer solar cells based on the copolymers of benzodithiophene and thienopyrroledione. Chem. Mater. 22 (2010) 2696–2698. DOI:10.1021/cm100417z |
| [20] | C. Piliego, T.W. Holcombe, J.D. Douglas, et al. Synthetic control of structural order in N-alkylthieno. J. Am. Chem. Soc. 132 (2010) 7595–7597. DOI:10.1021/ja103275u |
| [21] | G. Zhang, Y. Fu, Q. Zhang, et al. Benzo. Chem. Commum. 46 (2010) 4997–4999. DOI:10.1039/c0cc00098a |
| [22] | A. Najari, S. Beaupre, P. Berrouard, et al. Synthesis and new characterization of thieno. Adv. Funct. Mater. 21 (2011) 718–728. DOI:10.1002/adfm.201001771 |
| [23] | B.R. Aich, J. Lu, S. Beaupre, et al. Control of the active layer nanomorphology by using co-additives towards high-performance bulk heterojunction solar cells. Org. Electron. 13 (2012) 1736–1741. DOI:10.1016/j.orgel.2012.05.001 |
| [24] | B. Cabanetos, A.E. Labban, J.A. Bartelt. Linear side chains in benzo. J. Am. Chem. Soc. 135 (2013) 4656–4659. DOI:10.1021/ja400365b |
| [25] | K. Lu, J. Fang, H. Yan, et al. A facile strategy to enhance absorption coefficient and photovoltaic performance of two-dimensional benzo[1, 2-b:4, 5-b'] dithiophene and thieno[3, 4-c]pyrrole-4, 6-dione polymers via subtle chemical structure variations. Org. Electro 14 (2013) 2652–2661. DOI:10.1016/j.orgel.2013.07.006 |
| [26] | C. Zhang, H. Li, J. Wang, et al. Low-bandgap thieno. J. Mater. Chem. A 3 (2015) 11194–11198. DOI:10.1039/C5TA02376A |
| [27] | X. Hu, M. Shi, L. Zuo, et al. Synthesis, characterization, and photovoltaic property of a low band gap polymer alternating dithienopyrrole and thienopyrroledione units. Polymer 52 (2011) 2559–2564. DOI:10.1016/j.polymer.2011.03.057 |
| [28] | Y. Zhang, J. Zou, H. Yip, et al. Conjugated polymers based on C, Si and Nbridged dithiophene and thienopyrroledione units: synthesis, field-effect transistors and bulk heterojunction polymer solar cells. J. Mater. Chem. 21 (2011) 3895–3902. DOI:10.1039/c0jm03927f |
| [29] | T. Chu, J. Lu, S. Beaupre, et al. Bulk heterojunction solar cells using thieno. J. Am. Chem. Soc. 133 (2011) 4250–4253. DOI:10.1021/ja200314m |
| [30] | T. Chu, J. Lu, S. Beaupre, et al. Effects of the molecular weight and the side-chain length on the photovoltaic performance of dithienosilole/thienopyrrolodione copolymers. Adv. Funct. Mater. 22 (2012) 2345–2351. DOI:10.1002/adfm.v22.11 |
| [31] | C.M. Amb, S. Chen, K.R. Graham, et al. Dithienogermole as a fused electron donor in bulk heterojunction solar cells. J. Am. Chem. Soc. 133 (2011) 10062–10065. DOI:10.1021/ja204056m |
| [32] | Z.R. Owczarczyk, W.A. Braunecker, A. Garcia, et al. 5, 10-Dihydroindolo[3, 2-b]indole-based copolymers with alternating donor and acceptor moieties for organic photovoltaics. Macromolecules 46 (2013) 1350–1360. DOI:10.1021/ma301987p |
| [33] | T. Ikai, T. Kudo, M. Nagaki, et al. Fine tuning of frontier orbital energy levels in dithieno. Polymers 55 (2014) 2139–2145. DOI:10.1016/j.polymer.2014.03.021 |
| [34] | P. Berrouard, F. Grenier, J. Pouliot, et al. Synthesis and characterization of 5-octylthieno. Org. Lett. 13 (2011) 38–41. DOI:10.1021/ol1027514 |
| [35] | X. Qiao, Q. Wu, H. Wu, et al. High performance thin film transistors based on bithieno. Polym. Chem. 7 (2016) 807–815. DOI:10.1039/C5PY01995H |
 2016, Vol. 27
2016, Vol. 27 


