In order to resolve the problems of the traditional energy crisis and the consequent environmental pollution, efficient usage of solar energy by converting it into electricity is one of the most charming approaches. In the past few decades, great progress has been made in the field of photovoltaic applications. Recently, polymer solar cells (PSCs) have attracted the wide attention due to their advantages of low cost, flexibility, and light weight [1], which are regarded as the new generation of large-area solar cells by using the spin-coating, inkjet-printing, spray-coating, gravurecoating, or roller-casting processes, etc. [2]. At present, the bulk heterojunction (BHJ) device structure composed of both conjugated polymer donors and fullerene derivatives, where efficient charge separation is enabled by a large-area donor-acceptor (D-A) interface, has been regarded as the popular and effective architecture [3]. Enormous efforts have been made for integrative innovations on delicate material design as well as diverse device engineering to improve the photovoltaic performance of PSCs. State-of-the-art devices for single-junction PSCs have been certified records of over 10%, which is generally deemed as the entry point for commercial applications, although efficiency losses are still accounted from lab-scale cells to large-area modules [4-7].
1.1. Fundamental principles of PSCsTypical PSCs possess a sandwich structure, where a photoactive layer is clipped between the cathode and anode. A relatively more complicated or efficient structure (Fig. 1) involves an additional anode or cathode interfacial layer (AIL/CIL) inserted between the photoactive layer and the electrodes [3, 8]. Nowadays, the most studied PSCs are based on a BHJ configuration, where the photoactive layer contains an electron donor (D) and an electron acceptor (A). Four fundamental steps are included to describe the commonly accepted working mechanism of BHJ PSCs: (1) light absorption and generation of highly localized, tightly bounded Frenkel excitons; (2) exciton diffusion to the D-A interface; (3) exciton dissociation at the D-A interface: first creating charge transfer (CT) states or so-called polaron pairs, and then CT states fully dissociate into free charge carriers and (4) charge transport and collection [9-12]. Fig. 1 shows a schematic representation of above two typical BHJ device structures, illustrating the components involved in the mechanistic steps. The current-voltage curves also define the primary quantity and device performance of a solar cell [10, 12]. A complete understanding of the working mechanism paves the way to realize the highly efficient PSCs.

|
Download:
|
| Figure 1. (a) Device structures of PSCs. (b) The energy levels of the photoinduced charge transfer within PSCs. (c) J–V cures of a photovoltaic cell in the dark and under illumination. | |
In searching for high-performance PSCs, material designation attracted the continuous interests, and a great deal of research efforts in the last decades have been devoted to developing new conjugated polymer donors to extend absorption and harvest more solar energy. For an ideal polymer donor, appropriate energylevels, broad absorption ability and high charge carrier mobility are of great importance, which will directly affect open-circuit voltage (Voc), short-circuit current density (Jsc) and fill factor (FF) of the related solar cells [13]. A deep HOMO level is desirable for obtaining a high Voc because the Voc of BHJ devices is directly proportional to the gap between the HOMO level of donor and the LUMO level of acceptor in the active layer [14, 15]. In order to realize a satisfactory performance, polymer donors which contain an electron-donating (D) block and an electron-accepting (A) block have been tailored to improve the Voc, while keeping a low band gap with broad absorption and thus allowing for a high Jsc. Rational choice of the D and A units to obtain a desirable "push-pull" effect of the alternating donor and the acceptor segments will induce an intra and/or intermolecular charge transfer (ICT), giving rise to a lower energy absorption band and a better photo-response [16]. In addition, the delicate side chain engineering would further finely tune the energy levels, crystallinity and solubility of the corresponding polymers [14, 17]. Bear all above in mind, it is important to carry out detailed research work to explore the structure-property relationship of the photovoltaic polymers. Thus, a fundamental understanding of molecular designation and the benefits of versatile polymer syntheses allows for the effective tailoring of the intrinsic properties of photovoltaic polymers to serve for the desired purpose and address the application requirements [11].
1.2. The functions of fluorine atom in polymeric donor materialsAmong the various material design strategies, introducing the fluorine atoms to the conjugated polymers has become a promising method for enhancing the efficiency of their polymer solar cells (PSCs). Fluorine atom has attracted wide attention as a modified group used for constructing highly efficient photovoltaic polymers due to many versatile unique characteristics: (1) fluorine atom is the smallest electron-withdrawing group with slightly larger van der Waals radius than that of hydrogen (1.35Å vs. 1.2Å ), and smaller than cyano and trifluoromethyl groups commonly used as electron-deficient substituents; (2) fluorine atom is the strongest electronegative element with a Pauling electronegativity of 4.0, much larger than that of hydrogen (2.2) [18-20]. It is well known that the introduction of an electron-withdrawing group into the polymer backbone can lower its energy levels [21]. Thus, the fluorination can simultaneously lower the LUMO and HOMO levels of the resulting polymers while incurring only a minor effect on their optical band-gaps. As expected, BHJ PSCs based on these fluorinated copolymers would exhibit the higher Voc and PCE values than the corresponding non-fluorinated derivatives [22, 23]. Since it is only one small atom in size, it can be introduced onto the polymer backbone without any deleterious steric effects [24]. Moreover, an enhanced inter-/intramolecular interaction among polymer backbones can be found frequently due to the supramolecular interactions, such as C-F…H, F…S, C-F…pF, and so on [18, 25]. The torsional angles within polymer backbones also would be minimized and the planarity should be enhanced [26]. Therefore, fluorination is always helpful to reduce intramolecular distances in comparison to non-fluorinated polymers, facilitating the formation of highly ordered solid-state structure with a more closed co-facial p-p stacking, leading to increase the crystallinity [20, 27-29]. At the same time, these would lead to a more delocalized LUMO wave function that both polaron and exciton delocalization and their transport characteristics can be improved, resulting in higher carrier mobility for polymers and preferable morphology of their blend films with PCBM in PSCs, giving rise to the higher Jsc, FF and PCE [22, 25, 30-33]. In addition, fluorinated polymers exhibit great thermal stability and elevated resistance to oxidative degradation because the fluorine atoms can lower both the energy levels [18, 29, 34]. These features hold exceptional promise in enhancing the efficiency as well as the lifetime for polymer-fullerene PSCs, which are highly desired for practical applications [35].
In this review, recent progress towards highly efficient PSCs by rational design and reasonable modification of polymer donor materials with fluorination strategy on acceptor or donor skeletons are critically discussed, which will help us make deeply understanding of the fluorination effects and the structure- property relationships of the fluorinated photovoltaic polymers for efficient PSC applications.
2. Fluorination of acceptor building blocks 2.1. Thienothiophene-containing copolymersThieno[3, 4-b]thiophene (TT) has been widely used for building D-A conjugated copolymers for PSCs. Low band-gap copolymers(PTB1-6) were first synthesized and studied by Yu group [36, 37]. These polymers were stabilized by a quinoidal structure, providing a more rigid backbone with better π-π stacking property featuring a short packing distance, resulting in a high hole mobility [12]. The side chains also affect the absorption and carrier mobility, as well as the miscibility with fullerene derivatives (e.g. PC61BM and PC71BM). The high PCE of 6.1% was obtained from PTB 4:PC61BM devices [37]. Further optimization of polymer structure would lead to preparation of PTB7 with fluorination on the TT skeleton, whose HOMO and LUMO levels were decreased the to -5.15 and -3.31 eV, respectively. When mixed with PC71BM, a PCE of 7.4% was then achieved [2]. With the skilful device [38, 39] and interfacial engineering [40], the performance could notably be improved to more than 10% recently. In 2009, Hou et al. reported a series of BDT-TT based copolymers, by replacing the carboxyl side chains on the TT unit with more electron-withdrawing carbonyl group and attaching fluorine atom on the TT skeleton at the same time, the HOMO level was further decreased and the Voc was improved step-by-step without Jsc loss. PBDTTT-CF device exhibited a PCE of 7.73% [22].
Beside the TT decorations, more research efforts focused on the modifications of BDT units for this type of copolymers. Chen et al. reported PTB7-Th as the donor material by incorporating the twodimensional (2D) side chains to improve the planarity, thereby extending the absorption range along with increased absorption coefficient, giving rise to a high PCE up to 9.35% due to the enhanced charge generation and collection [41]. When using an inverted off-centre spin-coating method, the performance was dramatically improved up to 10.95% because of the more favourably vertical D:A composition gradient [42]. Replacing the alkyl side chains of the DBT-T units with alkylthiol groups increased the carrier mobility and down-shifted the HOMO level with slightly red-shifted absorption. Thus, the PSCs based on PBDTT-S-TT demonstrated a PCE of 8.42% without any additives [43]. Further elevated PCE of 9.48% could be obtained by adopting linear alkylthiol side chains to reduce the steric hindrance of PBDTS1 [44]. After substitution of the TT units with carbonyl groups, a deeper HOMO of -5.44 eV for PBDTT-S-TT-CF was obtained, thereby resulting in a higher Voc of 0.89 V and PCE of 9.58% [15]. PBDTS1 analogue of PBDT-TSR with a regioregular structure showed an improved absorption and an enhanced p-p stacking property, exhibited a high PCE of 10.2% [45]. When alkylfuryl and alkylselenyl groups were used as the side chains, distinctly different steric hindrances with different dihedral angles would be formed between PBDTTT-EFF and PBDTTT-EFS. Their desired steric hindrance would thus afford an inferior morphology than the alkylthienyl counterparts [46]. Inserting different number of fluorine atoms onto the side chains of BDT skeleton could simultaneously optimize the Voc, Jsc and FF, and PBT-3F devices exhibited the higher PCE of 8.6% than those of PBT-0F, PBT-1F, PBT- 2F [47].
In addition to side chain engineering, some research work also focused on the modifications of polymer backbones. By using thiophene group as the p-bridge, PBT-OP showed minimal difference with the optical absorption, molecular packing, blend morphology and charge transport properties of PBT-T, but significant impact on the HOMO level, which produced a larger DVoc of 0.18 V [48]. Replacing the BDT with benzodifuran (BDF) units lowered the HOMOs of the resulting copolymers [49, 50]. The reason was that the smaller size of oxygen atom induced a stronger molecular aggregation [49]. When fusing the BDT units with two or more thiophene cycles, the p-conjugation could be expanded to lower the positive charge density and exciton binding energy. The efficiency of PTDBD2 was elevated to 7.6% by fine-tuning the alkyl chains on the polymer backbones because of the improvement of the compatibility between the copolymer and PCBM [51]. The chemical structures of TT-based copolymers are described in Fig. 2.
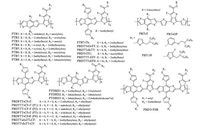
|
Download:
|
| Figure 2. The chemical structures of TT-containing copolymers | |
2.2. Benzothiadiazole-containing copolymers
Among the variable electron-deficient building blocks, 2, 1, 3- benzothiadiazole (BT) has attracted much attention for its special feature for controlling the energy levels in D-A copolymer applications. Mü hlbacher et al. reported a low band-gap copolymer of PCPDT-BT, which was the first donor candidate with BT as the acceptor block [52]. Subsequently, Neher et al. modified the BT skeleton with fluorine atoms, and achieved a high Voc and PCE of 6.16% from PCPDT-FBT devices [33]. When incorporated with two fluorine atoms, the solubility of PCPDT-DFBT became much poorer due to the enhanced F…H, F…F interactions [53]. Replacing thecarbon bridge (CPDT) with Si (DTS) or Ge (DTG) also caused poor device performance [54, 55], mainly because that a larger bridge atom favoured the reduced molecular tension, more planar geometry and higher degree of aggregation [56]. Yang et al. inserted an oxygen atom into the CPDT skeleton and developed a new donor block of dithienopyran (DTP). The resulting polymer of PDTP-DFBT exhibited the significantly improved solubility and processability, presenting a high PCE up to 8.0% [57].
BDT-BT based copolymers also attracted wide attention in recent years. You et al. reported the first fluorinated copolymer of BDT-BT with exceptional device performance in PSCs. The difluorination effect reduced the HOMO levels and improved the interchain interactions as well as the absorption coefficient, facilitating a PCE up to 7.2% [18]. Our group attached thiophene- containing side chains onto the BDT skeleton to further lower HOMO level, and copolymerized with BT derivatives with electron-rich or electron-deficient side chains. PDBT-FBT with the relatively electron-richer BT units benefited a higher Jsc, while the more electron-deficient BT analogue with fluorine atoms exhibited a higher Voc. After optimization of the phase separation of blend films, PDBT-FBT exhibited a higher PCE of 6.21% [30]. The effect of fluorine atom number on the device performance was also investigated in the succeeding research work. The continuously elevated Voc could be obtained by incorporation of more fluorine atoms. The improvements in Jsc and FF were likely due to the suppressed charge recombination by fluorination. In particular, PBnDT-DTffBT device showed a greater face-on orientation for its stronger p-p stacking along with a higher purity of polymer- fullerene domains, giving rise to a higher PCE of 8.3% [32, 58]. However, using the thiophene bridges instead of furan groups could cause serious phase separation with a large domain size [25]. Qin et al. devoted extensive efforts to control the solubility and steric hindrance by using a complex molecular structure of D1-A-D2-A, which would fine-tune their dipole orientations. Effective controlling the regioregularity of the backbones and the amount of nonchromophoric side chains, the molecular interactions was optimized to achieve a high device performance of 7.80% [59]. Besides, extending the p-conjugation length with TT units would elevate the photovoltaic properties by reducing the bandgap to enhance the absorption effectively [60]. With rational side chain engineering, a PCE of 9.44% could be obtained for copolymer of PBDTTS-TTDffBT [1]. In addition, the fluorinated BT was also chosen as side chains to be attached onto BDT skeleton, and the related copolymers exhibited a moderate device performance due to the narrow photo-response range [61, 62].
PTh4BT derivatives have already been investigated widely and exhibited a promising device performance in PSCs. Compared with the nonfluorinated counterpart, PTh4FBT exhibited an ordered structure because of the enhanced non-covalent interactions. The PCE from its inverted devices was 6.8% [28]. The change of length and branching positions as well as the number of thiophene rings would show a great influence on the overall device performance [4, 7, 63]. By using the hot spin-coating technology, Yan et al. exquisitely controlled the aggregation of PffBXT4T-2DT and obtained high PCEs of 10.5% and 10.8% when blended with PC71BM and TC71BM acceptors, respectively [6, 64]. If using a hydrocarbon processing solvent-additive system of TMB/PN, PffBT4T-C9C13 device exhibited an elevated PCE of 11.7% with a reduced domain size and increased domain purity [4]. Woo et al. reported another series of semi-crystalline and low band-gap fluorinated copolymers, PPD-TBTs, for fabrication of highly efficient PSCs. The PCE was 9.39% from a -300 nm thick active layer in single-cells and the efficiency was negligible changed at -1 mm thickness of PPDT2FBT:PC71BM film [20]. While replacing the alkoxylthienyl side chains with akylthiofuryl groups, the main-chain planarity was broken with a larger torsional angle due to the absence of the attractive O…S coulomb interaction and an increase of steric hindrance, leading to drastically decreased device performance [65]. Yang et al. reported two fluorinated diketopyrrolopyrrole (DPP)-BT copolymers with strong intermolecular interactions. The resulting copolymers exhibited well balanced bipolar charge mobility over 0.1 cm2V-1 s-1 [66].
Apart from those mentioned material designations, the fusion of p-conjugated backbones also has been developed as a promising strategy for efficient photovoltaic copolymers. It was reported that the CPDT and its derivatives of DTS, DTG as well as dithienopyrrole (DTP) were copolymerized with BT units, but the resulting copolymers showed an inferior device performance because of the poor morphology induced by their strong aggregation. When using a fused aromatic ring, such as indacenodithiophene (IDT) [67], silaindacenodithiophene (SiIDT) [19], a ubiquitous photovoltaic property could also be observed, especially in fluorinated BTcontaining copolymers. A feasible way is developed to increase the steric hindrance with relatively large side chains of 4-n-hexylphenyl groups. For example, PIDT-DFBT exhibited an improved PCE of 6.8% [68]. The chemical structures of BT-containing copolymers are summarized in Fig. 3.

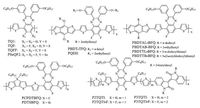
|
Download:
|
| Figure 3. The chemical structures of BT-containing copolymers. | |
2.3. Quinoxaline-containing polymers
The quinoxaline (Qx) skeleton has been extensively investigated to develop efficient acceptors in recent years due to its strong electron-deficient feature. Wang et al. reported a low band-gap polymer of TQ1 and applied it in PSC applications. TQ1 showed the promising photophysical property with a broad absorption, while the energy levels matched well with the requirements for PCBMbased PSCs. A PCE of 6.0% with a relatively high Voc of 0.9 V was achieved by simply processed device fabrication conditions [69]. However, the fluorinated counterparts exhibited a reduced device performance with the incorporation of some fluorine atoms [70]. Chen et al. reported a fluorinated copolymer of PBDT-TFQ, which possessed the excellent light harvest ability and an efficient photogeneration of charge-separated states when blended with PC71BM. The introduction of fluorine atoms on the Qx moiety could also lowered the energy levels of the related copolymers. On the other side, the high carrier mobility and better continuous percolation pathways for charge transport would contribute to a superior PCE of 8.0% [71]. Our group developed a series of D-A copolymers containing monofluorinated Qx units for PSC applications, which provided a good example for fine-tuning their thermal stability, absorption range, energy level, charge transport, as well as photovoltaic properties by side chain engineering strategy by considering both the branching degree and dimensionality of the side chains [17]. We also investigated the bridging atom effect on the photovoltaic performance of this type of copolymers by employing C-bridged CPDT or Si-bridged DTS units as the donor building blocks. Although PDTSBFQ showed a slightly blue-shifted absorption and a little larger band-gap, it exhibited a unique aggregation and a better crystallinity. The improved molecular ordering and lower-lying HOMO level guaranteed a PCE of 5.92% with high Jsc and Voc values [56]. Wang et al. reported the fluorinated copolymers based on a D-A1-D-A2 backbone of Qxisoindigo (ID), which exhibited the finely tuned conjugation length with low-lying HOMO levels and strong absorption coefficients. Superior photovoltaic performance was achieved for the P3TQTI-Fbased devices with a PCE of 7.0% [72]. The chemical structures of Qx-containing copolymers are listed in Fig. 4.
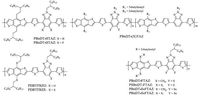
|
Download:
|
| Figure 4. The chemical structures of Qx-containing copolymers | |
2.4. Benzotriazole-containing copolymers
Benzo[d][1, 2, 3]triazoles (TAZ) is another typical electrondeficient block for building the D-A conjugated copolymers. Youet al. synthesized two large band-gap (-2.0 eV) copolymers for usage in photovoltaic applications based on TAZ and BDT units, which possessed desirable hole mobility and low-lying HOMO and LUMO levels. PBnDT-FTAZ devices exhibited a PCE more than 7%, and the device efficiency above 6% was still maintained at 1 mm thickness of the active layer [24]. PBnDT-(X)TAZ with increase the amount of fluorine atoms would contribute to the steady improvement of hole mobility induced by the adjustment of the molecular packing property [73]. Our group demonstrated two novel TAZ-containing conjugated copolymers, PBDTFBZO and PBDTFBZS, which exhibited good absorptions, deep energy levels with large band-gaps around 1.8 eV. The best PCE of 7.74% was obtained for PBDTFBZS devices. When combination of a low bandgap copolymer, the tandem PSCs based on PBDTFBZS showed an elevated PCE up to 9.4% [74]. However, other work demonstrated that an increased F…S interactions would induce the large phase aggregations if using the difluorinated TAZ instead of monofluorinated TAZ in the related copolymers [75]. Although changingthe thiophene bridges with selenophene units could successfully lower the band-gap and increase the Jsc of the corresponding PSCs, the overall device performance was still limited by the poor FF values [75]. The chemical structures of TAZ-containing copolymers are listed in Fig. 5.
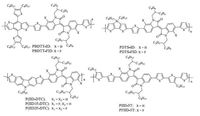
|
Download:
|
| Figure 5. The chemical structures of TAZ-containing copolymers. | |
2.5. Isoindigo-containing copolymers
Our group first reported the design and synthesis of the D-A copolymers with fluorinated isoindigo (FID) units along with their detailed characterization for photovoltaic applications. PBDTT-FID exhibited a deepHOMOlevel, small band-gap, and excellent carrier mobility, which made its PSCs exhibit an impressively high PCE of 7.04% [76]. In order to deeply investigate the synergetic fluorination effect, we also reported another copolymer of PDTS-FID with reduced band-gap and extended absorption, replacing the BDT donor unit with DTS [77]. Geng et al. reported some similar FIDcontaining copolymers, which featured a highly rigid backbone. Particularly, the fluorination strengthened their planarity and rigidity, but the related copolymers exhibited a lower mobility due to their regio-random configurations. Obviously, fluorination would shift down the HOMO levels of this type of copolymers, and thus induce high Voc values from their PSCs. However, it could also reduce the solubility and low Jsc sometimes. The high PCE of 8.0% was finally achieved despite the Voc and Jsc variations [78]. Detrembleur et al. reported a series of copolymers based on monofluorinated ID, in which the fluorination effect, spacer length and molar mass were studied simultaneously on their blend microstructure and overall device performance. In this work, fluorination and BDT incorporation would favour the high Voc values, but the relatively low Jsc values were observed due to their poor solubility and inferior miscibility with PCBM. Replacing the BDT blocks with thiophene units could increase the molecular weights of the corresponding copolymers and elevate the miscibility with PCBM. Therefore, the best PCE of 5.03% was then achieved for PFIID-5T devices [79]. The chemical structures of IDcontaining copolymers are summarized in Fig. 6.
|
Download:
|
| Figure 6. The chemical structures of ID-containing copolymers. | |
3. Fluorination in donor building blocks
Fluorination of the donor blocks was also found as an effective approach to elevate the photovoltaic property of the related copolymers. In 2011, Yu et al. reported a series of photovoltaic copolymers, PTBFs, with fluorinated BDT donor units. Fluorination of the donor segments would also lower both theHOMOand LUMO energy levels with slightly enlarged band-gaps (about 0.1-0.2 eV larger). PTBF1 (PTB7) with mono-fluorinated TT blocks showed the best device performance. However, PTBF3 with the perfluorinated backbone led to a poor compatibility with PC71BM because of the enhanced self-organization and fluorophobicity feature. Furthermore, they found that perfluorination of the polymeric backbone would also result in poor photochemical stability against singletoxygen attack [29]. However, there was another promising case if the BDT unit was fluorinated on its conjugated side chains. Hou et al. reported a series of D-A polymers with fluorine atoms on the skeleton of the TT unit and/or the thienyl side chains. They found that fluorination had less influence on their optical properties, but a positive effect on lowering their energy levels. Accordingly, PSCs based on the trifluorinated copolymer, PBT-3F, exhibited an enhanced PCE of 8.6% [47]. Thus, the introduction of fluorine atoms onto the appropriate positions is of great importance. Yang et al. substituted the BDT unit with fluorinated alkoxyphenyl groups and prepared a new copolymer of PBDTPF-DTBT, which exhibited desirable device performance of 7.02% [13]. Zou et al. investigated effect of the position of fluorine atom onto the alkoxyphenyl side chains of BDT unit. The meta-substituted PBOm- FPO favoured the more face-on direction and more suitable crystallinity with proper fibril feature. The facilitated charge generation guaranteed a high PCE of 8.0% [80]. Increase the number of fluorine atoms on the conjugated side chains, the higher Voc would be expected, giving rise to a PCE up to 8.24% [81]. Besides of the fluorination on the BDT units, the thiophene fluorination also showed a positive aspect. With suitable number of fluorine atoms, the copolymer of 3F exhibited a better device performance originated from a stronger tendency for a face-on orientation, exhibiting a high PCE of 9.14% [82]. Liu et al. reported a fluorinated thiophene-containing copolymer of PBTff4T-2OD (2F). With meticulous aggregation and morphology control, its PSCs showed a PCE of 10.4% [6]. Subsequently, they further investigated the fluorination on the donor and acceptor skeletons and developed two TAZ-based copolymers (PffT2-FTAZ and PT2-FTAZ), in which they found that fluorination on the donor units was more effective in lowering the LUMO level than HOMO level, reducing the bandgap for extension of their absorption range. At the same time, the fluorination on donor units also would cause a strong Jaggregation, contributing to increasing the crystallinity and hole mobility of the related copolymers. Therefore, PffT2-FTAZ devices exhibited a higher PCE of 7.8% than that (2.8%) of PT2-FTAZ devices [83]. Fluorination of other donor blocks (e.g. IDT) would also be studied for constructing efficient photovoltaic copolymers with the similar principle [84]. The chemical structures of copolymers with fluorinated donor blocks are summarized in Fig. 7.

|
Download:
|
| Figure 7. The chemical structures of copolymers with fluorinated donor blocks. | |
4. Conclusion
In summary, fluorination is a useful strategy to design polymer donor materials to improve the efficiency and stability of PSCs. Elucidation of the structure-property correlations would certainly facilitate widespread utilization of this approach for efficient solar cell fabrications. The reviewed literatures demonstrate that the amount and position (backbones and/or conjugated side chains, donor and/or acceptor units) will significantly affect the photophysical and photovoltaic properties of the related copolymers. Undoubtedly, fluorination lowers the energy levels, which is expected to obtain a high Voc. Fluorination also promotes stronger intermolecular interactions and induce the higher ordered molecular packing because of the supramolecular interactions, such as C-F…H, F…S, and C-F…$\pi $F and so forth, which will further improve the hole mobility of the prepared copolymers. However, inappropriate fluorine amount and position will lead to balance the trade-off of molecular aggregation and phase separation for efficient charge generation and separation. Although the obtained results highlight the structurally designation of the polymer donors and optimization of their PSCs, getting a highly efficient device for practical application is far from that simple. A small structural perturbation of the existing D-A conjugated copolymers can indeed significantly influence their device parameters (e.g. Voc, Jsc, and FF). Therefore, a successful material designation for highperformance PSCs needs comprehensive consideration of all above key factors. This review will be important to evaluate and formulate requirements for molecular structures with the fluorination strategy for high-performance polymer solar cells.
Acknowledgments This work was supported by the NSFC (Nos. 51573107, 21432005), the Youth Science and Technology Foundation ofSichuan Province (No. 2013JQ0032), the Foundation of State Key Laboratory of Polymer Materials Engineering (Nos. sklpme2014-3- 05, sklpme2015-2-01), the Synergistic Innovation Joint Foundation of CAEP-SCU (No. XTCX2014008), and the Fundamental Research Funds for the Central Universities (Nos. 2012SCU04B01, YJ2011025).| [1] | J.W. Jung, F. Liu, T.P. Russell, W.H. Jo. Medium bandgap conjugated polymer for high performance polymer solar cells exceeding 9% power conversion efficiency. Adv. Mater. 27 (2015) 7462–7468. DOI:10.1002/adma.201503902 |
| [2] | Y.Y. Liang, Z. Xu, J.B. Xia, et al. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 22 (2010) E135–E138. DOI:10.1002/adma.200903528 |
| [3] | M. He, J. Jung, F. Qiu, Z.Q. Lin. Graphene-based transparent flexible electrodes for polymer solar cells. J. Mater. Chem. 22 (2012) 24254–24264. DOI:10.1039/c2jm33784c |
| [4] | J.B. Zhao, Y.K. Li, G.F. Yang, et al. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 1 (2016) 15027. DOI:10.1038/nenergy.2015.27 |
| [5] | R. Po, G. Bianchi, C. Carbonera, A. Pellegrino. All that glisters is not gold: an analysis of the synthetic complexity of efficient polymer donors for polymer solar cells. Macromolecules 48 (2015) 453–461. DOI:10.1021/ma501894w |
| [6] | Y.H. Liu, J.B. Zhao, Z.K. Li, et al. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 5 (2014) 5293. DOI:10.1038/ncomms6293 |
| [7] | H.W. Hu, K. Jiang, G.F. Yang, et al. Terthiophene-based D-A polymer with an asymmetric arrangement of alkyl chains that enables efficient polymer solar cells. J. Am. Chem. Soc. 137 (2015) 14149–14157. DOI:10.1021/jacs.5b08556 |
| [8] | Z.C. Hu, K. Zhang, F. Huang, Y. Cao. Water/alcohol soluble conjugated polymers for the interface engineering of highly efficient polymer light-emitting diodes and polymer solar cells. Chem. Commun. 51 (2015) 5572–5585. DOI:10.1039/C4CC09433F |
| [9] | L.Y. Lu, T.Y. Zheng, Q.H. Wu, et al. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 115 (2015) 12666–12731. DOI:10.1021/acs.chemrev.5b00098 |
| [10] | B.C. Thompson, J.M.J. Fréchet. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 47 (2008) 58–77. DOI:10.1002/(ISSN)1521-3773 |
| [11] | Y.J. Cheng, S.H. Yang, C.S. Hsu. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 109 (2009) 5868–5923. DOI:10.1021/cr900182s |
| [12] | L.Y. Lu, L.P. Yu. Understanding low bandgap polymer PTB7 and optimizing polymer solar cells based on it. Adv. Mater. 26 (2014) 4413–4430. DOI:10.1002/adma.v26.26 |
| [13] | W.C. Chen, Z.K. Du, L.L. Han, et al. Efficient polymer solar cells based on a new benzo. Mater. Chem. A 3 (2015) 3130–3135. DOI:10.1039/C4TA06350C |
| [14] | D.L. Liu, W.C. Zhao, S.Q. Zhang, et al. Highly efficient photovoltaic polymers based on benzodithiophene and quinoxaline with deeper HOMO levels. Macromolecules 48 (2015) 5172–5178. DOI:10.1021/acs.macromol.5b00829 |
| [15] | C.H. Cui, Z.C. He, Y. Wu, et al. High-performance polymer solar cells based on a 2D-conjugated polymer with an alkylthio side-chain. Energy Environ. Sci. 9 (2016) 885–891. DOI:10.1039/C5EE03684D |
| [16] | K. Feng, X.P. Xu, Z.J. Li, et al. Low band gap benzothiophene-thienothiophene copolymers with conjugated alkylthiothieyl and alkoxycarbonyl cyanovinyl side chains for photovoltaic applications. Chem. Commun. 51 (2015) 6290–6292. DOI:10.1039/C4CC10062J |
| [17] | X.P. Xu, Y.L. Wu, J.F. Fang, et al. Side-chain engineering of benzodithiophenefluorinated quinoxaline low-band-gap co-polymers for high-performance polymer solar cells. Chem. Eur. J. 20 (2014) 13259–13271. DOI:10.1002/chem.201403153 |
| [18] | H.X. Zhou, L.Q. Yang, A.C. Stuart, et al. Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7% efficiency. Angew. Chem. Int. Ed. 50 (2011) 2995–2998. DOI:10.1002/anie.201005451 |
| [19] | B.C. Schroeder, Z.G. Huang, R.S. Ashraf, et al. Silaindacenodithiophene-based low band gap polymers-the effect of fluorine substitution on device performances and film morphologies. Adv. Funct. Mater. 22 (2012) 1663–1670. DOI:10.1002/adfm.v22.8 |
| [20] | T.L. Nguyen, H. Choi, S.J. Ko, et al. Semi-crystalline photovoltaic polymers with efficiency exceeding 9% in a 300 nm thick conventional single-cell device. Energy Environ. Sci. 7 (2014) 3040–3051. DOI:10.1039/C4EE01529K |
| [21] | Y. Zhang, S.C. Chien, K.S. Chen, et al. Increased open circuit voltage in fluorinated benzothiadiazole-based alternating conjugated polymers. Chem. Commun. 47 (2011) 11026–11028. DOI:10.1039/c1cc14586j |
| [22] | H.Y. Chen, J.H. Hou, S.Q. Zhang, et al. Polymer solar cells with enhanced opencircuit voltage and efficiency. Nat. Photonics 3 (2009) 649–653. DOI:10.1038/nphoton.2009.192 |
| [23] | M.J. Zhang, X. Guo, W. Ma, H. Ade, J.H. Hou. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv. Mater 27 (2015) 4655–4660. DOI:10.1002/adma.v27.31 |
| [24] | S.C. Price, A.C. Stuart, L.Q. Yang, H.X. Zhou, W. You. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J. Am. Chem. Soc. 133 (2011) 4625–4631. DOI:10.1021/ja1112595 |
| [25] | C.H. Duan, A. Furlan, J.J. van Franeker, et al. Wide-bandgap benzodithiophene-benzothiadiazole copolymers for highly efficient multijunction polymer solar cells. Adv. Mater. 27 (2015) 4461–4468. DOI:10.1002/adma.v27.30 |
| [26] | F. Livi, N.K. Zawacka, D. Angmo, et al. Influence of side chain position on the electrical properties of organic solar cells based on dithienylbenzothiadiazole-altphenylene conjugated polymers. Macromolecules 48 (2015) 3481–3492. DOI:10.1021/acs.macromol.5b00589 |
| [27] | Z.P. Fei, P. Boufflet, S. Wood, et al. Influence of backbone fluorination in regioregular poly (3-alkyl-4-fluoro) thiophenes. J. Am. Chem. Soc. 137 (2015) 6866–6879. DOI:10.1021/jacs.5b02785 |
| [28] | J.F. Jheng, Y.Y. Lai, J.S. Wu, et al. Influences of the non-covalent interaction strength on reaching high solid-state order and device performance of a low bandgap polymer with axisymmetrical structural units. Adv. Mater. 25 (2013) 2445–2451. DOI:10.1002/adma.v25.17 |
| [29] | H.J. Son, W. Wang, T. Xu, et al. Synthesis of fluorinated polythienothiophene-cobenzodithiophenes and effect of fluorination on the photovoltaic properties. J. Am. Chem. Soc. 133 (2011) 1885–1894. DOI:10.1021/ja108601g |
| [30] | Q. Peng, X.J. Liu, D. Su, et al. Novel benzo. Adv. Mater. 23 (2011) 4554–4558. DOI:10.1002/adma.201101933 |
| [31] | A.C. Stuart, J.R. Tumbleston, H.X. Zhou, et al. Fluorine substituents reduce charge recombination and drive structure and morphology development in polymer solar cells. J. Am. Chem. Soc. 135 (2013) 1806–1815. DOI:10.1021/ja309289u |
| [32] | N. Wang, Z. Chen, W. Wei, Z.H. Jiang. Fluorinated benzothiadiazole-based conjugated polymers for high-performance polymer solar cells without any processing additives or post-treatments. J. Am. Chem. Soc. 135 (2013) 17060–17068. DOI:10.1021/ja409881g |
| [33] | S. Albrecht, S. Janietz, W. Schindler, et al. Fluorinated copolymer PCPDTBT with enhanced open-circuit voltage and reduced recombination for highly efficient polymer solar cells. J. Am. Chem. Soc. 134 (2012) 14932–14944. DOI:10.1021/ja305039j |
| [34] | S. Guo, J. Ning, V. Kö rstgens, et al. The effect of fluorination in manipulating the nanomorphology in PTB7:PC71BM bulk heterojunction systems. Adv. Energy Mater. 5 (2015) 1401315. DOI:10.1002/aenm.201401315 |
| [35] | Z. Li, J.P. Lu, S.C. Tse, et al. Synthesis and applications of difluorobenzothiadiazole based conjugated polymers for organic photovoltaics. J. Mater. Chem. 21 (2011) 3226–3233. DOI:10.1039/c0jm04166a |
| [36] | Y.Y. Liang, Y. Wu, D.Q. Feng, et al. Development of new semiconducting polymers for high performance solar cells. J. Am. Chem. Soc. 131 (2009) 56–57. DOI:10.1021/ja808373p |
| [37] | Y.Y. Liang, D.Q. Feng, Y. Wu, et al. Highly efficient solar cell polymers developed via fine-tuning of structural and electronic properties. J. Am. Chem. Soc. 131 (2009) 7792–7799. DOI:10.1021/ja901545q |
| [38] | H.Q. Zhou, Y. Zhang, J. Seifter, et al. High-efficiency polymer solar cells enhanced by solvent treatment. Adv. Mater. 25 (2013) 1646–1652. DOI:10.1002/adma.201204306 |
| [39] | Z.C. He, C.M. Zhong, S.J. Su, et al. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 6 (2012) 591–595. |
| [40] | X.H. Ouyang, R.X. Peng, L. Ai, X.Y. Zhang, Z.Y. Ge. Efficient polymer solar cells employing a non-conjugated small-molecule electrolyte. Nat. Photonics 9 (2015) 520–524. DOI:10.1038/nphoton.2015.126 |
| [41] | S.H. Liao, H.J. Jhuo, Y.S. Cheng, S.A. Chen. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with lowbandgap polymer (PTB7-Th) for high performance. Adv. Mater. 25 (2013) 4766–4771. DOI:10.1002/adma.v25.34 |
| [42] | J. Huang, J.H. Carpenter, C.Z. Li, J.S. Yu, H. Ade. Highly efficient organic solar cells with improved vertical donor-acceptor compositional gradient via an inverted off-center spinning method. Adv. Mater. 28 (2016) 967–974. DOI:10.1002/adma.v28.5 |
| [43] | C.H. Cui, W.Y. Wong, Y.F. Li. Improvement of open-circuit voltage and photovoltaic properties of 2D-conjugated polymers by alkylthio substitution. Energy Environ. Sci. 7 (2014) 2276–2284. DOI:10.1039/C4EE00446A |
| [44] | L. Ye, S.Q. Zhang, W.C. Zhao, H.F. Yao, J.H. Hou. Highly efficient 2D-conjugated benzodithiophene-based photovoltaic polymer with linear alkylthio side chain. Chem. Mater. 26 (2014) 3603–3605. DOI:10.1021/cm501513n |
| [45] | H.F. Yao, W.C. Zhao, Z. Zheng, et al. PBDT-TSR: a highly efficient conjugated polymer for polymer solar cells with a regioregular structure. J. Mater. Chem. A 4 (2016) 1708–1713. DOI:10.1039/C5TA08614K |
| [46] | S.Q. Zhang, L. Ye, W.C. Zhao, et al. Side chain selection for designing highly efficient photovoltaic polymers with 2D-conjugated structure. Macromolecules 47 (2014) 4653–4659. DOI:10.1021/ma500829r |
| [47] | M.J. Zhang, X. Guo, S.Q. Zhang, J.H. Hou. Synergistic effect of fluorination on molecular energy level modulation in highly efficient photovoltaic polymers. Adv. Mater. 26 (2014) 1118–1123. DOI:10.1002/adma.201304427 |
| [48] | M.J. Zhang, X. Guo, W. Ma, et al. An easy and effective method to modulate molecular energy level of the polymer based on benzodithiophene for the application in polymer solar cells. Adv. Mater. 26 (2014) 2089–2095. DOI:10.1002/adma.201304631 |
| [49] | L.J. Huo, L. Ye, Y. Wu, et al. Conjugated and nonconjugated substitution effect on photovoltaic properties of benzodifuran-based photovoltaic polymers. Macromolecules 45 (2012) 6923–6929. DOI:10.1021/ma301254x |
| [50] | P.S. Huang, J. Du, S.S. Gunathilake, et al. Benzodifuran and benzodithiophene donor-acceptor polymers for bulk heterojunction solar cells. J. Mater. Chem. A 3 (2015) 6980–6989. DOI:10.1039/C5TA00936G |
| [51] | H.J. Son, L.Y. Lu, W. Chen, et al. Synthesis and photovoltaic effect in dithieno. Adv. Mater. 25 (2013) 838–843. DOI:10.1002/adma.v25.6 |
| [52] | D. Mühlbacher, M. Scharber, M. Morana, et al. High photovoltaic performance of a low-bandgap polymer. Adv. Mater. 18 (2006) 2884–2889. DOI:10.1002/(ISSN)1521-4095 |
| [53] | Y.X. Li, J.Y. Zou, H.L. Yip, et al. Side-chain effect on cyclopentadithiophene/ fluorobenzothiadiazole-based low band gap polymers and their applications for polymer solar cells. Macromolecules 46 (2013) 5497–5503. DOI:10.1021/ma4009302 |
| [54] | H. Medlej, A. Nourdine, H. Awada, et al. Fluorinated benzothiadiazole-based low band gap copolymers to enhance open-circuit voltage and efficiency of polymer solar cells. Eur. Polym. J. 59 (2014) 25–35. DOI:10.1016/j.eurpolymj.2014.07.006 |
| [55] | C.P. Yau, Z.P. Fei, R.S. Ashraf, et al. Influence of the electron deficient co-monomer on the optoelectronic properties and photovoltaic performance of dithienogermole-based co-polymers. Adv. Funct. Mater. 24 (2014) 678–687. DOI:10.1002/adfm.201302270 |
| [56] | X.P. Xu, K. Li, Z.J. Li, et al. The enhanced performance of fluorinated quinoxalinecontaining polymers by replacing carbon with silicon bridging atoms on the dithiophene donor skeleton. Polym. Chem. 6 (2015) 2337–2347. DOI:10.1039/C4PY01622J |
| [57] | L.T. Dou, C.C. Chen, K. Yoshimura, et al. Synthesis of 5H-dithieno. Macromolecules 46 (2013) 3384–3390. DOI:10.1021/ma400452j |
| [58] | J. Lee, S.B. Jo, M. Kim, et al. Donor-acceptor alternating copolymer nanowires for highly efficient organic solar cells. Adv. Mater. 26 (2014) 6706–6714. DOI:10.1002/adma.v26.39 |
| [59] | T.S. Qin, W. Zajaczkowski, W. Pisula, et al. Tailored donor-acceptor polymers with an A-D1-A-D2 structure: controlling intermolecular interactions to enable enhanced polymer photovoltaic devices. J. Am. Chem. Soc. 136 (2014) 6049–6055. DOI:10.1021/ja500935d |
| [60] | X.C. Wang, Z.G. Zhang, H. Luo, et al. Effects of fluorination on the properties of thieno. Polym. Chem. 5 (2014) 502–511. DOI:10.1039/C3PY00940H |
| [61] | P. Shen, H.J. Bin, L. Xiao, Y.F. Li. Enhancing photovoltaic performance of copolymers containing thiophene unit with D-A conjugated side chain by rational molecular design. Macromolecules 46 (2013) 9575–9586. DOI:10.1021/ma401886a |
| [62] | Y.S. Huang, F. Wu, M. Zhang, et al. Synthesis and photovoltaic properties of conjugated polymers with an asymmetric 4-(2-ethylhexyloxy)-8-(2-ethylhexylthio)benzo. Dyes Pigments 115 (2015) 58–66. DOI:10.1016/j.dyepig.2014.12.012 |
| [63] | Z.H. Chen, P. Cai, J.W. Chen, et al. Low band-gap conjugated polymers with strong interchain aggregation and very high hole mobility towards highly efficient thickfilm polymer solar cells. Adv. Mater. 26 (2014) 2586–2591. DOI:10.1002/adma.v26.16 |
| [64] | W. Ma, G.F. Yang, K. Jiang, et al. Influence of processing parameters and molecular weight on the morphology and properties of high-performance PffBT4T-2OD:PC71BM organic solar cells, Adv. Energy Mater., 2015, 5:. Adv. Energy Mater 5 (2015) . |
| [65] | M.A. Uddin, T.H. Lee, S.H. Xu, et al. Interplay of intramolecular noncovalent coulomb interactions for semicrystalline photovoltaic polymers. Chem. Mater. 27 (2015) 5997–6007. DOI:10.1021/acs.chemmater.5b02251 |
| [66] | J. Lee, M. Jang, S.M. Lee, et al. Fluorinated benzothiadiazole (BT) groups as a powerful unit for high-performance electron-transporting polymers. ACS Appl. Mater. Interfaces 6 (2014) 20390–20399. DOI:10.1021/am505925w |
| [67] | H. Bronstein, J.M. Frost, A. Hadipour, et al. Effect of fluorination on the properties ofa donor-acceptor copolymer for use in photovoltaic cells and transistors. Chem. Mater. 25 (2013) 277–285. DOI:10.1021/cm301910t |
| [68] | J.J. Intemann, K. Yao, H.L. Yip, et al. Molecular weight effect on the absorption, charge carrier mobility, and photovoltaic performance of an indacenodiselenophene-based ladder-type polymer. Chem. Mater. 25 (2013) 3188–3195. DOI:10.1021/cm401586t |
| [69] | E. Wang, L.T. Hou, Z.Q. Wang, et al. An easily synthesized blue polymer for highperformance polymer solar cells. Adv. Mater. 22 (2010) 5240–5244. DOI:10.1002/adma.201002225 |
| [70] | W.L. Zhuang, H.Y. Zhen, R. Kroon, et al. Molecular orbital energy level modulation through incorporation of selenium and fluorine into conjugated polymers for organic photovoltaic cells. J. Mater. Chem. A 1 (2013) 13422–13425. DOI:10.1039/c3ta13040a |
| [71] | H.C. Chen, Y.H. Chen, C.C. Liu, et al. Prominent short-circuit currents of fluorinated quinoxaline-based copolymer solar cells with a power conversion efficiency of 8.0%. Chem. Mater. 24 (2012) 4766–4772. DOI:10.1021/cm302861s |
| [72] | Q. Tao, Y.X. Xia, X.F. Xu, et al. D-A1-D-A2 copolymers with extended donor segments for efficient polymer solar cells. Macromolecules 48 (2015) 1009–1016. DOI:10.1021/ma502186g |
| [73] | W.T. Li, S. Albrecht, L.Q. Yang, et al. Mobility-controlled performance of thick solar cells based on fluorinated copolymers. J. Am. Chem. Soc. 136 (2014) 15566–15576. DOI:10.1021/ja5067724 |
| [74] | K. Li, Z.J. Li, K. Feng, et al. Development of large band-gap conjugated copolymers for efficient regular single and tandem organic solar cells. J. Am. Chem. Soc. 135 (2013) 13549–13557. DOI:10.1021/ja406220a |
| [75] | R.L. Uy, L. Yan, W.T. Li, W. You. Tuning fluorinated benzotriazole polymers through alkylthio substitution and selenophene incorporation for bulk heterojunction solar cells. Macromolecules 47 (2014) 2289–2295. DOI:10.1021/ma5001095 |
| [76] | Y.C. Yang, R.M. Wu, X. Wang, et al. Isoindigo fluorination to enhance photovoltaic performance of donor-acceptor conjugated copolymers. Chem. Commun. 50 (2014) 439–441. DOI:10.1039/C3CC47677D |
| [77] | Z.G. Wang, J. Zhao, Y. Li, Q. Peng. Low band-gap copolymers derived from fluorinated isoindigo and dithienosilole: synthesis, properties and photovoltaic applications. Polym. Chem. 5 (2014) 4984–4992. DOI:10.1039/C4PY00273C |
| [78] | Y.F. Deng, J. Liu, J.T. Wang, et al. Dithienocarbazole and isoindigo based amorphous low bandgap conjugated polymers for efficient polymer solar cells. Adv. Mater. 26 (2014) 471–476. DOI:10.1002/adma.201303586 |
| [79] | M. Tomassetti, F. Ouhib, A. Wislez, et al. Low bandgap copolymers based on monofluorinated isoindigo towards efficient polymer solar cells. Polym. Chem. 6 (2015) 6040–6049. DOI:10.1039/C5PY00693G |
| [80] | J. Yuan, Y.P. Zou, R.L. Cui, et al. Incorporation of fluorine onto different positions of phenyl substituted benzo. Macromolecules 48 (2015) 4347–4356. DOI:10.1021/acs.macromol.5b00564 |
| [81] | G.W. Li, X. Gong, J.C. Zhang, et al. 4-Alkyl-3, 5-difluorophenyl-substituted benzodithiophene-based wide band gap polymers for high-efficiency polymer solar cells. ACS Appl. Mater. Interfaces 8 (2016) 3686–3692. DOI:10.1021/acsami.5b08769 |
| [82] | J.W. Jo, J.W. Jung, E.H. Jung, et al. Fluorination on both D and A units in D-A type conjugated copolymers based on difluorobithiophene and benzothiadiazole for highly efficient polymer solar cells. Energy Environ. Sci. 8 (2015) 2427–2434. DOI:10.1039/C5EE00855G |
| [83] | Z.K. Li, H.R. Lin, K. Jiang, et al. Dramatic performance enhancement for large bandgap thick-film polymer solar cells introduced by a difluorinated donor unit. Nano Energy 15 (2015) 607–615. DOI:10.1016/j.nanoen.2015.05.016 |
| [84] | S.S. Chen, K.C. Lee, Z.G. Zhang, et al. An indacenodithiophene-quinoxaline polymer prepared by direct arylation polymerization for organic photovoltaics. Macromolecules 49 (2016) 527–536. DOI:10.1021/acs.macromol.5b02324 |
 2016, Vol. 27
2016, Vol. 27 


