Recently,organic light-emitting diodes (OLEDs) have attracted considerable attention owing to their perspective applications in both full-color flat-panel displays and solid-state lighting [1]. Thanks to the research enthusiasm in the past three decades,great success has been achieved in developing high-performance OLEDs for practical applications [2]. Nevertheless,considering that OLEDs are a kind of energy conversion devices,to kick off their commercialization,it is crucial to develop devices with more boosted efficiency and improved reliability.
As the key efficiency parameter of OLEDs,external quantum efficiency (EQE) is a product of several separate terms [3]:
| $EQE={{\eta }_{op}}\times IQE={{\eta }_{op}}\times \left( \gamma \times {{\varphi }_{PL}}\times {{\eta }_{r}} \right)$ |
where ηop is the optical out-coupling factor,IQE is the internal quantum efficiency,γ is the charge balance factor,φPL is the photoluminescence (PL) quantum yield,and ηr is the possibility that an exciton is formed as a singlet one finally. In general,ηop is assumed to be 0.2 in a device without out-coupling enhancement,because it correlates highly with the refractive index of the emissive medium; an ideal γ of 1.0 can be achieved by circumspect structural design of OLEDs with appropriate charge-transporting layers,host-guest system as well as electrode materials; a maximum φPL of 1.0 could be reached through proper molecular design of the emitting material. However,as the recombination of charge carriers in OLED will initially generate singlet and triplet excitons with 25% and 75% probability,respectively [4],the hr will severely limit the EQE if the 75% triplet excitons could not be utilized in an OLED. As a consequence,phosphorescent OLEDs (PhOLEDs) capable of harvesting both singlet and triplet excitons (ηr is assumed to be 1.0) have attracted much attention. Nonetheless,phosphorescent rare metal complexes are associated with rather high cost and limited resource. Moreover,by now,deep-blue phosphors with high efficiency are still scarce,and the stability and longevity of blue PhOLEDs still cannot meet with the requirement for practical applications [5].
In contrast,although fluorescence OLEDs (FOLEDs) are often considered to show relatively low efficiencies due to their deficiency in triplet exciton harvesting (ηr is frequently assumed to be 0.25),recent research results have unveiled that through rational molecular design,fluorescent materials capable of utilizing both singlet and triplet excitons could also be implemented. Hence the exploitation of such FOLED materials has emerged to be one of the hottest research issues due to their combined high efficiency and low cost.
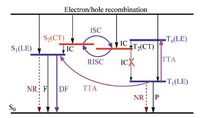
With regard to the conversion from triplet to singlet excitons in FOLEDs,three possible mechanisms have been reported,namely thermally activated delayed fluorescence (TADF) [6],hybridized local and charge-transfer excited states (HLCT) [7a],and tripletfusion delayed fluorescence (TFDF) [8] (vide Scheme 1). For TADF materials (also named as E-type delayed fluorescence materials),they should possess not only an excited state with charge-transfer (CT) feature,but also a quite small singlet-triplet energy gap (ΔEST),so that thermally activated reverse intersystem crossing (RISC) processes from T1 to S1 states could occur efficiently in the electroluminescence (EL) process (vide Scheme 1a),leading to a possible maximum ηr of 1.0 in TADF-OLEDs. However,to achieve a small ΔEST,the hole and electron wavefunctions of the compound should be well-separated spatially,which will result in a relatively small radiative rate constant (kf) hence low φPL [9]. As a consequence,the conflict between the small ΔEST and the high kf and φPL within TADF luminogens makes it difficult to design high-performance TADF materials rationally. In addition,despite the fact that many TADF-OLEDs have been demonstrated to show impressive high EQEmax at relatively low brightness,they generally suffer from severe efficiency roll-off at higher driving current [9b]. This should be ascribed to the accumulation of long-lived T1 excitons at higher driving current,which will lead to adverse triplet-triplet and singlet-triplet quenching processes. Hence currently,TADF-OLEDs are still at an early stage toward practical applications. For HLCT materials (also called as “hot-exciton” OLED materials),they must possess excited states with combined and compatible local excited (LE) and CT characteristics,together with a much lower-lying LE-charactered T1 state hence suppressed T2→T1 internal conversion (IC) processes (vide Scheme 1b). Taking advantages of the relatively small ΔEST between 1CT and 3CT states as well as the large energy gap between 3CT and 3LE (T1) states,the “hot” electronically injected 3CT excitons in HLCT-OLEDs could be converted efficiently into 1CT followed 1LE ones via RISC and IC processes,leading to a possible ηr of 1.0. Additionally,the more competitive kRISC (3CT→1CT) than kIC (3CT→3LE) would endow HLCT-OLEDs with suppressed accumulation of T1 excitons hence insignificant efficiency roll-off; while the highly radiative 1LE excitons of HLCT materials will be beneficial to the acquirement of high φPL [7b]. Nevertheless,the great challenge of HLCT-OLED materials lies in the rational molecular design of HLCT materials,since they have to violate the Kasha’s rule. Moreover,the efficiencies of HLCT-OLEDs hitherto reported are still unsatisfactory.

|
Download:
|
| Scheme. 1. Summary of processes in harvesting the triplet excitons for luminescence in FOLED devices. (a) TADF; (b) HLCT; and (c) TFDF. Here, DF denotes delayed fluorescence; IC denotes internal conversion process; PF denotes prompt fluorescence; ISC denotes intersystem crossing; RISC denotes reverse intersystem crossing, NR denotes non-radiative relaxation; ΔEST(CT) denotes the singlet-triplet energy splitting; and Ph denotes phosphorescence. | |
On the contrary,for TFDF materials (also named as P-type delayed fluorescence materials),their prerequisite is just 2E(T1) > E(S1) (vide Scheme 1c),which could be fulfilled by many LE-featured conjugated molecular systems. Consequently,rational molecular design is easier to be actualized for TFDF than TADF and HLCT materials. As a minimum of two triplet excitons is needed to produce one singlet exciton through TF,the possible ηr in a TFDFOLED is 0.625. It is noteworthy that although according to some previous reports,TF process may only contribute to the device efficiency at relatively low current densities (J) [10, 11],some latest literatures have revealed that negligible efficiency roll-off or even favorable efficiency roll-up could be observed in TFDF-OLEDs [12],which are of great importance in practical applications. In fact,despite the numerous research efforts on the exploitation of highperformance PhOLED and TADF-OLED materials,the majority of current practical blue OLEDs still rely on TFDF materials [8].
In light of the key role TF plays in achieving highly efficient FOLEDs,it is necessary to summarize and discuss the TFDF mechanism,the molecular design principles of TFDF materials and the research progress in TFDF-OLEDs. In this review,we start with a description of some basic principles of TFDF in triplet exciton harvesting. Then,we present the molecular design and EL properties of TFDF materials. We expect this review could provide valuable insights into the design strategies for high-performance TFDF-OLED materials.
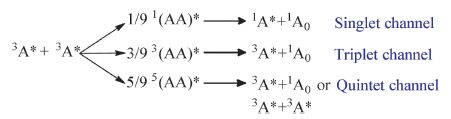
2. TF mechanism and evidence of TF processes in FOLEDsAs illustrated below,the collision of two uncorrected triplet excitons will generally result in nine equally probable statistical outcomes through exciton interaction pairs n(AA)* [13].
|
Yet for many organic molecules (e.g.,anthracene,tetracene,rubrene,etc.),their quintet state energy levels are relatively high,making the quintet channel quite deficient [14]. As a consequence,the prevented quintet channel will change the overall spin statistics in TTA process as follows [7, 15]:

|
It should be noted that in the triplet channel,one free triplet exciton will be regenerated for recycled fusion,hence the final TFinduced singlet yield is calculated to be 0.2 [7]. Taking into consideration that the initial composition of triplet excitons in OLEDs is 75%,the TF process therefore will contribute an extra 0.75×0.2 = 15% of singlets,and the total ηr of TFDF-OLEDs may become 0.4 [7].
Similarly,for compounds whose 2E(T1) < E(TN),their triplet channel in TTA may also be suppressed efficiently due to the endothermicity [7],leading to the result of 3A* + 3A*→1/2 1(AA)*→1A* + 1A0,hence a final TF-induced singlet yield of 0.5. Under this situation,the possible ηr in a TFDF-OLED is 0.625,including 0.25 from the original singlets and 0.375 (75%×0.5) from TF contributions [7]. It is noteworthy that very recently,Monkman et al. [15] have identified a new TADF-induced TF process to generate singlets. As illustrated in Scheme 2,for TFDF materials whose 2E(T1) > E(TN),although normally,their TN excitons generated through TF via the triplet channel will be wasted to a large extent,if they possess a CT excited state structure together with a quite small ΔEST(CT),the TN excitons could be converted directly to singlets through the RISC process,leading to enhanced singlet yields.
|
Download:
|
| Scheme. 2. TADF-induced TF process for ICT-featured TFDF materials whose 2E(T1) > E(TN). | |
In general,three experimental techniques are employed to identify whether TF process contributes to the triplet harvesting in OLEDs: (1) The luminance-current behaviors of an OLED [16]; (2) The time-resolved PL and/or EL spectra of the material and/or device [3, 17]; and (3) The mageto-EL (MEL) behaviors of an OLED [18]. Theoretically,if TF process contributes to the efficiency of an OLED,a super-linear luminance-current behavior should be observed [16]. Yet for most OLEDs,they will suffer from efficiency roll-off at relatively high J due to the presence of other processes consuming singlet and triplet excitons,e.g.,singlet-polaron annihilation [10],triplet-polaron interactions [11],etc. Hence in devices whose TTA contribution is not large enough,a super-linear luminance-current characteristic may be indiscernible [19]. In addition,since TADF and TFDF are both long-time-scaled processes,it is difficult to distinguish TFDF from TADF merely through time-resolved PL and/or EL measurements. In contrast,although both TADF and TFDF are highly spin-dependent processes capable of generating sizable MEL responses,it is easy to differentiate TFDF from TADF due to their quite different MEL characteristics. As a consequence,MEL has been considered as a powerful non-contact and non-destructive probing tool to identify the TF process in OLEDs [19].
3. TFDF-OLED materials 3.1. Early observations of TTA process in OLEDsAlthough the first observation of TFDF phenomenon could be dated to 1960’s in solution [20] and crystal samples [21] of polycyclic aromatic hydrocarbon compounds (e.g.,anthracene,phenanthrene),it was not until 1998 that Kido et al. [22] at Yamagata University proposed the first surmise of the TTA-related enhancement of efficiency of FOLEDs,because they found that an OLED with tris(4-methyl-8-quinolinolato)aluminum(III) (Almq3) as the host material showed a high EQEmax of 7.1%,which exceeds the theoretical EQEmax limit of 5.0%. This pioneer work,despite the lack of experimental evidences,has prompted people to think about the harvesting of triplet excitons in FOLEDs.
In 2002,Ganzorig et al. [16] reported their experimental findings that the substantial luminance component of a Alq3-based OLED scales quadratically with its current density. This result verified the presence of bimolecular TTA processes in this device. Popovic et al. [23] have also demonstrated successfully the contribution of TTA processes in a Alq3-based OLED based on the observation of delayed EL. In fact,by applying a reverse bias,they could even distinguish whether the delayed EL originates from TTA processes or recombination of trapped charges. Recently,Mayr [24] has also observed delayed EL originating from TTA processes in a high performance Alq3-based device (EQEmax = 6.9%). Note that this device showed nearly negligible efficiency roll-off in a remarkably broad range of current density from 0.1 to 100 mA cm-2. Another experimental technique capable of identifying the presence of TTA process in OLEDs is MEL [18]. Both Bussmann [25] and Xiong [26] have reported their observations of significant TTA-related high magnetic field effect on EL,which could be enhanced at lower temperatures and higher current densities. All these experimental findings confirmed that Alq3 derivatives are capable of harvesting triplets in OLEDs via TF processes.
Although early investigations of TFDF-OLEDs were mainly focused on Alq3 and its derivatives,with the deepening of the understanding on TTA processes in OLEDs,a variety of novel TFDFOLED materials have been designed and exploited rationally,and the most attractive ones are those bearing fused polycyclic aromatic core(s) such as anthracene [27] and rubrene [10]. In general,these compounds possess an excited state with LEfeatured π-π* transition structure rather than CT one. As shown in Fig. 1,for both anthracene [28] and rubrene [29],the sum of energies of their two triplets is larger than their singlet exciton,i.e.,E(S1)≤2E(T1),making them capable of up-converting triplets into singlets through TF. In fact,for rubrene,the sum of energies of its two triplets is even smaller than the energy of its one TN exiton,i.e.,2E(T1) < E(TN),making the triplet channel of the TF process energetically unfavorable. Consequently,rubrene should be a quite promising TFDF material to harvest triplets in OLEDs efficiently.

|
Download:
|
| Figure 1. Molecular structure and excited state energy level data of anthracene and rubrene. | |
In comparison with LE-featured TFDF materials,much less research attentions have been paid to CT ones. Nevertheless,recent research results have revealed that through rational molecular design,CT compounds could also act as high-performance TFDFOLED materials. Hence in this review,we will describe the development of TFDF materials according to their different excited state structures,i.e.,LE and CT.
3.2. TFDF-OLED materials with LE charactersFor most LE-featured TFDF-OLED materials,anthracene core(s) could be found in their molecular structures. In fact,although anthracene is not only the first-reported OLED material [30],but also the first-reported TFDF compound [20],OLEDs with it as the emissive layer could just show rather poor EL performance,which should be ascribed to its serious concentration quenching (CQ) arising from the intense π-π stacking interactions. Nevertheless,recent research results have revealed that the CQ of anthracene derivatives could be alleviated effectively through modifications at its C-2,C-9 and C-10 positions,resulting in high-performance TFDF-OLED materials capable of harvesting triplet excitons [31].
In 2002,by substituting 2-naphthyl groups at both the C-9 and C-10 positions of its anthracene core,Shi and Tang [27] developed compound 1 as an excellent OLED host material (Fig. 2). Later,Luo [32] attributed the high EL performance of 1 to the efficient TF processes within the OLED according to transient EL characterization. These results suggested that anthracene should be a promising structural unit for the construction of TFDF-OLED materials. Compound 2 bearing an additional methyl at the C-2 position was found to show more suppressed crystallization hence better EL performance than 1 [33]. A sky-blue OLED with 2 as the host material was reported to show an EQE of 8.7% and a luminance efficiency (LE) of 16.2 cd A-1 at 20 mA cm-2. Through transient EL characterization,Kido et al. [34] also attributed the high performance of 2-based OLEDs to the TF contribution. Note that the 2-based blue OLED fabricated by Kido et al. not only shows a high efficiency,but also displays a negligible efficiency roll-off (EQE@100 cd m-2 = 8.2%; EQE@1000 cd m-2 = 7.7%). For 3 bearing a phenyl substituent at the C-2 position,its EL performance was found to be even higher than 1 and 2: the sky-blue 3-based OLED exhibits an EQE of up to 9.1%,in which the TF process could contribute up to 32% of the overall efficiency [35].

|
Download:
|
| Figure 2. Molecular structure of LE-featured TFDF-OLED materials 1-17 bearing anthracene core(s). | |
By introducing bulky meta-positioned m-terphenyl and/or 1,3,5-triphenylbenzene groups at the C-9 and C-10 positions of anthracene,Park’s group [36] constructed three deep blue compounds 4-6 (Fig. 2). In comparison with 2,4-6 possess more suppressed molecular packing,hence much enhanced EL performance. It is worth noting that the deep-blue 6-based OLED shows a high EQE of 7.2% at 10 mA cm-2 as well as satisfactory CIE 1931 coordinates of (0.156,0.088),which are very close to the National Television System Committee (NTSC) standard. Yokoyama attributed the high efficiency of this device to not only the TF process,but also the out-coupling enhancement by dipole orientation of the emitters [37].
Since blue OLEDs often suffer from retarded charge carrier injection/transport in the emissive layer (EML) due to the intrinsic wide-band-gap nature of the fluorophor,the grafting of holetransporting triphenylamine or carbazole units is an effective approach to enhance the EL performance of the emitters due to the better hole-injection/transportation [31]. For example,a nondoped deep-blue OLED with 7 as the EML was found to show relatively high performance [EQE of 4.61% and LE of 3.64 cd A-1 at 10 mA cm-2; CIE1931: (0.151,0.086)] [38]. Lee et al. [39] attributed the satisfactory EL performance of 7 to the presence of TTA processes. Suzuki [40] reported another anthracene derivative 8,blue OLED using it as the host material shows an EQEmax of as high as 11.9%. According to time-resolved EL measurement results,they attributed the high efficiency of this device to the harvesting of triplet excitons via TTA processes. Besides,the authors pointed out that to increase the triplet exciton density for efficient TF process,the host compound should possess a lower T1 energy level than those of the guest and carriertransporting materials,so that triplets could be mainly confined to the host compound.
Diarylethene is also an important structural unit for the construction of high-performance OLED materials. In 2013,Monkman [3] synthesized compounds 9 and 10 bearing a diarylethene skeleton,an anthracene core and a triphenylamine hole-injection group concurrently. A green OLED with 9 as the emissive dopant exhibits an EQEmax of 6.0%,which far exceeds its theoretical EQEmax of 3.6%. In fact,transient EL measurements indicated that TF contributes nearly 60% of the singlets produced in this device. The authors attributed the strong TF capability of 9 to its energy level alignment of E(S1) < 2E(T1) < E(TN),by which triplet channel could be prevented effectively. It should be pointed out here that although 9 and 10 are considered as CT-featured compounds by the authors,we classify them here as LE-featured materials due to their rather weak PL solvatochromism.
OLED materials bearing two anthracene cores were also found to show TTA characters. With bisanthracene derivative 11 as the host material,Kanno [41] fabricated a green FOLED whose EQE and LE increase with increasing current density at low J of 0-2 mA cm-2,but level off at EQE of 10% and LE of 30 cd A-1 in a wide J range of 2- 100 mA cm-2. The authors tentatively attributed the high performance of this device to the TF property of anthracene cores. Fukagawa [42] carried out comparison studies on bisanthracene derivatives 12 and 13 to elucidate the correlation between TF properties and intermolecular interactions of the host materials. A device based on compound 13 showing more alleviated intermolecular stacking than 12 was found to display better performance (EQEmax: 7.2% vs. 5.0%). Further transient EL characterization indicated that in the 13-based OLED,a more intense delayed component was observed. Thus the authors ascribed the less efficient TF processes in 12-based device to the formation of excimers with lower T1 energy levels. To achieve non-coplanar molecular structures in anthracene derivatives,the introduction of more rigid and bulky segments such as tetraphenylsilane seems to be an effective strategy. In 2008,Lee et al. [43] synthesized bisanthracene derivatives 14-16 with tetraphenylsilane as the bridging unit,and the OLED using 15 as the host showed an EQEmax of 6.3%. Lee [39] suggested that the contribution of TTA may be one possible reason accounting for the high EL performance of 15.
In 2014,Kido [44] reported their exciting findings that a donoracceptor- type bisanthracene compound 17 is an excellent deepblue OLED guest material. The 5 wt%-doped 17-based OLED showed an impressive EQE of 12% and maximum luminance (Lmax) of >4500 cd m-2,together with a standard deep-blue color index of (0.15,0.06). In fact,this is one of the best deep-blue OLEDs reported so far in scientific literatures. Based on transient EL measurements,the authors suggested that TTA processes may play a key role in achieving this high efficiency. The only imperfection of this device lies in its efficiency roll-off,for example,its EQE drops to 4.2% at a brightness of 1000 cd m-2.
In addition to anthracene derivatives,rubrene has also been demonstrated to be an excellent TFDF-OLED material. With the aid of transient EL characterization,Forrest [10] systematically investigated the utilization and deactivation processes of singlet and triplet excitons in rubrene-based OLEDs. Because of its energy level alignment of 2E(T1) ≈ E(S1) (vide Fig. 1),the adverse singlet fission (SF) and favorable TF processes will coexist in rubrene. Therefore,rubrene should act as the host rather than the emitter in OLED applications,so that the singlets of rubrene could be deactivated via fast resonant FÖ rster energy transfer to the guest compound,leading to suppressed SF in rubrene. In addition,in an OLED using rubrene as the host,it was found that the triplets could positively contribute to its efficiency at only lower J of <2.2 A cm-2,while further increased J would result in decreased efficiency. Forrest ascribed this to the adverse singlet-triplet annihilation at higher driving current in the device. Note that these observations were quite similar to Kondakov’s experimental findings on OLEDs based on anthracene derivatives [11]. By using rubrene as the co-host material,highly efficient red FOLEDs showing EQEs of >11% have been demonstrated successfully [17, 45]. According to transient EL measurement results,TF processes could even contribute ~50% of the EL emission in an OLED with 0.5 wt% perylene derivative doped rubrene as theEML[17]. As the T1 energy level of perylene was calculated to be 0.2 eV higher than that of rubrene,taking into consideration that the guest concentration is as low as 0.5 wt%,the authors believed that only rubrene molecules were involved in the TF processes in the EML of this device.
In 2014,Cheng [12] reported their findings that a pyrene derivative 18 and four triphenylene derivatives 19-22 are also quite perspective TFDF-OLED materials (Fig. 3). A blue OLED whose EML is composed of 5 wt% of 20 and 95 wt% of 18 could exhibit an EQEmax of 10.2% and a LEmax of 12.3 cd A-1,together with CIE1931 coordinates of (0.14,0.14). Moreover,this OLED shows an intriguing efficiency roll-up rather than roll-off when the luminance was increased from 10 to 30,000 cd m-2. Through transient EL characterization,the authors attributed this abnormal efficiency roll-up to the larger contributions from TTA at higher current densities. Very recently,a bipolar gallium(III) complex 23 has also been verified to show TTA character through transient PL and EL characterizations [46]. Using 23 as the host material,Duan et al. implemented a NIR OLED with EQEmax of up to 2.1% and negligible efficiency roll-off.

|
Download:
|
| Figure 3. Molecular structure of anthracene-free LE-featured TFDF-OLED materials 18-23. | |
Thanks to the numerous research efforts,many new LEfeatured TTA materials have been exploited successfully,and highperformance TFDF-OLEDs with EQEs far exceeding the theoretical limit (up to 12%) have been demonstrated. In comparison with LEfeatured TFDF materials,the development of TFDF materials bearing a CT excited state structure lags far behind. Nevertheless,according to recent research findings,CT compounds also could act as quite promising candidates as TFDF-OLED materials.
3.3. TFDF-OLED materials with CT charactersThe first discovery revealing that typical CT molecular systems may act as TFDF-OLED materials appeared in 2013. Jankus et al. [47] found that for a blue OLED whose emissive species is an intermolecular CT-featured exciplex formed between compounds 24 and 25 (Fig. 4),its EQEmax could reach 2.7%,almost twice as high as the 25% singlet production predicts of 1.4%,i.e.,almost 50% of the EL should arise from additional singlets converted from triplet states. Yet according to transient PL and EL experimental results,the enhanced efficiency of this device should be attributed to the harvesting of triplets via TFDF rather than TADF processes,despite the fact that the ΔEST of this binary exciplex system is nearly zero. The reason is that the 3LE energy level of compound 24 is lowerlying than the 3CT energy level of the exciplex,hence the 3CT excitons will be quenched seriously into 3LE excitons of 24,leading to suppressed TADF but enhanced TFDF in the device.

|
Download:
|
| Figure 4. Molecular structure of CT-featured TFDF-OLED materials 24-31. | |
Although subsequently,Dias [48] unveiled through photophysical measurements that for molecules with intramolecular CT (ICT) characters,they could also display TFDF character,it was not until 2014 that the first report of ICT-featured TFDF-OLED material appeared [49a]. Using photophysics and MEL as experimental tools,we identified that in dilute solution,the ICT-featured naphthalimide derivative 26 shows TADF,but when being used as the OLED emitter,26 could up-convert triplets to singlets via TF rather than RISC processes,despite its quite small ΔEST of 0.18 eV. We also attributed the TFDF properties of 26 in OLEDs to its energy level alignment of E(3LE) < E(3CT). Owing to the contributions of TF processes,OLED with 26 as the host material could exhibit an EQEmax of 3.6%,far exceeding its theoretical limit of 2.5%. Encouraged by these scientific findings,recently,we have exploited another ICT naphthalimide compound 27 whose E(3LE) is also less than E(3CT) [49b]. In addition,it possessed much alleviated CQ,and could form a highly efficient energy transfer pair with 26. Consequently,an heavily-doped (6 wt%) OLED with 27 and 26 as the guest and host materials,respectively,exhibited relatively high EL performance (LEmax = 7.73 cd A-1,Lmax = 31,940 cd m-2,EQEmax = 5.83%). Note that the singlet production predict of the EQE of this device is only 3.0%. The high efficiency of this device was attributed to the simultaneous harvesting of triplet excitons through both host and guest materials.
Recently,great research breakthroughs have also been achieved in developing high performance deep-blue ICT TFDF-OLED materials. Using compound 28 as the guest emitter,Suh [50] fabricated an OLED with EQEmax up to 10.2% and Lmax > 5000 cd m-2,and its CIE1931 coordinates were (0.15,0.10). Besides its high efficiency,this device showed satisfactory efficiency stability (with EQE of 9.5% at 1000 cd m-2). Although the ΔEST of 28 (0.2 eV) is small enough to facilitate the RISC process,the authors attributed the excellent device performance to the contribution from TF processes due to the lack of temperature dependence of the decay profiles and total fluorescence intensities as well. Very recently,Cheng et al. [51] have also exploited three high performance TFDF-OLED materials 29-31. By using 4,40- bis(N-carbazolyl)-1,10-biphenyl (CBP) and 29 as the host and guest compounds respectively,a deep-blue OLED with an EQEmax of 9.1% and CIE1931 coordinates of (0.15,0.09) was acquired,yet this device suffered from serious efficiency roll-off. Nevertheless,by replacing CBP into another host with not only more balanced carrier-transporting capability but also TFDF property,the resulting 29-based deep-blue OLED showed a high EQEmax of 7.7% together with an intriguing efficiency roll-up. In fact,the performance of this device is among the best deep-blue FOLEDs reported so far.
4. Summary and perspectivesAlthough in comparison with TADF-OLED and HLCT-OLEDs whose ηr could be as high as 1.0,TFDF-OLEDs show a lower ηr of 0.625,they have been considered to be better candidates with regard to the acquirement of highly efficient and reliable FOLEDs for practical applications. In fact,currently,the majority of the practical OLEDs still rely on TFDF materials for creating efficient blue light. In consideration of the key role TFDF materials play in the harvesting of triplet excitons in OLEDs,many researcher efforts have been devoted to the exploitation of high-performance TFDFOLED materials (the device structures and performances of representative TFDF-OLED materials are summarized in Table 1),and encouraging progress have been made to cope with the crucial problems in OLEDs,such as efficiency,efficiency roll-off as well as carrier injection/transport. When TFDF materials are used as the non-doped emitters as well as host compounds,both suppressed intermolecular interactions and appropriate triplet energy levels of EML and carrier-transport layers should be indispensable to actualize efficient triplet harvesting. When they are used as the guest fluorophores,relatively high doping-levels (≥5 wt%) together with the employment of TTA-featured host compounds will be propitious to the enhancement of EL efficiency,because triplet excitons could be harvested simultaneous by both the host and guest compounds.
|
|
Table 1 Device structures, singlet and triplet energy levels and EL performance of representative TFDF-OLED materials. |
In spite of these encouraging progresses made in TFDF-OLED materials,the development of TFDF materials with maximized contribution of TTA process remains a challenge due to the lack of in-depth mechanistic understanding. Additionally,although a variety of TFDF-FOLEDs showing efficiencies exceeding the spinstatistical limit have been achieved,many of them suffer from obvious efficiency roll-off,and deep-blue devices with more boosted efficiency and improved reliability are still on the wish list. Consequently,the understanding of the TF mechanism and the exploration of high-performance deep-blue TFDF materials deserve additional investigations. Furthermore,despite the fact that the development of CT-featured TFDF materials lags far behind LEstructured ones,taking into consideration that CT compounds generally possess more balanced carrier injection/transport properties than their LE counterparts,and CT compounds with small ΔEST may yield more singlets than LE one in case that their 2E(T1) > E(TN),it is reasonable to expect they should be quite promising TFDF-OLED materials. In fact,by using a CT material as the guest compound,the EQE of deep-blue TFDF-OLEDs has been put to a new level of 9.5%@1000 cd m-2. Therefore,further research efforts should be devoted to the exploitation of highperformance CT-featured TFDF-OLED materials.
Acknowledgment This work is supported by National Natural Science Foundation of China (No. 21372168).| [1] | C.W. Tang, S.A. VanSlyke. Organic electroluminescent diodes. Appl. Phys. Lett. 51 (1987) 913–915. DOI:10.1063/1.98799 |
| [2] | K.T. Kamtekar, A.P. Monkman, M.R. Bryce. Recent advances in white organic lightemitting materials and devices (WOLEDs). Adv. Mater. 22 (2010) 572–582. DOI:10.1002/adma.v22:5 |
| [3] | C.J. Chiang, A. Kimyonok, M.K. Etherington, et al. ultrahigh efficiency fluorescent single and bi-layer organic light emitting diodes: the key role of triplet fusion. Adv. Funct. Mater. 23 (2013) 739–746. DOI:10.1002/adfm.v23.6 |
| [4] | P.W. Atkins, R.S. Friedman. Friedman, Molecular Quantum Mechanics[M]. New York: Oxford University Press, 2005 . |
| [5] | (a) Y. Chi, P.T. Chou, Transition-metal phosphors with cyclometalating ligands: fundamentals and applications, Chem. Soc. Rev. 39(2010) 638-655; (b) P.T. Chou, Y. Chi, M.W. Chung, et al., Harvesting luminescence via harnessing the photophysical properties of transition metal complexes, Chem. Soc. Rev. 255(2011) 2653-2665. |
| [6] | Y. Tao, K. Yuan, T. Chen, et al. Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv. Mater. 26 (2014) 7931–7958. DOI:10.1002/adma.v26.47 |
| [7] | (a) D.H. Hu, L. Yao, B. Yang, et al., Reverse intersystem crossing from upper triplet levels to excited singlet: a ‘hot excition’ path for organic light-emitting diodes, Philos. Trans. A: Math. Phys. 373(2015) 20140318; (b) L. Yao, B. Yang, Y.G. Ma, Progress in next-generation organic electroluminescent materials: material design beyond exciton statistics, Sci. China Chem. 57(2014) 335-345. |
| [8] | D.Y. Kondakov. Triplet-triplet annihilation in highly efficient fluorescent organic light-emitting diodes: current state and future outlook. Philos. Trans. A: Math. Phys. Eng. Sci. 373 (2015) 20140321. DOI:10.1098/rsta.2014.0321 |
| [9] | (a) P. Rajamalli, N. Senthilkumar, P. Gandeepan, et al., A new molecular design based on thermally activated delayed fluorescence for highly efficient organic light emitting diodes, J. Am. Chem. Soc. 138(2015) 628-634; (b) S. Hirata, Y. Sakai, K. Masui, et al., Highly efficient blue electroluminescence based on thermally activated delayed fluorescence, Nat. Mater. 14(2015) 330-336. |
| [10] | Y.F. Zhang, S.R. Forrest. Triplets contribute to both an increase and loss in fluorescent yield in organic light emitting diodes. Phys. Rev. Lett. 108 (2012) 267404. DOI:10.1103/PhysRevLett.108.267404 |
| [11] | D.Y. Kondakov. Characterization of triplet-triplet annihilation in organic lightemitting diodes based on anthracene derivatives. J. Appl. Phys. 102 (2007) 114504. DOI:10.1063/1.2818362 |
| [12] | P.Y. Chou, H.H. Chou, Y.H. Chen, et al. Efficient delayed fluorescence via triplettriplet annihilation for deep-blue electroluminescence. Chem. Commun. 50 (2014) 6869–6871. DOI:10.1039/c4cc01851f |
| [13] | J. Jortner, S.I. Choi, J.L. Katz, et al. Triplet energy transfer and triplet-triplet interaction in aromatic crystals. Phys. Rev. Lett. 11 (1963) 323–326. DOI:10.1103/PhysRevLett.11.323 |
| [14] | B. Dick, B. Nickel. Accessibility of the lowest quintet state of organic molecules through triplet-triplet annihilation; an INDO CI study. Chem. Phys. 78 (1983) 1–16. DOI:10.1016/0301-0104(83)87001-3 |
| [15] | V. Jankus, M. Aydemir, F.B. Dias, et al., Generating light from upper excited triplet states: a contribution to the indirect singlet yield of a polymer OLED, helping to exceed the 25% singlet exciton limit, Adv. Sci. 3(2016), http://dx.doi.org/10.1002/advs.201500221. |
| [16] | C. Ganzorig, M. Fujihira. A possible mechanism for enhanced electrofluorescence emission through triplet-triplet annihilation in organic electroluminescent devices. Appl. Phys. Lett. 81 (2002) 3137–3139. DOI:10.1063/1.1515129 |
| [17] | D.Y. Kondakov, T.D. Pawlik, T.K. Hatwar, et al. Triplet annihilation exceeding spin statistical limit in highly efficient fluorescent organic light-emitting diodes. J. Appl. Phys. 106 (2009) 124510. DOI:10.1063/1.3273407 |
| [18] | P. Chen, Z.H. Xiong, Q.M. Peng, et al. Magneto-electroluminescence as a tool to discern the origin of delayed fluorescence: reverse intersystem crossing or triplettriplet annihilation. Adv. Opt. Mater. 2 (2014) 142–148. DOI:10.1002/adom.201300422 |
| [19] | J. Xiang, Y.B. Chen, W.Y. Jia, et al. Realization of triplet-triplet annihilation in planar heterojunction exciplex-based organic light-emitting diodes. Org. Electron. 28 (2016) 94–99. DOI:10.1016/j.orgel.2015.10.017 |
| [20] | C.A. Parker, C.G. Hatchard. Delayed fluorescence from solutions of anthracene and phenanthrene. Proc. R. Soc. Lond. A: Math. Phys. Sci. 269 (1962) 574–584. DOI:10.1098/rspa.1962.0197 |
| [21] | R.G. Kepler, J.C. Caris, P. Avakian, et al. Triplet excitons and delayed fluorescence in anthracene crystals. Phys. Rev. Lett. 10 (1963) 400–402. DOI:10.1103/PhysRevLett.10.400 |
| [22] | J. Kido, Y. Iizumi. Fabrication of highly efficient organic electroluminescent devices. Appl. Phys. Lett. 73 (1998) 2721–2723. DOI:10.1063/1.122570 |
| [23] | Z.D. Popovic, H. Aziz. Delayed electroluminescence in small-molecule-based organic light-emitting diodes: evidence for triplet-triplet annihilation and recombination-center-mediated light-generation mechanism. J. Appl. Phys. 98 (2005) 013510. DOI:10.1063/1.1937472 |
| [24] | C. Mayr, T.D. Schmidt, W. Brütting. High-efficiency fluorescent organic lightemitting diodes enabled by triplet-triplet annihilation and horizontal emitter orientation. Appl. Phys. Lett. 105 (2014) 183304. DOI:10.1063/1.4901341 |
| [25] | A.H. Davis, K. Bussmann. Large magnetic field effects in organic light emitting diodes based on tris(8-hydroxyquinoline aluminum)(Alq3)/N,N'-di(naphthalen-1-yl)-N,N'-diphenylbenzidine (NPB) bilayers. J. Vac. Sci. Technol. A 22 (2004) 1885–1891. |
| [26] | (a) R. Liu, Y. Zhang, Y.L. Lei, et al., Magnetic field dependent triplet-triplet annihilation in Alq3-based organic light emitting diodes at different temperatures, J. Appl. Phys. 105(2009) 093719; (b) Y.L. Lei, Y. Zhang, R. Liu, et al., Driving current and temperature dependent magnetic-field modulated electroluminescence in Alq3-based organic light emitting diode, Org. Electron. 10(2009) 889-894. |
| [27] | J.M. Shi, C.W. Tang. Anthracene derivatives for stable blue-emitting organic electroluminescence devices. Appl. Phys. Lett. 80 (2002) 3201–3203. DOI:10.1063/1.1475361 |
| [28] | (a) H. Zhang, H. Tong, Y.L. Zhao, et al., Synthesis, crystal structures and photoluminescence of anthracene- and pyrene-based coumarin derivatives, Spectrochim. Acta A: Mol. Biomol. Spectrosc. 150(2015) 316-320; (b) K.A. Nguyen, J. Kennel, R. Pachter, A density functional theory study of phosphorescence and triplet-triplet absorption for nonlinear absorption chromophores, J. Chem. Phys. 117(2002) 7128-7136. |
| [29] | L. Ma, K.K. Zhang, C. Kloc, et al. Singlet fission in rubrene single crystal: direct observation by femtosecond pump-probe spectroscopy. Phys. Chem. Chem. Phys. 14 (2012) 8307–8312. DOI:10.1039/c2cp40449d |
| [30] | M. Pope, H.P. Kallmann, P. Magnante. Electroluminescence in organic crystals. J. Chem. Phys. 38 (1963) 2042–2043. DOI:10.1063/1.1733929 |
| [31] | M.R. Zhu, C.L. Yang. Blue fluorescent emitters: design tactics and applications in organic light-emitting diodes. Chem. Soc. Rev. 42 (2013) 4963–4976. DOI:10.1039/c3cs35440g |
| [32] | Y.C. Luo, H. Aziz. Correlation between triplet-triplet annihilation and electroluminescence efficiency in doped fluorescent organic light-emitting devices. Adv. Funct. Mater. 20 (2010) 1285–1293. DOI:10.1002/adfm.v20:8 |
| [33] | C.H. Liao, M.T. Lee, C.H. Tsai, et al. Highly efficient blue organic light-emitting devices incorporating a composite hole transport layer. Appl. Phys. Lett. 86 (2005) 203507. DOI:10.1063/1.1931052 |
| [34] | Y.J. Pu, G. Nakata, F. Satoh, et al. Optimizing the charge balance of fluorescent organic light-emitting devices to achieve high external quantum efficiency beyond the conventional upper limit. Adv. Mater. 24 (2012) 1765–1770. DOI:10.1002/adma.201104403 |
| [35] | D.Y. Kondakov. Role of triplet-triplet annihilation in highly efficient fluorescent devices. J. Soc. Info. Display 17 (2009) 137–144. DOI:10.1889/JSID17.2.137 |
| [36] | S.K. Kim, B. Yang, Y.Q. Ma, et al. Exceedingly efficient deep-blue electroluminescence from new anthracenes obtained using rational molecular design. J. Mater. Chem. 18 (2008) 3376–3384. DOI:10.1039/b805062g |
| [37] | D. Yokoyama, Y. Park, B. Kim, et al. Dual efficiency enhancement by delayed fluorescence and dipole orientation in high-efficiency fluorescent organic lightemitting diodes. Appl. Phys. Lett. 99 (2011) 123303. DOI:10.1063/1.3637608 |
| [38] | I. Cho, S.H. Kim, J.H. Kim, et al. Highly efficient and stable deep-blue emitting anthracene-derived molecular glass for versatile types of non-doped OLED applications. J. Mater. Chem. 22 (2012) 123–129. DOI:10.1039/C1JM14482K |
| [39] | W.C. Chen, C.S. Lee, Q.X. Tong. Blue-emitting organic electrofluorescence materials: progress and prospective. J. Mater. Chem. 3 (2015) 10957–10963. |
| [40] | T. Suzuki, Y. Nonaka, T. Watabe, et al. Highly efficient long-life blue fluorescent organic light-emitting diode exhibiting triplet-triplet annihilation effects enhanced by a novel hole-transporting material. Jpn. J. Appl. Phys. 53 (2014) 052102. DOI:10.7567/JJAP.53.052102 |
| [41] | K. Okumoto, H. Kanno, Y. Hamada, et al. Green fluorescent organic light-emitting device with external quantum efficiency of nearly 10%. Appl. Phys. Lett. 89 (2006) 063504. DOI:10.1063/1.2266452 |
| [42] | H. Fukagawa, T. Shimizu, N. Ohbe, et al. Anthracene derivatives as efficient emitting hosts for blue organic light-emitting diodes utilizing triplet-triplet annihilation. Org. Electron. 13 (2012) 1197–1203. DOI:10.1016/j.orgel.2012.03.019 |
| [43] | Y.Y. Lyu, J. Kwak, O. Kwon, et al. Silicon-cored anthracene derivatives as host materials for highly efficient blue organic light-emitting devices. Adv. Mater. 20 (2008) 2720–2729. DOI:10.1002/adma.v20:14 |
| [44] | J.Y. Hu, Y.J. Pu, F. Satoh, et al. Bisanthracene-based donor-acceptor-type lightemitting dopants: highly efficient deep-blue emission in organic light-emitting devices. Adv. Funct. Mater. 24 (2014) 2064–2071. DOI:10.1002/adfm.v24.14 |
| [45] | J.P. Spindler, W.J. Begley, T.K. Hatwar, et al. 30.4: high-efficiency fluorescent redand yellow-emitting OLED devices. SID Int. Symp. Digest Tech. Pap. 40 (2009) 420–423. DOI:10.1889/1.3256804 |
| [46] | J. Xue, C. Li, L.J. Xin, et al. High-efficiency and low efficiency roll-off near-infrared fluorescent OLEDs through triplet fusion. Chem. Sci. 7 (2016) 2888–2895. DOI:10.1039/C5SC04685H |
| [47] | V. Jankus, C.J. Chiang, F. Dias, et al. Deep blue exciplex organic light-emitting diodes with enhanced efficiency; P-type or E-type triplet conversion to singlet excitons?. Adv. Mater. 25 (2013) 1455–1459. DOI:10.1002/adma.v25.10 |
| [48] | F.B. Dias, K.N. Bourdakos, V. Jankus, et al. Triplet harvesting with 100% efficiency by way of thermally activated delayed fluorescence in charge transfer OLED emitters. Adv. Mater. 25 (2013) 3707–3714. DOI:10.1002/adma.v25.27 |
| [49] | (a) J. Zhou, P. Chen, X. Wang, et al. Charge-transfer-featured materials-promising hosts for fabrication of efficient OLEDs through triplet harvesting via triplet fusion, Chem. Commun., 2014,50: 7586-7589; (b) X.J. Zheng, Q.M. Peng, J. Lin, et al., Simultaneous harvesting of triplet excitons in OLEDs by both guest and host materials with an intramolecular charge-transfer feature via triplet-triplet annihilation. J. Mater. Chem. C 3 (2015) 6970–6978. |
| [50] | S.J. Cha, N.S. Han, J.K. Song, et al. Efficient deep blue fluorescent emitter showing high external quantum efficiency. Dyes Pigm. 120 (2015) 200–207. DOI:10.1016/j.dyepig.2015.04.020 |
| [51] | Y.H. Chen, C.C. Lin, M.J. Huang, et al. Superior upconversion fluorescence dopants for highly efficient deep-Blue electroluminescent devices. Chem. Sci. 7 (2016) 4044–4051. DOI:10.1039/C6SC00100A |
 2016, Vol. 27
2016, Vol. 27 



