Poly(arylene ether)s (PAEs) have emerged as high-performance engineering plastics since the first successful preparation with high molecular weight by R. N. Johnson et al. in 1967 [1-3].ue to existence of the rigid and thermally stable arylene units, flexible and heat resistant oxygen ether, and/or extra polar bonds (e.g. - (C=O)-, -(O=S=O)-) [4], these amorphous or semi-crystalline engineering plastics possess excellent properties, such as high thermal and oxidative stability, good mechanical strength, superior electrical insulating ability and high glass transition temperatures. Up to now, there has been a great family of PAE products including poly(arylether ketone), poly(aryl ether sulfone), poly(- arylene ether nitrile) and so on, which have been widely used in electronic and electrical products, aerospace industry and mechanical manufacturing fields [5].
It should be noted that, the application of PAEs has been recently extended from engineering plastics to optoelectronic functional materials. Apart from high thermal and morphological stability, PAEs possess well-defined conjugated moieties and localized excited state between two saturated oxygen atoms [6].herefore, their optical properties can be modulated easily and reliably to realize wide bandgap blue emission.n addition, unlike conventional π-conjugated polymers (π-CPs) that are synthesized through cross-coupling reactions catalyzed by transition metal catalyst, PAEs can be synthesized from difluorinate and dihydroxy monomers by means of classical polycondensation conditions with base in polar solvents (Fig. 1). Simple work-up of washing by water and precipitating in poor solvents can readily produce the desired polymer with high purity for use in optoelectronic devices without any residual catalyst contamination, which is always an annoying problem for metal-catalyzed conjugated polymers [7].

|
Download:
|
| Figure 1. Comparison between p-conjugated polymers and PAEs. | |
In this review, we will demonstrate the new application of PAEs in organic light-emitting diodes (OLEDs). Various kinds of functional PAEs including fluorescent polymers, host polymers and phosphorescent polymers are outlined, and their molecular design, synthesis and device performance are overviewed.
2. PAE-based fluorescent polymersIn contrast to traditional fluorescent π-CPs, PAE-based fluorescent polymers can be considered as incorporating smallmolecular fluorescent emitters with well-defined structure into a polymeric skeleton through insulated oxygen atoms. As the fluorescent emitters take advantages of structural uniformity and regularity, the PAEs possess well-defined optical properties.n this sense, PAEs should be able to take advantages of both polymeric and small-molecular materials.hat is, the optical properties of the polymers are mainly determined by the small-molecular emitters, and their thermal properties and morphology in the solid state can be modulated through designing appropriate polymer structure. Although a series of condensation light-emitting polymers (such as polyesters [8, 9], polyamides [10-12], and polyurethanes [13]) have been reported with small-molecular fluorescent emitters as building blocks for the polymer main chain, only the PAEs show good electroluminescent properties comparable or even higher than those of fully π-CPs, probably due to their relatively high photoluminescence efficiency and weak charge trapping effect [14, 15].
2.1. PAEs with fluorescent main chainPPV-based PAEs:he early studies of the light-emitting PAEs are concentrated on poly(phenylenevinylene) (PPV) derivatives.n 1993, . E. Earasz et al. [6] reported a polyaryl alkyl ether (P1, ig.) by combining phenylenevinylene oligomers with flexible-chain aliphatic oligomeric segments in the main chain. A differential scanning calorimeter (DSC) trace of the copolymer displays a glass transition at 341 ℃. No melting or other thermal transition in the measured temperature range of 303-490 ℃ was observed.he results implied that P1 was an amorphous homogeneous copolymer.mportantly, the copolymer showed the maximum absorption/ emission wavelength similar to that of the conjugated oligomeric segment in the main chain.he uniform conjugation length of P1 determined the spectral purity of the electroluminescence (EL) of the OLED device, which in this case was in the blue region.or comparison, in conventional full-conjugated PPVs, it was difficult to control the effective conjugation length to realize blue emission because of the random distribution of chemical and morphological defect in the chain.
To improve the carrier-transporting ability, our group reported novel blue-emitting PPV-based PAEs containing N, N, N, N-tetraphenyl- phenylenediamine (TPPA) and phenylenevinylene moieties in the backbone (P2) [16].he ether linkage in the backbone can increase the thermal stability and break the conjugation to achieve blue emission. Moreover, the incorporation of thePPA moiety can improve the hole-transporting ability of the resultant polymer, sincePPA and its derivatives have been widely used as building blocks for hole-transporting materials in OLEDs.he introduced bulkyPPA side chain can also reduce the selfquenching of excitons caused by the interchain interaction. As a result, a bright blue emission with a peak at 450 nm in film was observed for P2.he single-layer device showed an emissive maxima at 442 nm together with a maximum brightness of 144 cd m-2 and a luminous efficiency of 0.2 cd A-1.
To further improve the charge balance in the emissive layer, a bipolar PAE (P3) was synthesized by incorporating the octafluorobiphenyl moiety as the electron transporting unit and carbazole as the hole-transporting unit into the main chain [17].he conjugation length of the polymer was also limited to the blueemission region by ether linkage. P3 was completely soluble in common organic solvents and exhibited good thermal stability up to 308 ℃. Although the OLED device made from P3 showed somewhat poor device performance and high turn-on voltage (30- 35 V), a pure blue EL peaked at 458 nm was obtained, corresponding to its photoluminescence (PL).
Quinoline -based PAEs: Beyond the PPV-based PAEs, .. Parker reported a new class of soluble and high-efficiency blue PAE (P4) based on the quinoline segment [18]. Multilayer devices with P4 as the emitting layer revealed an internal quantum efficiency higher than 4% as well as the EL emission located at 450 nm. Considering the fact that the quinoline segment had high electron affinity and excellent electron-transporting ability, a hole-transporting tetraphenyldiaminobiphenyl (TPD) unit was then incorporated into the backbone by Alex K-Y. Jen et al. to improve the charge balance [19].he formed polymer P5 possessed excellent thermal stability with a glass transition temperature of 196 ℃, and a decomposition temperature of 445 ℃. Unfortunately, the PL spectrum of P5 was red-shifted to 547 nm, attributable to the charge transfer effect.ts corresponding device performance was strongly dependent on the used hole- and electron-transporting layers, indicative of the electron-dominant nature of P5.herefore, even higher amount of hole-transporting units should be introduced to further balance the charge flux.
Oligophenylenes-based PAEs:wo novel PAEs consisting of alternate isolated chromophores (dicyano-p-quaterphenyl (P6) and bis(trifluoromethyl)-p-quaterphenyl (P7)) had been synthesized and characterized by Yun Chen et al. [20].he PL spectra of the two polymers showed maximum peaks at 407 nm and 413 nm in the film state, respectively, corresponding to those of pquaterphenyl segments. Here the π-quaterphenyl substituted by cyano and trifluoromethyl groups and carbazole acted as the electron and hole transporting units, respectively.heir highest occupied molecular orbital and lowest unoccupied molecular orbital (HOMO/LUMO) energy levels measured by cyclic voltammetry were estimated to be -5.23/-3.25 eV for P6 and -5.41/- 3.32 eV for P7.herefore, the decreased charge injection barriers from opposite electrodes could be expected although their EL performance was not reported.
Oligofluorene-based PAEs: Our group reported a series of blue light-emitting PAEs (P8 and P9) containing ter- or penta-fluorenes in the main chain via nucleophilic substitution polycondensation reaction [21].he energy levels and carrier transport properties of the polymers were tuned by introducing hole-transporting triarylamine groups in the side chains and electron-transporting oxadiazole units in the main chain.t was found that the optical properties of the polymers were dominantly determined by the ter- or penta-fluorenes segments, and thus both the polymers showed high PL quantum yields of 88%-100%.SC characterizations indicated that they were robust polymers with high glass transition temperatures up to 156 ℃. And P9 containing pentafluorenes exhibited comparable EL properties to the fullyconjugated fluorene-based polymers. With a single-layer device structure, deep blue emission was achieved for P9 along with an external quantum efficiency (EQE) of 1.4% and Commissionnternationale de L’Eclairage (CIE) coordinates of (0.17, 0.09).

|
Download:
|
| Figure 2. Chemical structures of PAEs with fluorescent main chain. | |
2.2. PAEs with fluorescent side chain
In the continuing work of light-emitting PAEs, our group reported a series of PAEs with light-emitting side chain [22-25]. As depicted in Fig. 3, the polyaryl ether main chain acted as the molecular skeleton, while the side chain acted as the emitting moieties.he main chain and side chain were separated from each other by saturated sp3 carbon atoms. Compared with the PAEs containing emitters in the backbone, therefore, the PAEs with emitters in the side chains took advantages of ease of modulation of their optical properties.

|
Download:
|
| Figure 3. PAEs with fluorescent side chain. | |
Four PAEs (P10-P13) containing ter- or penta-fluorene units in the side chains were firstly designed and synthesized for blue light emission [22]. All the PAEs showed optical properties identical to the corresponding side chains.o investigate their EL properties, the devices with a single-layer configuration were fabricated.t was found that the turn-on voltage was 3.5 V for P12 and P13, much lower than P10 and P11 (6.5-10 V) without the diphenylamine groups.his implied that the incorporation of diphenylamine to oligofluorene terminals could significantly reduce the hole-injection barrier in OLEDs. Moreover, the single-layer devices based on P12 and P13 gave luminous efficiencies of 1.2 and 2.0 cd A-1 at a brightness of 300 cd m-2 with the CIE coordinates of (0.15, 0.13) and (0.19, 0.20), respectively.
To further modify the optical and electrical properties of the emitters, novel blue light-emitting PAEs (P14-P16) comprising of bipolar oligofluorene pendants as chromophores were designed and synthesized [23].n this case, pyrimidine and arylamine moieties were utilized as the electron acceptor and electron donor, respectively.hrough varying end-cappers from hydrogen to carbazole and diphenylamine, the emission color of the resultant polymers could be finely tuned to cover from deep blue to greenish blue, and their HOMO and LUMO levels could be simultaneously modulated to facilitate charge injection to improve the device performance. Among these polymers, P15 with bifluorene bridge and carbazole end-capper obtained the best trade-off between the efficiency and emission wavelength, revealing a peak luminous efficiency as high as 1.26 cd/A and CIE coordinates of (0.17, 0.17).
On the other hand, a well-known “host-dopant” concept in vacuum-deposited OLEDs was applied to design highly efficient light-emitting PAEs.or example, P17 containing pentafluorene as the host and distyrylarylene derivative (BCzVF) as the dopants was prepared for efficient pure-blue light emission by condensation polymerization (Fig. 4) [24]. Color tuning could be achieved through efficient FÖrster energy transfer from the deeπ-blue pentafluorene host to the pure-blue BCzVF dopant. Single-layer devices based on P17 exhibited voltage-independent and stable pure-blue emission with CIE coordinates of (0.15, 0.15), a maximum brightness of 3576 cd m-2, and a maximum luminous efficiencies of.15 cd A-1, respectively.mportantly, the performance of P17 surpassed that of the host-only polymer P11, which could be reasonably ascribed to the energy transfer from the host to the dopant.

|
Download:
|
| Figure 4. Design principles and chemical structures of PAEs with a host-dopant feature. | |
Based on the blue PAEs with a host-dopant feature, white lightemitting PAEs (P18) were further developed by introducing an additional orange emitters in the side chains [25]. By carefully tuning the feed ratios of the emitting species, the excited state energy can be transferred efficiently from the deeπ-blue unit to the light-blue and orange ones, while the energy transfer between light blue and orange units can be ignored because of the extremely low content of the orange species. With the simultaneous blue and orange emission, white EL was observed for P18.he single-layer device of P18 displayed a high luminous efficiency of 7.96 cd A-1, a maximum brightness of 9950 cd m-2 accompanied with CIE coordinates of (0.33, 0.44).
3. PAE-based host polymersHost materials are critical for phosphorescent OLEDs (PhOLEDs) since phosphorescent emitters generally show concentration quenching effect and thus should be dispersed in suitable hosts to achieve high efficiency [26, 27]. Basically, there are several requirements for host materials.tirstly, triplet energy (ET) of the host should be higher than that of the dopant to prevent energy back transfer from dopant to host [28]. Secondly, charge injection/ transporting capability of the host should be balanced to enhance exciton formation ratio [29]. However, there is a conflict between the high triplet energy and charge injection for most polymer hosts.hat is, a polymer with high ET always shows wide energy band gap along with large charge injection barriers, leading to unbalanced carrier flux in the emissive layer.or instance, the typical host poly(N-vinylcarbazole) (PVK) has a high ET of.9 eV but with a very deep HOMO level of about -5.9 eV [30]. By contrast, the π-CPs, such as polycarbazoles (PCzs [31]) and polyfluorenes (PΦf [32]), possess good carrier injection capabilities, but their ETs are lower than.70 eV because of the large conjugation extent.his makes them only suitable for green and red PhOLEDs.
Therefore, the development of novel polymeric hosts with both high ETs and good carrier injection capabilities used for blue PhOLEDs is challenging, but of great significance. Recently, different from the conjugated polymers and non-conjugated ones, a partially-conjugated PAE host was designed by our group to meet such requirements [33]. As shown in Fig. 5, the high ET was within our expectation because the π-conjugation of the backbone could be interrupted by the saturated oxygen atoms. Meanwhile, the electron-transporting arylphosphine oxide and hole-transporting carbazole units were simultaneously introduced into the main chain and side chain, respectively, to endow the polymers with bipolar characteristic. As a result, a bipolar polymer P19 was obtained with a high ET of.96 eV.his ET surpassed most polymeric hosts ever reported, and was sufficiently higher than that of the blue phosphor, Irpic (ET =.62 eV).n addition, compared with PVK, its HOMO and LUMO levels were tuned to -5.7 and -2.3 eV, respectively, beneficial to improve both the hole and electron injection capability. Single-layer blue-emitting PhOLEDs fabricated from P19 doped withIrpic achieved higher EL efficiency by 100 times than those using PVK as the host.urthermore, by introduction of a hole/exciton blocking layer, a double-layer device with a satisfactory efficiency of3.3 cd A-1 at a practical brightness of 116 cd m-2 was realized at a dopant concentration as low as 5 wt%.hese values allowed this PAE polymer among the most efficient solution-processable hosts for blue triplet emitters. Most importantly, the obtained promising performance suggests that PAEs may show great advantages over conventional π-CPs in the design of wide-bandgap optoelectronic polymers.
4. PAE-based phosphorescent polymersAlthough high-performance PhOLEDs have been realized by doping phosphorescent dyes into polymer hosts as presented in Section 3, these physical blend systems may suffer from phase segregation under long-term device operation. An alternative is to develop electrophosphorescent polymers (PhPs), where the phosphorescent dyes are incorporated into the polymeric main chain or side chain via a covalent bond.n literatures, most efforts have been paid to red and green PhPs based on conjugated polymers [34-40]. However, owing to the lack of suitable hightriplet- energy polymer hosts that can be used as the molecular design platform, blue PhPs are rare, and the device efficiency is extremely poor [41, 42].n fact, as one of the three primary colors, blue PhPs are indispensable components for low cost full-color displays as well as white light sources.n view of these points, the redesign of blue PhPs is highly desirable.
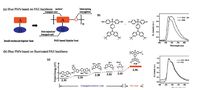
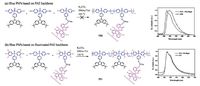
Considering that the tradeoff between high triplet energy and suitable HOMO/LUMO levels was realized on P19, our group further reported blue PhPs based on the PAE-based polymer host [43].nitially we attempted to directly introduceIrpic to the side chain of P19 to obtain P20 (Fig. 6). Unfortunately, the resultant polymer exhibited a bathochromic shift of 11-18 nm relative to the physical blend of P19 doped withIrpic.he possible reason lies in that the atoms inIrpic are not endurable to the harsh polycondensation condition under a strong base in a polar solvent at high temperature (165 ℃), resulting in the observed red shift after the leave from the C^N ligand.o solve this problem, an activated fluorinated poly(arylene ether phosphine oxide) backbone (FPCzPO) was used to construct the blue PhPs (P21).n this case, the polymers could be synthesized under a milder temperature of 120 ℃. Compared with the counterparts prepared at 165 ℃, unexpected bathochromic shift was successfully avoided, and the emission of P21 matched well with the tetheredIrpic. A state-ofart luminous efficiency as high as 19.4 cd A-1 was achieved, comparable to the corresponding physical blend system.he result indicates that PCzPO is an excellent platform for the development of high-performance blue PhPs.
|
Download:
|
| Figure 5. PAE-based host and the triplet energy comparison with conjugated counterparts [33]. | |
|
Download:
|
| Figure 6. Synthesis of PAE-based blue PhPs and their corresponding EL spectra [43]. | |
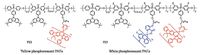
Apart from blue PhPs, yellow PhPs are also necessary to generate white emission when mixed with blue ones. On the basis of the same fluorinated PAE hostPCzPO, a series of novel yellow PhPs had been designed and synthesized by grafting a yellowemitting iridium complex (fbi)2Ir(acac) to the polymer side chain (Fig. 7) [44].ue to the efficient energy transfer, the EL fromPCzPO was almost completely quenched, even if ther complex content incorporated into the polymer was as low as mol%. With anr loading of 3 mol%, P22 exhibited the best device performance with a peak luminous efficiency of 10.4 cd A-1, superior to that of the previously reported yellow PhPs with polyfluorene as the main chain.
|
Download:
|
| Figure 7. Chemical structures of PAE-based yellow and white PhPs. | |
With efficient blue and yellow PhPs in hand, further work was performed on highly efficient all-phosphorescent single whiteemitting polymers (SWPs) by simultaneously grafting blue and yellow phosphors intoPCzPO [45].ifferent from the traditional all-fluorescent and fluorescent/phosphorescent hybrid ones reported in the literatures [35, 46-49], all-phosphorescent SWPs were supposed to take advantages of high efficiency because all the singlet and triplet excitons can be fully utilized by the phosphorescent dopants. As shown in Fig. 7, Irpic and (fbi)2Ir(acac) [50] were selected as the blue and yellow phosphors, respectively, for the design of all-phosphorescent SWPs. Meanwhile, in view of the compatibility ofIrpic, the low temperature polycondensation condition (120 ℃) was adopted. However, the synthesis of SWPs was still of challenge, for the yellow emission part of the resultant polymers displayed a hypsochromic shift of 10 nm compared with (fbi)2Ir(acac).his abnormity may be caused by the ancillary ligand exchange between the blue and yellowr complex.o avoid the color change, a “two-step addition” strategy was applied for the polymerization.n this case, the monomer containingIrpic was firstly copolymerized with the fluorinated and hydroxy monomers, and the monomer containing (fbi)2Ir(acac) was successively added to continue the polymerization.With this modifiedmethod, a series of all-phosphorescent SWPs (P23) were successfully prepared by tailoring the feed ratios of monomers. P23 showed normal blue emission fromFIrpic and yellowemission from(fbi)2Ir(acac).Among the SWPs, P23 with 7.5 mol%Irpic and 0.7 mol% (fbi)2Ir(acac) in the side chain possessed the best device performance with a maximum luminous efficiency of 18.4 cd A-1, a maximum power efficiency of 8.5 lm W-1, and a peak EQE of 7.1%. Even at a brightness of 1000 cd/ m2, it still remained as high as 14.2 cd A-1, indicating a slow efficiency roll-off.his was the highest performance ever reported for SWPs at that time. By comparison to the phosphorescent bichromophoric block copolymers with non-conjugated polystyrene as backbone (EQE = 1.5%) [51], the efficiency was improved by about 4.7 folds. Most importantly, the efficiency was also much better than those of all-fluorescent and fluorescent/phosphorescent hybrid SWPs, shedding light on the significance of all-phosphorescent SWPs based on PAEs.
5. ConclusionIn this review, we have summarized the new applications of PAEs in OLEDs. Generally, by suitable molecular design, highperformance optoelectronicmaterials can be obtained based on the traditional PAE skeletons via classic condensation polymerization synthetic procedure.he performance of PAE-based fluorescent polymers is comparable to the fully-conjugated polymers. Nevertheless, PAE-based hosts show unique advantages over conjugated polymers, because the oxygen interruption can realize high triplet energy. With a PAE host as the basic backbone, furthermore, a series of monochromic or white PhPs have been designed and synthesized by grafting iridium complexes into the polymer side chain.
Despite these achievements, significant progress is still needed for PAE-based polymers.irstly, the chemical and physical properties of the PAEs should be further explored to optimize the device performance.or fluorescent PAEs, the polymer skeleton and the emitting chromophores should be carefully chosen to boost the device efficiency and enhance the color purity.or PAE-based hosts, it is necessary to finely tailor their chemical structures to tune not only the triplet energy within a wide range to meet the requirements of different color dopants, but also the HOMO/LUMO levels to match well the adjacent layers and further reduce the charge injection barriers. While for PAE-based PhPs, phosphorescent dopants with suitable emission wavelength, high PL quantum efficiency as well as short triplet lifetime should be searched. Secondly, besides the molecular design, the polymerization methodology is another important issue for PAE-based materials. Because phosphorscent complexes are not durable under harsh conditions, some novel strategies using mild polymerization conditions should be developed for the synthesis of PAEs.inally, although PAEs are basically thermal and chemical stable, the lifetimes and reliabilities of OLEDs based on PAEs have not been well addressed yet.n general, high EL efficiency, various emission colors, good color purity, and long operational lifetime are basic requirements for these materials. We believe that, with continuous efforts on the research of PAE-based OLED materials, they will be one of most promising candidates for the future display and lighting products.
Acknowledgment The authors are grateful to the National Natural Science Foundation of China (Nos. 51573182, 51203149, 21204084, 91333205) and the 973 Project (No.015CB655000) for financial support of this research.| [1] | W.F. Hale, A.G. Farnham, R.N. Johnson, R.A. Clendinning. Poly(aryl ethers) by nucleophilic aromatic substitution. II. Thermal stability, J. Polym. Sci.. Part A: Polym. Chem. 5 (1967) 2399–2414. DOI:10.1002/pol.1967.150050917 |
| [2] | R.N. Johnson, A.G. Farnham. Poly(aryl ethers) by nucleophilic aromatic substitution. III. Hydrolytic side reactions, J. Polym. Sci.. Part A: Polym. Chem. 5 (1967) 2415–2427. DOI:10.1002/pol.1967.150050918 |
| [3] | R.N. Johnson, A.G. Farnham, R.A. Clendinning, W.F. Hale, C.N. Merriam. Poly(aryl ethers) by nucleophilic aromatic substitution. I. Synthesis and properties, J. Polym. Sci.. Part A: Polym. Chem. 5 (1967) 2375–2398. DOI:10.1002/pol.1967.150050916 |
| [4] | S. Rahmani, A.S.Z. Mahani. Synthesis and characterization of novel poly(arylene ether)s containing 1, 3, 4-oxadiazole and triazole units through click chemistry. Macromol. Res. 23 (2015) 1018–1025. DOI:10.1007/s13233-015-3133-y |
| [5] | M.G. Dhara, S. Banerjee. Fluorinated high-performance polymers: poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 35 (2010) 1022–1077. DOI:10.1016/j.progpolymsci.2010.04.003 |
| [6] | Z. Yang, I. Sokolik, F.E. Karasz. A soluble blue-light-emitting polymer. Macromolecules 26 (1993) 1188–1190. DOI:10.1021/ma00057a047 |
| [7] | K.T. Nielsen, K. Bechgaard, F.C. Krebs. Removal of palladium nanoparticles from polymer materials. Macromolecules 38 (2005) 658–659. DOI:10.1021/ma047635t |
| [8] | C. Hosokawa, N. Kawasaki, S. Sakamoto, T. Kusumoto. Bright blue electroluminescence from hole transporting polycarbonate. Appl. Phys. Lett. 61 (1992) 2503–2505. DOI:10.1063/1.108162 |
| [9] | T. Burnell, J.A. Cella, P. Donahue, et al. Synthesis and electrooptical properties of copolymers derived from phenol-functionalized telechelic oligofluorenes. Macromolecules 38 (2005) 10667–10677. DOI:10.1021/ma0518353 |
| [10] | I.K. Spiliopoulos, J.A. Mikroyannidis. Synthesis of soluble, blue-light-emitting rigid-rod polyamides and polyimides prepared from 2', 6', 3''', 5'''-tetraphenylor tetra(4-biphenylyl)-4, 4""-diamino-p-quinquephenyl. Macromolecules 31 (1998) 515–521. DOI:10.1021/ma970490o |
| [11] | G.S. Liou, S.H. Hsiao, N.K. Huang, Y.L. Yang. Synthesis, photophysical, and electrochromic characterization of wholly aromatic polyamide blue-light-emitting materials. Macromolecules 39 (2006) 5337–5346. DOI:10.1021/ma0608469 |
| [12] | C. Hamciuc, E. Hamciuc, M. Homocianu, A. Nicolescu, I.D. Carja. Blue lightemitting polyamide and poly(amide-imide)s containing 1, 3, 4-oxadiazole ring in the side chain. Dyes Pigments 114 (2015) 110–123. DOI:10.1016/j.dyepig.2014.10.018 |
| [13] | C.H. Ku, C.H. Kuo, M.K. Leung, K.H. Hsieh. Carbazole-oxadiazole containing polyurethanes as phosphorescent host for organic light emitting diodes. Eur. Polym. J. 45 (2009) 1545–1553. DOI:10.1016/j.eurpolymj.2009.01.024 |
| [14] | S.W. Hwang, Y. Chen. Photoluminescent and electrochemical properties of novel poly(aryl ether)s with isolated hole-transporting carbazole and electron-transporting 1, 3, 4-oxadiazole fluorophores. Macromolecules 35 (2002) 5438–5443. DOI:10.1021/ma012181a |
| [15] | S.H. Chen, Y. Chen. Poly(p-phenylenevinylene) derivatives containing electrontransporting aromatic triazole or oxadiazole Segments. Macromolecules 38 (2005) 53–60. DOI:10.1021/ma048990m |
| [16] | H.C. Li, Y.F. Hu, Y.G. Zhang, et al. Novel thermally stable blue-light-emitting polymer containing N, N, N', N'-tetraphenyl-phenylenediamine units and its intramolecular energy transfer. Chem. Mater. 14 (2002) 4484–4486. DOI:10.1021/cm0255203 |
| [17] | T. Ahn, H.K. Shim. Synthesis and luminescent properties of blue light emitting polymers containing both hole and electron transporting units. Macromol. Chem. Phys. 202 (2001) 3180–3188. DOI:10.1002/(ISSN)1521-3935 |
| [18] | I.D. Parker, Q. Pei, M. Marrocco. Efficient blue electroluminescence from a fluorinated polyquinoline. Appl. Phys. Lett. 65 (1994) 1272–1274. DOI:10.1063/1.112092 |
| [19] | Y.Q. Liu, H. Ma, A.K.Y. Jen. Synthesis and characterization of a novel bipolar polymer for light-emitting diodes. Chem. Commun. (1998) 2747–2748. |
| [20] | S.W. Hwang, Y. Chen. Synthesis and electrochemical and optical properties of novel poly(aryl ether)s with isolated carbazole and p-quaterphenyl chromophores. Macromolecules 34 (2001) 2981–2986. DOI:10.1021/ma001855z |
| [21] | G.X. Jiang, J. Wu, B. Yao, et al. Synthesis and characterization of blue-lightemitting poly(aryl ether)s containing oligofluorenes in the main chain. Macromolecules 39 (2006) 7950–7958. DOI:10.1021/ma0612273 |
| [22] | G.X. Jiang, B. Yao, Y.H. Geng, et al. Poly(aryl ether)s containing ter- and pentafluorene pendants for efficient blue light emission. Macromolecules 39 (2006) 1403–1409. DOI:10.1021/ma052282z |
| [23] | G.X. Jiang, C.L. Bian, J.Q. Ding, L.X. Wang. Synthesis and characterization of blue light-emitting poly(aryl ether)s containing pyrimidine-incorporated oligofluorene pendants with bipolar feature. Chin. J. Polym. Sci. 31 (2013) 787–797. DOI:10.1007/s10118-013-1279-7 |
| [24] | C.L. Bian, G.X. Jiang, H. Tong, et al. Pure blue electroluminescent poly(aryl ether)s with dopant-host systems, J. Polym. Sci.. Part A: Polym. Chem. 49 (2011) 3911–3919. DOI:10.1002/pola.24828 |
| [25] | C.L. Bian, G.X. Jiang, Y.X. Cheng, Z.Y. Xie, L.X. Wang. Poly(aryl ether)s for efficient white electroluminescence with simultaneous bicolor emission. Acta Polym. Sin. (2012) 334–343. |
| [26] | Y. Kawamura, S. Yanagida, S.R. Forrest. Energy transfer in polymer electrophosphorescent light emitting devices with single and multiple doped luminescent layers. J. Appl. Phys. 92 (2002) 87–93. DOI:10.1063/1.1479751 |
| [27] | M.A. Baldo, D.F. O'Brien, Y. You, et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395 (1998) 151–154. DOI:10.1038/25954 |
| [28] | M. Sudhakar, P.I. Djurovich, T.E. Hogen-Esch, M.E. Thompson. Phosphorescence quenching by conjugated polymers. J. Am. Chem. Soc. 125 (2003) 7796–7797. DOI:10.1021/ja0343297 |
| [29] | H.H. Chou, C.H. Cheng. A highly efficient universal bipolar host for blue, green, and red phosphorescent OLEDs. Adv. Mater. 22 (2010) 2468–2471. DOI:10.1002/adma.v22:22 |
| [30] | M.K. Mathai, V.E. Choong, S.A. Choulis, B. Krummacher, F. So. Highly efficient solution processed blue organic electrophosphorescence with 14 lm/W luminous efficacy. Appl. Phys. Lett. 88 (2006) 243512. DOI:10.1063/1.2212060 |
| [31] | A. van Dijken, J.J.A.M. Bastiaansen, N.M.M. Kiggen, et al. Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes: polymer hosts for high-efficiency light-emitting diodes. J. Am. Chem. Soc. 126 (2004) 7718–7727. DOI:10.1021/ja049771j |
| [32] | K. Brunner, A. van Dijken, H. Börner, et al. Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes: tuning the HOMO level without influencing the triplet energy in small molecules. J. Am. Chem. Soc. 126 (2004) 6035–6042. DOI:10.1021/ja049883a |
| [33] | S.Y. Shao, J.Q. Ding, T.L. Ye, et al. A novel, bipolar polymeric host for highly efficient blue electrophosphorescence: a non-conjugated poly(aryl ether) containing triphenylphosphine oxide units in the electron-transporting main chain and carbazole units in hole-transporting side chains. Adv. Mater. 23 (2011) 3570–3574. DOI:10.1002/adma.201101074 |
| [34] | J. Liu, L.J. Bu, J.P. Dong, et al. Green light-emitting polyfluorenes with improved color purity incorporated with 4, 7-diphenyl-2, 1, 3-benzothiadiazole moieties. J. Mater. Chem. 17 (2007) 2832–2838. DOI:10.1039/b700004a |
| [35] | C.H. Chien, S.F. Liao, C.H. Wu, et al. Electrophosphorescent polyfluorenes containing osmium complexes in the conjugated backbone. Adv. Funct. Mater. 18 (2008) 1430–1439. DOI:10.1002/adfm.v18:9 |
| [36] | J. Liu, L. Chen, S.Y. Shao, et al. Highly efficient red electroluminescent polymers with dopant/host system and molecular dispersion feature: polyfluorene as the host and 2, 1, 3-benzothiadiazole derivatives as the red dopant. J. Mater. Chem. 18 (2008) 319–327. DOI:10.1039/B712562C |
| [37] | Z.H. Ma, J.Q. Ding, B.H. Zhang, et al. Red-emitting polyfluorenes grafted with quinoline-based iridium complex: "simple polymeric chain, unexpected high efficiency". Adv. Funct. Mater. 20 (2010) 138–146. DOI:10.1002/adfm.v20:1 |
| [38] | Z.H. Ma, L.C. Chen, J.Q. Ding, et al. Green electrophosphorescent polymers with poly(3, 6-carbazole) as the backbone: a linear structure does realize high efficiency. Adv. Mater. 23 (2011) 3726–3729. DOI:10.1002/adma.v23.32 |
| [39] | J. Liu, L. Yu, C.M. Zhong, et al. Highly efficient green-emitting electrophosphorescent hyperbranched polymers using a bipolar carbazole-3, 6-diyl-co-2, 8-octyldibenzothiophene-S, S-dioxide-3, 7-diyl unit as the branch. RSC Adv. 2 (2012) 689–696. DOI:10.1039/C1RA00610J |
| [40] | S.Y. Shao, Z.H. Ma, J.Q. Ding, et al. Spiro-linked hyperbranched architecture in electrophosphorescent conjugated polymers for tailoring triplet energy back transfer. Adv. Mater. 24 (2012) 2009–2013. DOI:10.1002/adma.201104544 |
| [41] | S. Tokito, M. Suzuki, F. Sato, M. Kamachi, K. Shirane. High-efficiency phosphorescent polymer light-emitting devices. Org. Electron. 4 (2003) 105–111. DOI:10.1016/j.orgel.2003.08.005 |
| [42] | Y.M. You, S.H. Kim, H.K. Jung, S.Y. Park. Blue electrophosphorescence from iridium complex covalently bonded to the poly (9-dodecyl-3-vinylcarbazole): suppressed phase segregation and enhanced energy transfer. Macromolecules 39 (2006) 349–356. DOI:10.1021/ma052015t |
| [43] | S.Y. Shao, J.Q. Ding, L.X. Wang, X.B. Jing, F.S. Wang. Highly efficient blue electrophosphorescent polymers with fluorinated poly(arylene ether phosphine oxide) as backbone. J. Am. Chem. Soc. 134 (2012) 15189–15192. DOI:10.1021/ja305634j |
| [44] | S.Y. Shao, J.Q. Ding, L.X. Wang, X.B. Jing, F.S. Wang. Synthesis and characterization of yellow-emitting electrophosphorescent polymers based on a fluorinated poly(-arylene ether phosphine oxide) scaffold. J. Mater. Chem. 22 (2012) 24848–24855. DOI:10.1039/c2jm34421a |
| [45] | S.Y. Shao, J.Q. Ding, L.X. Wang, X.B. Jing, F.S. Wang. White electroluminescence from All-phosphorescent single polymers on a fluorinated poly(arylene ether phosphine oxide) backbone simultaneously grafted with blue and yellow phosphors. J. Am. Chem. Soc. 134 (2012) 20290–20293. DOI:10.1021/ja310158j |
| [46] | G.L. Tu, Q.G. Zhou, Y.X. Cheng, et al. White electroluminescence from polyfluorene chemically doped with 1, 8-napthalimide moieties. Appl. Phys. Lett. 85 (2004) 2172–2174. DOI:10.1063/1.1793356 |
| [47] | J. Liu, S.Y. Shao, L. Chen, et al. White electroluminescence from a single polymer system: improved performance by means of enhanced efficiency and red-shifted luminescenceofthe blue-light-emittingspecies. Adv.Mater. 19 (2007) 1859–1863. DOI:10.1002/(ISSN)1521-4095 |
| [48] | J.X. Jiang, Y.H. Xu, W. Yang, et al. High-efficiency white-light-emitting devices from a single polymer by mixing singlet and triplet emission. Adv. Mater. 18 (2006) 1769–1773. DOI:10.1002/(ISSN)1521-4095 |
| [49] | J. Liu, L. Chen, S.Y. Shao, et al. Three-color white electroluminescence from a single polymer system with blue, green and red dopant units as individual emissive species and polyfluorene as individual polymer host. Adv. Mater. 19 (2007) 4224–4228. DOI:10.1002/(ISSN)1521-4095 |
| [50] | Q. Wang, J.Q. Ding, D.G. Ma, et al. Harvesting excitons via two parallel channels for efficient white organic leds with nearly 100% internal quantum efficiency: fabrication and emission-mechanism analysis. Adv. Funct. Mater. 19 (2009) 84–95. DOI:10.1002/adfm.v19:1 |
| [51] | D.A. Poulsen, B.J. Kim, B. Ma, C.S. Zonte, J.M.J. Fréchet. Site isolation in phosphorescent bichromophoric block copolymers designed for white electroluminescence. Adv. Mater. 22 (2010) 77–82. DOI:10.1002/adma.v22:1 |
 2016, Vol. 27
2016, Vol. 27 


