Luminescent materials with room temperature phosphorescence (RTP) have attracted increasing attention because of their fundamental importance [1] and wide applications in organic light-emitting diodes (OLEDs) [2], chemo- and bio-sensing [3], bioimaging [4], photodynamic therapy [5], and anti-counterfeiting techniques [6].istinguished from fluorogens [7], their longlived triplet excitons can exclude the interference from shortlived cellular autofluorescence background when used in bioimaging [4], and also can be potentially fully utilized in OLEDs. So far, RTP luminogens, however, are mainly inorganic and organometallic compounds with rare and expensive elements [8], such as rare earth metals, platinum, and iridium, although pure organic luminogens take intriguing advantages of low cost, relative ease of synthesis and processing, high stability, and facile functionalization.t is understandable that phosphorescence is generated by the occurrence of spin flip from lowest triplet excited (T1) state to the ground (S0) state, which is a quantum mechanically forbidden process. Without precious metals, the rate of phosphorescence is so slow that the longlived triplet excitons are inclined to undergo nonradiative decays through molecular motions (rotation and vibration), collision processes, and interactions with quenchers like humidity and oxygen.herefore, phosphorescence from pure organic luminogens is normally observed under cryogenic (e.g. 77 K) and inert conditions [9].
Todiminish thenonradiativedeactivations, differentapproaches, for instance, adsorption onto solid substrates or embedded into silica glasses, chelation, incorporation into polymer chains, and inclusion into surfactants or cyclodextrins have been developed to realize RTP [10].n most cases, however, merely inefficient phosphorescence is obtained.n010, ang and coworkers observed a unique phenomenon of crystallization-induced phosphorescence (CIP) in a series of pure organic luminogens, such as benzophenone (BP), methyl 4, 4´-bromobenzoate (MBB), and 4, 40-dibromobiphenyl (DBBP0) [11]. Namely, these luminogens are nonphosphorescent (some even virtually nonemissive) in solution and amorphous states, however, they demonstrate efficient RTP upon crystallization [11].he discovery of CIP phenomenon opens up a crystal engineering approach to achieve efficient RTP from pure organic luminogens.t is found that restriction of intramolecular motions (RIM) is mainly accountable for the CIP phenomenon, which is analogous to themechanism of aggregation-induced emission (AIE) [12]. Meanwhile, effective isolation from oxygen and moisture via the self-protective crystal lattice further promotes the RTP emission. Later, similarphenomenonwas reported byKimandcoworkers [13]. Subsequently, on the basis of fundamental considerations of carbonyl groups, heteroatoms, and heavy atom effect [1a, 14], efficient RTP from pure organic luminogens was received through further (co)crystallization, doping/trapping in rigid matrices, creating intermolecular interactions between luminogens and hosts, and even singlet fission [15-28].n this review, we focus on the recent progress of CIP luminogens, aiming to show a brief picture on this exciting renewed area.
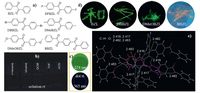
2. CIP of benzophenones and benzilsIt is well known that BP is phosphorescent at cryogenic temperatures, whose intersystem crossing (ISC) efficiency is approaching unity.In 2010, ang and coworkers discovered the unique CIP phenomenon of BP [11]. BP molecules exhibit merely negligible fluorescence in neither protic nor aprotic solvents, or amorphous states when absorbed onto thin-layer chromatography (TLC) plates or incorporated into polymethyl methacrylate (PMMA) films under ambient conditions, while being highly phosphorescent upon crystallization, with solution and crystal efficiencies (Φf and Φc) of 0.001% and 15.9%, respectively. Similar phenomenon is observed in its derivatives (DFBP, CBP, BBP, BBP, and ABP), MBB, as well asBBP´ (Fig. 1), with phosphorescence efficiency (Φp) up to 40%.he nonluminescence of these compounds in solution and amorphous states is ascribed to the collisions and/or active intramolecular motions, which can effectively annihilate the exciton energies via nonradiative relaxations even in rigid polymer films. When cooled to 77 K, however, the solutions, polymer films, and spots onLC plates all exhibit strong phosphorescence, indicative of the crucial role of structure rigidification (Fig. 1c). Notably, the spot ofFBP dotted on TLC plate with six times forms a crystalline outer rim that is emissive at room temperature.

|
Download:
|
| Figure 1. (a) Chemical structures and (b) photographs of luminescent crystals for BP and its derivatives, MBB, and DBBP′ . (c) Photographs of DFBP in solutions [from left to right: n-hexane, tetrahydrofuran (THF), dichloromethane (DCM), acetonitrile, ethanol] and PMMA films, and on TLC plate at room temperature and 77 K taken under UV irradiation. (d) Perspective view of molecular packing arrangement and intermolecular interactions inFBP crystals. Reproduced with permission from Ref. [11]. | |
|
Download:
|
| Figure 2. (a) Chemical structures of BZL and its derivatives. Photographs for (b) BZL in varying oxygen-free solvents (365 nm) and (c) onLC plates (upper:54 nm, bottom: 365 nm), and (d) crystals of BZL and its derivatives (365 nm) taken under UV light at ambient conditions. (e) Molecular packing of BZL in crystals. Reproduced with permission from Ref. [17]. | |
Based on above results, it is rational to speculate that some special interactions in the crystalline state have helped to restrict intramolecular motions of the molecules, thus generating rigidified molecular conformations and turning on the RTP emission.urther scrutinization of their crystals confirms it. As exampled in Fig. 1d, abundant C-H…O (2.724, .777Å) and C-H…F (2.796Å) hydrogen bonds are present in theFBP crystals, which seriously lock the molecular conformations and restrict the molecular motions. Crystal structures of the other compounds show similar multiple intermolecular interactions, like C-H…O, C-H…X (X =, Cl, Br), N-H…O, C-H…p hydrogen bonds, and C-Br…Br-C halogen bonds.he structural rigidification effect induced by such intermolecular interactions activates the RIM process and makes the molecules highly phosphorescent in the crystalline state at room temperature.n addition, those crystalline lattices can also protect the luminogens away from quenching substances as oxygen and moisture, thus further enhancing the phosphorescence emission.
Generally, carbonyl group and nonplanar conformations are favorable for effective spin-orbit coupling, thus facilitating theSC processes from lowest excited singlet (S1) state to T1 state and enhancing the probability of phosphorescence emission. Compared to BP, benzil (BZL) contains one more carbonyl group and even more twisted conformations.herefore, it is expected BZL is also CIP-active. Exactly, dissolving it in varying oxygen-free organic solvents or dotting it onLC plates, there is no visible emission; upon crystallization, however, intense green phosphorescence (521 nm) with the lifetime (<τ>) of 142 ms is observed (Fig.) [17].ig.e illustrates the fragmental molecular packing arrangement of BZL in the crystal. Clearly, numbers of nonclassic C-H…O hydrogen bonds (2.416, .417, .482, .483Å) between one molecule and six adjacent cousins form a 3D interactions network, which tightly fixes the benzene rings and carbonyl groups, thus stiffening the molecular conformations and diminishing the exciton energy dissipations through molecular motions and collisions. What’s more, the highly twisted conformations of BZL also prevent the formation of detrimental species such as excimers or exciplexes in the crystalline state, thus offering bright RTP.
Besides BZL, its derivatives likeFBZL, BBZL, MeBZL, MeOBZL, and BBZL (Fig.a) also demonstrate typical CIP characteristics, with RTP maxima at 500, 526, 505, 517, and 584 nm, respectively.he significantly red-shifted emission of BBZL is ascribed to its extended conjugation, and moreover greatly enhanced spin-orbit coupling and lowered energy difference between T1 and S0 states.
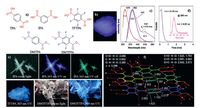
3. Crystallization-induced dual emission (CIDE) from aromatic acids and estersAbove results suggest that carbonyl group and effective intermolecular interactions are favorable for RTP emission at crystalline states owing to the promoted spin-orbit coupling and subsequent ISC processes and conformation rigidification. Aromatic acids are thus checked because of the presence of carboxyl group (-COOH) and expectable effective hydrogen bonds in the crystals.t is found that terephthalic acid (TPA, ig. 3a) is virtually nonemissive in solvents or dotted on TLC plate, but simultaneously emit boosted fluorescence (including prompt and delayed components) and phosphorescence upon crystallization at ambient conditions, demonstrating distinctive crystallization-induced dual emission (CIDE) behaviors [18]. As shown in Fig. 3d and c, PA fluoresces at 319 nm with extremely low Φs of 0.57% inHF, whereas its crystals give intense deep blue emission at 388 nm with Φc of 8.4%. With a delay time (td) of 0.5 ms, an apparent peak (392 nm) together with a shoulder (511 nm) assignable to delayed fluorescence (DF) and RTP is recorded.ransient photoluminescence (PL) measurement reveals the presence of both short and long lifetime emission species with <τ> values of 0.53 ns and 0.16 ms at 380 nm, respectively, thus confirming the existence of prompt and delayed fluorescence (Fig. 3d). Such dual emission in pure organic compounds, particularly those without metal or heavy atoms, remains rare.
|
Download:
|
| Figure 3. (a) Chemical structures of aromatic acids and esters with CIDE characteristics. (b) Photograph of crystalline powders ofPA taken under 365 nm UV light. (c) PL spectra ofPA solution and crystalline powders with td of 0 and 0.5 ms. (d) Emission decay profiles of crystalline powders ofPA monitored at 380 nm. (e) Photographs ofPA, FTPA, andMTFTPA taken under room light or UV light as indicated. (f)ragmental molecular packing arrangement inPA. Reproduced with permission from Ref. [18]. | |
Isomeric effect and fluorine-substitution impact on the emission without altering the CIDE characteristics, as evidenced by the emission behaviors of isophthalic acid (IPA) and tetrafluoroterephthalic acid (TFTPA) (Fig. 3) [18]. BothPA andFTPA are practically nonemissive in solutions (Φf ≤ 0.33%).heir crystals, however, emit strong blue light upon irradiation with emission maxima/Φc values of 380 nm/15.3% and 367 nm/2.0%, respectively. Moreover, after ceasing the UV photoexcitation, persistent green RTP (506 nm) with the <τ> value of 290 ms is detected by naked-eye (Fig. 3e).rom the views of molecular packing arrangement of these aromatic acids, takingPA for example (Fig. 3f), it becomes easy to understand the CIDE mechanism.he crystal ofIPA is full of intermolecular interactions, which form a 3D network.hese plentiful and powerful interactions firmly lock and rigidify the molecular conformations, thus remarkably decreasing the nonradiative vibrational dissipations occurred in solutions and enhancing both fluorescence and phosphorescence emissions.hese experimental results are also supported by the theoretical data. Calculations show thatPA in crystalline state has a much smaller energy gap between S1 and T2 states and a larger spin-orbit coupling than those in gas phase, which effectively promote theSC and reverseSC processes between S1 and T2 states, thus boosting the RTP andF emissions. Notably, the oscillator strength is increased by 55 times in crystals compared to that in gas phase, indicating strikingly enhanced radiative transition in crystals.
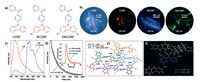
In analogy to acids, their corresponding esters (Fig. 3a) are also found to be CIDE-active.ig. 3e illustrates the bright blue light emission of dimethyl tetrafluoroterephthalate (DMTFTPA) crystals, in high contrast to the feeble emission in solutions.espite the absence of typical hydrogen bonds, there remain effective intermolecular interactions in the crystals of the esters, which induce structure rigidification and dual emission.
4. Persistent RTP from pure organic luminogensAbovePA crystals depict persistent RTP (<τ> ≈90 ms) at ambient conditions, which is even rarely found for pure organic luminogens [5, 19, 20]. Persistent RTP has significant fundamental importance and promising applications in high density data recording, high contrast background independent imaging, and anti-counterfeiting. However, there still lacks of universal design strategies. Since <τ> = 1/(kr + knr + kq) [29], based on the CIP phenomenon, it is expected that with suitable kr, persistent RTP can be achieved if thermally vibrational nonradiative deactivation and quenching can be minimized.o further explore pure organic luminogens with persistent RTP, we designed electron donoracceptor (D-A) structured CZBP consisting of typical CIP-active BP and carbazole with comparable sizes (Fig. 4a).or comparison, mono- and dibromo-substituted CZBP (BCZBP andBCZBP, ig. 4a) were also synthesized.he crystals of CZBP demonstrate bright white light upon UV illumination, and moreover an orange RTP emission lasting for several seconds after ceasing the photoexcitation is observed (Fig. 4b). PL measurement of CZBP crystals shows an emission peak at 436 nm, however, with a td of 0.5 ms, besides the original peak, three fresh ones emerge at 552, 569, and 597 nm (Fig. 4c), which belong toF and RTP, respectively.ime-resolved measurement also confirms the dual-emissive property of CZBP, whose<τ> values for prompt fluorescence and RTP are 3.52 ns and 517.87 ms (Fig. 4d), respectively.or bromo-substituted BCZBP andBCZBP, they also exhibit dual emission at crystalline state, with blue and green emissions at room temperature.he RTP emissions from the crystals of these three luminogens are ascribed to the presence of aromatic carbonyl and carbazole units, heavy atom, and remarkable intermolecular interactions. However, neither of BCZBP norBCZBP gives persistent RTP at ambient conditions, instead, both depict persistent phosphorescence at 77 K.hese results reveal that heavy atoms are not the predominant factor to the disappearance of persistent RTP.
|
Download:
|
| Figure 4. (a) Chemical structures and (b) photographs of luminescent crystals for CZBP, BCZBP, andBCZBP. (c) Prompt/delayed emission spectra and (d) phosphorescence/ fluorescence decay profiles of CZBP crystals. (e) Single-crystal structures and molecular packings of (e) CZBP and (f) BP. Reproduced with permission from Ref. [21]. | |
Fig. 4e and f present the crystal structures of CZBP and BP molecules. Both of them exhibit abundant C-H…π, C-H…H-C, and C-H…p intermolecular interactions, however, these molecular contacts in CZBP are much stronger owing to their smaller distances. Meanwhile, the fraction of “bonded” hydrogens (6/17) is much larger than that of BP molecules (2/10), which should be more effective to restrict the high energy nonradiative C-H vibration stretching (0.37 eV). Also, CZBP molecules possess a much denser crystal packing than BP molecules, as proved by their crystal densities (CZBP: 1.3325 g cm-3, BP: 1.2316 g cm-3). All above factors along with the isolation from quenchers by crystalline lattices collectively result in significantly diminished knr and kq, thus offering persistent RTP for CZBP.herefore, with proper kr, severe crystallization with dense molecular packing and effective short contacts may endow the compounds with longlasting RTP. Meanwhile, much shorter RTP lifetimes of brominesubstituted BCZBP andBCZBP is predominantly attributed to the less rigidified conformations rather than heavy atom effect as they can generate lasting phosphorescence at cryogenic temperature.
It is also noted that CZBP crystals demonstrate blue fluorescence and orange phosphorescence and therefore fascinating white light emission, which is seldom observed in a single pure organic compound, indicative of a fluorescence-phosphorescence dual emission strategy toward white light emission from a single molecule.
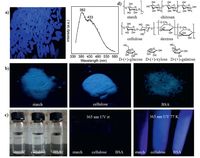
5. RTP from natural productsBesides conventional conjugated compounds, we discovered unprecedented bright emission from common rice upon irradiation, which peaks at 382 nm and 433 nm with <τ> values of 1.72 μs/2.9 ms and 1.78 ns/5.3 ms (Fig. 5a), respectively, suggesting its fluorescence-phosphorescence dual emission property [22]. Since rice is a mixture of different natural products, to more clearly illuminate the emission mechanism, we studied the photophysical properties of starch, the main component of rice.he aqueous solution and solid solution onto TLC plate of starch are nonluminescent. However, when cooled to 77 K, blue luminescence arises.ntriguingly, starch powders emit brightly even at room temperature (Fig. 5b).urther check of cellulose, a carbohydrate with similar structure to starch, shows analogous luminescent behaviors (Fig. 5b).hese exciting findings promoted us to examine other naturally occurring products.hen bovine serum albumin (BSA), a frequently used protein for experimental research, was also found to exhibit resembling emission behaviors (Fig. 5b and c).n the solid state, not only fluorescence, but also RTP is detected for starch, cellulose, and BSA, with RTP lifetimes at the microsecond levels [22].
|
Download:
|
| Figure 5. (a) Photograph of the rice taken under 365 nm UV light and its emission spectrum. Photographs of (b) solid powders and (c) solutions of starch, cellulose, and BSA taken under 365 nm UV light or room light at room temperature or 77 K as indicated. (d) Chemical structures of some examples of nonconventional luminogens with RTP. Reproduced with permission from Ref. [22]. | |
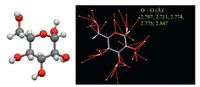
|
Download:
|
| Figure 6. Single crystal structure and intermolecular interactions around one molecule of D-(+)-glucose.he single crystal structure data are adopted from Ref. [30]. | |
Since there are no conventional bulky aromatic building blocks as emission centers, so the question comes out that what should be accountable for the bright light emission in the solid state.or starch and cellulose, apart from carbon and hydrogen atoms, there are only electron-rich oxygen atoms with lone pairs.t is thus believed that the oxygen units are responsible for their emissions.n the solid aggregates, such electron-rich atoms form clusters with overlapped electrons and thus extended conjugations with lowered energy gaps and rigidified conformations. Moreover, these cluster chromophores are readily excited and radiatively deactivated with the aid of the strong intra- and/or intermolecular interactions and physical constraints among polymer chains, which remarkably rigidify the molecular conformations, thus preventing the nonradiative deactivation channels and affording bright emissions. Meanwhile, the nonluminescence of these molecules in solution should be ascribed to the absence of chromophore with sufficient electronic conjugation and meanwhile, the rotations and vibrations of the groups carrying rambling and ramshackle lone pairs non-radiatively annihilate the excited states.his mechanism is also supported by the emission behaviors of chitosan, dextran, glycogen, xylose, and galactose (Fig. 5d), which exhibit visible emission in the solid states, particularly in the crystalline states. Single crystal structure of D-(+)-glucose [30] reveals the presence of abundant intermolecular interactions, and moreover multiple O…O short contacts (2.707, .711, .774, .775, .847Å, ig. 6). While these intermolecular interactions definitely contribute to the rigidification ofmolecular conformations, the O…O short contacts result in through space conjugation, which are crucial for the light emissions. And the RTP emission might be originated from the n-π* transition of the clusters owing to the presence of lone pairs.his single crystal structure analysis result duly validates our above assumption.
6. Other CIP luminogens with efficient RTPRecently, CIP phenomenon has also been observed by other researchers in diverse systems, and increasing pure organic CIP luminogens have been demonstrated.n this section, we will list some representative examples.t is known that heteroatoms are in favor of phosphorescence emission. As depicted in Fig. 7, a group of hetero sulfur (S) and tellurium (Te) containing pure organic luminogens with CIP characteristics were developed [23, 24]. A series of functionalized persulfurated benzene molecules (Fig. 7a) give no emission in solutions, however, they afford strong green RTP with efficiency up to 100% in the crystalline state [23].his is a consequence of decreased intramolecular motions, however, conformational and rotamer factors along with substituents may also take effect. He et al. reported several tellurophenes bearing pinacolboronate (BPin) side groups exhibit bright phosphorescence in solid state under ambient conditions (Fig. 7b).t is revealed that theeII and proximal BPin units are indispensable for the RTP emission [24]. Moreover, when doped into solution-cast PMMA films, these molecules exhibit striking phosphorescence with Φp of ~11.5%. Such RTP emission from amorphous state is hard to achieve, which is also caused by the conformation rigidification and isolation from oxygen and moisture.

|
Download:
|
| Figure 7. (a) Chemical structures of persulfurated benzene molecules. (b) Chemical structures of pinacolboronate-capped tellurophenes and photographs of B-Te-6-B in solution and solid states taken under 365 nm UV light. Photographs in (b) are reproduced with permission from Ref. [24]. | |
Researchers also endeavored to explore the effects of substituents and heavy atoms on the photophysical properties of CIP luminogens.or instance, Shimizu and coworkers reported a new class of pure organic CIP luminogens of 1, 4-bis(aroyl)-2, 5- dibromobenzenes (1a-1e, ig. 8a), their crystals emit intense RTP opposite with no emission in solutions or in doped polymer films. With varying substituents, the color of the phosphorescence can be tuned from blue to green with Φp values of 5~18% [25]. Shi and Zhao utilized and compared the heavy-atom effect in a series of dibromobenzene derivatives, they found that PhBr2C6Br2 and PhBr2C8Br2 with two more bromine atoms show much higherp (up to1.9%) than their PhBr2C6 and PhBr2C8 counterparts, since the heavy-atom interactions in the former are much stronger than those in the latter at crystalline state (Fig. 8b) [15]. And very recently, unlike twisted BP, BZL, and bis(aroyl)-2, 5-dibromobenzenes, Maity and coworkers discovered bright RTP from BaA crystals (Fig. 8c), regardless of its planar structure [26].

|
Download:
|
| Figure 8. Chemical structures and photographs taken under 365 nm UV light of (a) bis(aroyl)-benzene derivatives, (b) dibromobenzene derivatives, and (c) BaA. Reproduced with permission from (a) Ref. [25], (b) Ref. [15], and (c) Ref. [26]. | |
Kim et al. reported a new design principle to fabricate chromophores with triplet-producing aromatic aldehydes and triplet-promoting bromine, thus generating phosphorescent emission in the crystalline state by utilizing the directed heavy atom effect (DHAE) [13]. Although their planar conformations lead to the excimer-induced self-quenching to some extent and make the single component crystals as Br6A just exhibit a low RTP efficiency (~2.9%), when diluted into the analogous crystals as Br6 in which the aldehyde group is replaced by bromine as the host crystal, the RTP efficiency shows a significant increase (up to 55%) since the host provides the halogen-bonding, but non-quenching, scaffold for the chromophores. According to this design principle, they synthesized a class of compounds with heavy atoms and analogous hosts, achieving mixed crystals with tunable RTP colors from blue to orange (Fig. 9).

|
Download:
|
| Figure 9. Chemical structures of various brominated aromatic aldehydes and corresponding dibromo compounds and the photographs of their mixed crystals taken under UV irradiation. Reproduced with permission from Ref. [13]. | |
In addition to homocrystals, cocrystals also can exhibit efficient RTP with rational design. Jin et al. [27] successfully assembled cocrystals between 1, 4-diiodotetrafluorobenzene (DITFB) as the halogen bonding donor and polycyclic aromatic hydrocarbons [PAHs, like naphthalene (Nap), phenanthrene (phe), pyrene (Pyr), carbazole (Cz), fluorine (Flu), dibenzofuran (Dbf), and dibenzothiophene (Dbt)] as the π-type halogen bonding acceptors to realize bright RTP (Fig. 10a).ig. 10b demonstrates the strong green and orange RTP emission of Naπ-DITFB and Phe-DITFB cocrystals, respectively.n these cocrystals, heavy iodine atoms in the conformer significantly promote spin-orbit coupling, thus facilitating their phosphorescence emission. Moreover, ITFB conformer is also used as the “solid diluent” to reduce the self-quenching of the luminogens.herefore, constructing cocrystals betweenITFB and PAHs based on C-I…π halogen bonding and other intermolecular interactions offers a new approach to gain more CIP luminogens. Similarly, d’Agostino and coworkers [28] formulated the cocrystals ofPA-DITFB (1), PA-2DITFB (2), tStb-DITFB (3), and tStb-2DITFB (4) (DPA: diphenylacetylene, tStb: trans-stilbene, Fig. 10a), by mechanochemical method. As a result of the external heavy atom effect, and depending on the stoichiometry, cocrystals 1 and 3 exhibit both fluorescence and phosphorescence, whereas and 4 emit exclusive phosphorescence at room temperature.his work discloses the possibility to modulate photophysical properties of CIP cocrystals through stoichiometry.

|
Download:
|
| Figure 10. (a) Chemical structures of the conformer ITFB and other luminophors. (b) Phosphorescent excitation (blue lines) and emission spectra of Nap-DITFB (green line) and Phe-DITFB (yellow line). Reproduced with permission from (b) Ref. [27]. | |
7. Conclusion
The discovery of the CIP phenomenon for pure organic luminogens opens up a new approach for the fabrication of bright phosphors at roomtemperature through versatile crystal engineering. Based on the concept of spin-orbit coupling, people designed new kinds of possible phosphorescent luminogens by taking advantages of carbonyl groups, heteroatoms, and heavy atoms.n this review, we summarized the recent progress of pure organic CIP luminogens including conventional conjugated compounds and those without classic chromophores. Besides homocrystals, mixed crystals and cocrystals are also effective to generate efficiency RTP. Conformation rigidification aided by effective intermolecular interactions together with isolation from oxygen and moisture triggers bright RTP in crystals.hese phosphors with efficient RTP can be applied in multiple appealing fields like biological hypoxia, water/oxygen sensing, counterfeiting and smart optical recording, sometimes even inOLEDs.Despite the exciting advancements in CIP luminogens, there remainfundamentalandtechnical challenges: (1)ine modulation of the emission color and/or efficiency through molecular and (co)crystal engineering, for example, RGB emission and white light emission from (co)crystals; (2)abrication of CIP luminogenswithhigh RTP efficiency (Φp > 30%); (3) Construction of pure organic luminogens with persistent RTP, deciphering corresponding mechanism, and the balance between RTP efficiency and lifetime; (4) General design strategy toward CIP luminogens; (5) Realizing efficient RTP emission from nonconventional luminogens and mechanism understanding; (6)evelopment of potential applications in biological, medical, and optoelectronic areas. However, it is believed that further exploration in this renewed area would address these challenges, reveal more underlying fundamentals on the triplet exciton involved processes, and offer more RTP compounds with marvelous properties for diverse emerging applications.
Acknowledgment This work was financially supported by the National Natural Scienceoundation of China (No. 51473092) and the Shanghai Rising-Star Program (No. 15QA1402500). W.Z.Y. thanks the SMCChenxing Young Scholar Program of Shanghai Jiaoong University.| [1] | (a) S.K. Lower, M.A. El-Sayed, The triplet state and molecular electronic processes in organic molecules, Chem. Rev. 66(1966) 199-241; (b) C.J. Fischer, A. Gafni, D.G. Steel, J.A. Schauerte, The triplet-state lifetime of indole in aqueous and viscous environments: significance to the interpretation of room temperature phosphorescence in proteins, J. Am. Chem. Soc. 124(2002) 10359-10366. |
| [2] | (a) Y.G. Ma, H.Y. Zhang, J.C. Shen, C.M. Che, Electroluminescence from triplet metal-ligand charge-transfer excited state of transition metal complexes, Synth. Met. 94(1998) 245-248; (b) M.A. Baldo, D.F. O'brien, Y. You, et al., Highly efficient phosphorescent emission from organic electroluminescent devices, Nature 395(1998) 151-154. |
| [3] | D. Lee, J. Jung, D. Bilby, et al. A novel optical ozone sensor based on purely organic phosphor. ACS Appl. Mater. Interfaces 7 (2015) 2993–2997. DOI:10.1021/am5087165 |
| [4] | (a) Q.L.M. de Chermont, C. Chanéac, J. Seguin, et al., Nanoprobes with nearinfrared persistent luminescence for in vivo imaging, Proc. Natl. Acad. Sci. U. S. A. 104(2007) 9266-9271; (b) Q. Zhao, C.H. Huang, F.Y. Li, Phosphorescent heavy-metal complexes for bioimaging, Chem. Soc. Rev. 40(2011) 2508-2524. |
| [5] | G.Q. Zhang, G.M. Palmer, M.W. Dewhirst, C.L. Fraser. A dual-emissivematerials design concept enables tumour hypoxia imaging. Nat. Mater. 8 (2009) 747–751. DOI:10.1038/nmat2509 |
| [6] | Y.H. Deng, D.X. Zhao, X. Chen, et al. Long lifetime pure organic phosphorescence based on water soluble carbon dots. Chem. Commun. 49 (2013) 5751–5753. DOI:10.1039/c3cc42600a |
| [7] | (a) P.X. Liang, D. Wang, Z.C. Miao, et al., Spectral and self-assembly properties of a series of asymmetrical pyrene derivatives, Chin. Chem. Lett. 25(2014) 237-242; (b) P.Z. Chen, H.R. Zheng, L.Y. Niu, et al., A BODIPY analogue from the tautomerization of sodium 3-oxide BODIPY, Chin. Chem. Lett. 26(2015) 631-635; (c) C.C. Wang, S.Y. Yan, Y.Q. Chen, et al., Triphenylamine pyridine acetonitrile fluorogens with green emission for pH sensing and application in cells, Chin. Chem. Lett. 26(2015) 323-328. |
| [8] | (a) R. Shrivastava, J. Kaur, Studies on long lasting optical properties of Eu2+ and Dy3+ doped di-barium magnesium silicate phosphors, Chin. Chem. Lett. 26(2015) 1187-1190; (b) B. Wang, H. Lin, J. Xu, et al., Design, preparation, and characterization of a novel red long-persistent perovskite phosphor: Ca3Ti2O7: Pr3+, Inorg. Chem. 54(2015) 11299-11306; (c) V.W.W. Yam, V.K.M. Au, S.Y.L. Leung, Light-emitting self-assembled materials based on d8 and d10 transition metal complexes, Chem. Rev. 115(2015) 7589-7728. |
| [9] | S. Reineke, M.A. Baldo. Room temperature triplet state spectroscopy of organic semiconductors. Sci. Rep. 4 (2014) 3797. |
| [10] | (a) E.B. Asafu-Adjaye, S.Y. Su, Mixture analysis using solid substrate room temperature luminescence, Anal. Chem. 58(1986) 539-543; (b) S. Scypinski, L.J.C. Love, Room-temperature phosphorescence of polynuclear aromatic hydrocarbons in cyclodextrins, Anal. Chem. 56(1984) 322-327; (c) D. Levy, D. Avnir, Room temperature phosphorescence and delayed fluorescence of organic molecules trapped in silica sol-gel glasses, J. Photochem. Photobiol. A 57(1991) 41-63; (d) G.Q. Zhang, J.B. Chen, S.J. Payne, et al., Multi-emissive difluoroboron dibenzoylmethane polylactide exhibiting intense fluorescence and oxygen-sensitive room-temperature phosphorescence, J. Am. Chem. Soc. 129(2007) 8942-8943. |
| [11] | W.Z. Yuan, X.Y. Shen, H. Zhao, et al. Crystallization-induced phosphorescence of pure organic luminogens at room temperature. J. Phys. Chem. C 114 (2010) 6090–6099. DOI:10.1021/jp909388y |
| [12] | (a) J.D. Luo, Z.L. Xie, J.W.Y. Lam, et al., Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole, Chem. Commun. (2001) 1740-1741; (b) W.Z. Yuan, P. Lu, S.M. Chen, et al., Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: development of highly efficient light emitters in the solid state, Adv. Mater. 22(2010) 2159-2163; (c) J. Mei, N.L.C. Leung, R.T.K. Kwok, J.W.Y. Lam, B.Z. Tang, Aggregation-induced emission: together we shine, united we soar, Chem. Rev. 115(2015) 11718-11940. |
| [13] | O. Bolton, K. Lee, H.J. Kim, K.Y. Lin, J. Kim. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 3 (2011) 205–210. |
| [14] | (a) M.A. El-Sayed. Spin-orbit coupling and the radiationless processes in nitrogen heterocyclics, J. Phys. Chem., 1963,38: 2834-2838; (b) M.A. El-Sayed, Triplet state. Its radiative and nonradiative properties. Acc. Chem. Res. 1 (1968) 8–16. DOI:10.1021/ar50001a002 |
| [15] | H.F. Shi, Z.F. An, P.Z. Li, et al. Enhancing organic phosphorescence by manipulating heavy atom interaction. Cryst. Growth Des. 16 (2016) 808–813. DOI:10.1021/acs.cgd.5b01400 |
| [16] | G.P. Yong, Y.M. Zhang, W.L. She, Y.Z. Li. Stacking-induced white-light and bluelight phosphorescence from purely organic radical materials. J. Mater. Chem. 21 (2011) 18520–18522. DOI:10.1039/c1jm14690d |
| [17] | Y.Y. Gong, Y.Q. Tan, H. Li, et al. Crystallization-induced phosphorescence of benzils at room temperature. Sci. China Chem. 56 (2013) 1183–1186. DOI:10.1007/s11426-013-4930-9 |
| [18] | Y.Y. Gong, L.F. Zhao, Q.Peng, etal.. Crystallization-induced dualemission from metaland heavy atom-free aromatic acids and esters. Chem. Sci. 6 (2015) 4438–4444. DOI:10.1039/C5SC00253B |
| [19] | (a) S. Hirata, K. Totani, J.X. Zhang, et al., Efficient persistent room temperature phosphorescence in organic amorphous materials under ambient conditions, Adv. Funct. Mater. 23(2013) 3386-3397; (b) Z.F. An, C. Zheng, Y. Tao, et al., Stabilizing triplet excited states for ultralong organic phosphorescence, Nat. Mater. 14(2015) 685-690; (c) C.Y. Li, X. Tang, L.Q. Zhang, et al., Reversible luminescence switching of an organic solid: controllable on-off persistent room temperature phosphorescence and stimulated multiple fluorescence conversion, Adv. Opt. Mater. 3(2015) 1184-1190; (d) Z.Y. Yang, Z. Mao, X.P. Zhang, et al., Intermolecular electronic coupling of organic units for efficient persistent room-temperature phosphorescence, Angew. Chem. Int. Ed. 55(2016) 2181-2185. |
| [20] | (a) P.C. Xue, J.B. Sun, P. Chen, et al., Luminescence switching of a persistent roomtemperature phosphorescent pure organic molecule in response to external stimuli, Chem. Commun. 51(2015) 10381-10384; (b) X.P. Zhang, T.Q. Xie, M.X. Cui, et al., General design strategy for aromatic ketone-based single-component dual-emissive materials, ACS Appl. Mater. Interfaces 6(2014) 2279-2284. |
| [21] | Y.Y. Gong, G. Chen, Q. Peng, et al. Achieving persistent room temperature phosphorescence and remarkable mechanochromism from pure organic luminogens. Adv. Mater. 27 (2015) 6195–6201. DOI:10.1002/adma.201502442 |
| [22] | Y.Y. Gong, Y.Q. Tan, J. Mei, et al. Room temperature phosphorescence from natural products: crystallization matters. Sci. China Chem. 56 (2013) 1178–1182. DOI:10.1007/s11426-013-4923-8 |
| [23] | A. Fermi, G. Bergamini, R. Peresutti, et al. Molecular asterisks with a persulfurated benzene core are among the strongest organic phosphorescent emitters in the solid state. Dyes Pigments 110 (2014) 113–122. DOI:10.1016/j.dyepig.2014.04.036 |
| [24] | G. He, W. Torres Delgado, D.J. Schatz, et al. Coaxing solid-state phosphorescence from tellurophenes. Angew. Chem. Int. Ed. 53 (2014) 4587–4591. DOI:10.1002/anie.201307373 |
| [25] | M. Shimizu, A. Kimura, H. Sakaguchi. Room-temperature phosphorescence of crystalline 1, 4-bis (aroyl)-2, 5-dibromobenzenes. Eur. J. Org. Chem. 2016 (2016) 467–473. DOI:10.1002/ejoc.201501382 |
| [26] | S. Maity, P. Mazumdar, M. Shyamal, G.P. Sahoo, A. Misra. Crystal induced phosphorescence from benz(a)anthracene microcrystals at room temperature. Spectrochim. Acta Part A 157 (2016) 61–68. DOI:10.1016/j.saa.2015.12.002 |
| [27] | (a) H.Y. Gao, X.R. Zhao, H. Wang, X. Pang, W.J. Jin, Phosphorescent cocrystals assembled by 1,4-diiodotetrafluorobenzene and fluorene and its heterocyclic analogues based on C-I…π halogen bonding, Cryst. Growth Des. 12(2012) 4377-4387; (b) Q.J. Shen, H.Q. Wei, W.S. Zou, H.L. Sun, W.J. Jin, Cocrystals assembled by pyrene and 1,2-or 1, 4-diiodotetrafluorobenzenes and their phosphorescent behaviors modulated by local molecular environment, CrystEngComm 14(2012) 1010-1015; (c) H.Y. Gao, Q.J. Shen, X.R. Zhao, et al., Phosphorescent co-crystal assembled by 1,4-diiodotetrafluorobenzene with carbazole based on C-I…π halogen bonding, J. Mater. Chem. 22(2012) 5336-5343; (d) Q.J. Shen, X. Pang, X.R. Zhao, et al., Phosphorescent cocrystals constructed by 1,4-diiodotetrafluorobenzene and polyaromatic hydrocarbons based on C-I…π halogen bonding and other assisting weak interactions, CrystEngComm 14(2012) 5027-5034. |
| [28] | S. d'Agostino, F. Grepioni, D. Braga, B. Ventura. Tipping the balance with the aid of stoichiometry: room temperature phosphorescence versus fluorescence in organic cocrystals. Cryst. Growth Des. 15 (2015) 2039–2045. DOI:10.1021/acs.cgd.5b00226 |
| [29] | kr and knr are the rate constants for radiative (phosphorescence) and nonradiative deactivations from the T1 state, respectively, and kq is the rate constant based on quenching of the triplet excitons by interaction with the surroundings such as oxygen and humidity. |
| [30] | G.M. Brown, H.A. Levy. α-D-Glucose: precise determination of crystal and molecular structure by neutron-diffraction analysis. Science 147 (1965) 1038–1039. |
 2016, Vol. 27
2016, Vol. 27 


