Corannulene, a bowl-shape fragment of C60,was first synthesized in 1966 [1]; owing to the efforts of Siegel et al., it canbe produced in kilogram scale now [2]. Overthe past several decades, chemists have been fascinated by its unique topologi-cal structure, and endeavor to its functionalization and properties [3-6].hese derivatives have been applied in the fields of supramolecular chemistry [7-10], liquid crystals [11, 12], chiroptical activity [7, 13, 14], bottom-up synthesis of carbon nanotubes [15], radicals [16-20] and lithium-ion cells [21]. Although its semiconducting properties are also interest-ing and have attracted considerable attention on the synthesis and basic characterization, the device performance based on corannulene derivatives has not been demonstrated until recently [22-27].
Unlike planar polycyclic aromatic hydrocarbons (PAHs), cor- annulene has a dipole moment as high as.1ebye, resulting from the different electron densities on its concave and convex surfaces [28, 29].he dipole moment, the size of the bowl surface and bowl depth will significantly influence their packing in single crystals [5, 26].or corannulene, CH…π interactions instead of π-π interactions [30] dominate in single crystal, thus prohibiting intermolecular charge carrier transport. Molecular orbital energy decides the injection barrier of hole or electron to electrode. Pristine corannulene exhibits high electron affinity(-2.65 eV) and low ionization potential (-6.30eV) [24, 31]. Both values are far from the work functions of common metal electrodes (Au: 5.2 eV, Ag: 4.2 eV, Al: 4.2 eV) [32], indicating high injection barrier for both holes and electrons. Hence, modulating the packing and molecular orbital energy of corannulene derivatives are critical for their applications in organic electronics.ortunately, the rich chemistry of corannulene allows such on-demand rational design of different corannulene molecules. We and Pei et al. have developed a series of corannulene derivatives and used them as π-channel [22], n-channel [23] and ambipolar materials [26].n addition, we also used corannulene derivatives as non-fullerene acceptors in organic solar cells [24].n this review, we intend to provide an overview of the past, present of and future of the application of coranulene derivatives in organic electronics. We first summarize the functionalization to extend the π-conjugated framework of corannulene or incorporation of heteroatom on the backbone of corannulene from007 to016 (for those before007, see reviews [3-5, 33, 34]), then present the applications and device characterization of corannulene derivatives in organic electronics, and finally we evaluate the prospective of using corannulenes in organic electronics and outlines the treads for their future development.
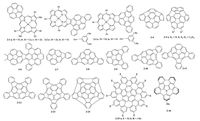
2. Extended π-conjugated or nitrogen-doped corannulene derivativesExtension of π-surface of the π-conjugated molecules both increases their intermolecular π-π interactions and effectively modulates their molecular optical and electrochemical properties [35, 36]. As aforementioned, the dominating presence of CH...π interactions in pristine corannulene single crystals will prohibit intermolecular charge carrier transport [30].o improve coran-nulene derivatives' intermolecular π-π interactions (electronic couplings) in the solid state, increasing the corannulene π- conjugated surface is effective [5, 33]. Lash et al. prepared cor- annulenoporphyrins 2-1 (Fig. 1) from nitrocorannulene in five steps [37]. Corannulenoporphyrins 2-1 exhibited significantly red- shifted absorption with onset absorption wavelength at about 680 nm. Later, Osuka et al. fused porphyrins into dibenzo[a, g]cor- annulene in just two steps by coupling porphyrins at different positions with dibenzo[a, g]corannulene, and then oxidatively fusing to give 5- or 6-membered rings linked dibenzo[a, g]cor- annulene-porphyrin 2-2 and 2-3 [38].he HOMO level of 2-2b is 5.14eV and that of 2-3b is 5.28 eV, both match the work function of gold (5.2 eV), indicating their potential applications as p- channel materials. Wu et al. used the straightforward palladium- catalyzed cyclization to construct the highly curved bulkybowls 2-4 and 2-5 [39, 40]. Both compounds showedD columnar stacking with large π-orbitals overlap, thus facilitating the intermolecular charge carrier transport. Siegel et al. fused graphene fragment into corannulene with the key step of activation of a C(Ar)-F bond to give compounds 2-6 [41]. Because of the steric hindrance, a meso pair of 5-helicenes existed in single crystals. And calculation revealed that the HOMOs mainly localized on graphenic region and the LUMOs on corannulene core, confirming that curvature changed the orbital levels and bandgap compared to a planar graphenic form [42, 43]. Scott et al. proved that highly distorted conjugated molecules, with curvatures surpassing that of C60, can be synthesized by solution phase synthetic approach instead of extreme conditions like high-temperature flash vacuum pyrolysis method [39].hey synthesized a series of multiindenocorannu- lenes (compounds 2-7 to 2-13) and investigated their optical properties, providing the basic ideas to extend corannulene π- system by Pd-catalyzed coupling reactions.
|
Download:
|
| Figure 1. π-surface extended corannulene derivatives. | |
In 2012, Scott et al. developed an elegant “dynamic covalent” [44-46] approach to 1, 3, 5, 7, 9-pentakis(Bpin)corannulene after 96 h throughr-catalyzed C-H polyborylations [47].he poly- borylated corannulene is an important building block for a series of π-conjugated extended corannulene derivatives. Later, Siegel et al. optimized the procedures using microwave-assisted method to decrease the reaction time from 96 h to 4 h compared with the routine bench-top preparation [48].he polyborylations of corannulene provide the multi positions for coupling reactions. 1, 3, 5, 7, 9-Pentakis(Bpin)corannulene coupled with-bromo-1, 3- dichlorobenzene followed by flash vacuum pyrolysis to give isomerically pure [5, 5]nanotube 2-14 [47].tami, Scott et al. also used 1, 3, 5, 7, 9-pentakis(Bpin)corannulene as a starting material to synthesize the defect nonplanar nanographene compound-15 with odd-membered rings [49, 50].
Doping of heteroatoms into conjugated systems is an efficient method to alter their intrinsic properties such as orbital energy and optical properties [51-53], and thus to influence their semicon-ducting properties.t has been proved that nitrogen-doping can significantly increase the mobilities of conjugated molecules [54, 55]. Several theoretical calculations on nitrogen-doped cor- annulenes have been reported [56, 57] and revealed that the substitution of carbon atoms with nitrogen atoms in corannulene lowered the HOMO-LUMO gap. Nervertheless, it is a big challenge to incorporate nitrogen atoms into corannulene due to the synthetic difficulties, albeit several attempts [58]. Recently, Ito et al. reported the first nitrogen-doped corannulene named 8-tert- butyl-6b2-azapentabenzo[bc, ef, hi, kl, no]corannulene (2-16). Com-pound 2-16 adopted columnar stacking in single crystal with a π-π distance of 3.69 A. And it showed a higher fluorescence quantum yield than that of corannulene (quantum yield is 0.24 for 2-16 and 0.07 for pristine corannulene) [59].
3. nfluence of energy levels and packing modes to mobility of corannulene derivativesElectron-deficient groups such as imide, fluorine, trifluoro- methyl and cyano groups are often introduced into molecular backbones not only to lower their LUMO levels, but also to form additional interactions such as F…H interactions sometimes, which may facilitate the charge carrier transport. Strauss, Lentz, Diederich et al. have developed a series of electron-withdrawing groups substituted corannuenes [60-65].hose who interested in corannulenes bearing electron-deficient groups can refer to an elegant review by Lentz et al. in014 [6].
Lentz et al. first investigated the mobilities of corannulene derivatives by electrodeless microwave conductivity measure-ment [61].hey found that the mobility of columnar packed trifluoromethylated corannulene was significantly larger than that of corannulene (Σμ > 0.9 cm2 V-1 s-1 for trifluoromethylated and Sm > 5 x 10-4 cm2 V-1 s-1 for corannulene).heir results em-phasized the significant influence of packing on charge carrier mobility, although they did not report the applications of corannulene derivatives in OFET devices.
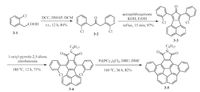
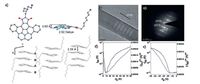
We and Pei et al. applied corannulene derivatives in OFETs devices, and the results revealed that both packing in the solid state and energy levels of corannulene derivatives lead to different charge carrier transporting properties [22, 23, 26]. Pei et al. used imide-fused corannulene derivative 3-5 as the active layer in OFETs [26].he synthesis is shown in Scheme 1. 3-3 was synthesized from commercial available 3-1 by two condensations. 3-5 was obtained from 3-3 by D-A reaction and Pd-catalyzed intramolecular coupling. After introducing imide group to corannulene, the molecular dipole moment has been significantly changed up to.92ebye and the LUMO level was lowered to -3.40 eV. By tuning the molecular dipole moment and choosing proper alkyl chain, 3-5 showed 1-D convex-concave columnar packing in the single crystal (Fig.a).he distance between π- stacking molecules is 3.39 -. 3-5 in good solvent will assemble along π-stacking direction into single-crystal microwires (Fig.b and c) when poor solvent is added.ntriguingly, OFETs devicesbased on these microwires showed ambipolar transporting properties with an electron mobility of 0.02 cm2 V-1 s-1 and a hole mobility of less than 0.01 cm2 V-1 s-1 in vacuum (Fig.d). Because of the doping of oxygen [66], it showed π-channel transporting property with a hole mobility of 0.05 cm2 V-1 s-1 (Fig.e) in air.t is the first time that the corannulene derivative is used in organic field-effect transistors.
|
Download:
|
| Scheme. 1. Synthesis of 3-5. | |
|
Download:
|
| Figure 2. (a) Single-crystal structure and packing of 3-5. (b)ransmission electron microscopy (TEM) image and (c) selective-area electron diffraction (SAED) pattern of 3-5 microwires. (d)ranfer characteristics of 3-5 microwire in vacuum and (e) under ambient conditions.Reprinted with permission from Ref. [24]. Copyright014, Royal Society of Chemistry. | |
Later, we developed pure π- or n-channel materials based on corannulene by introducing multi electron rich or electron deficient groups to conjugated backbones.o achieve pure hole injection, four electron-rich thiophenes were first fused into p system of corannulene by Stille coupling and then Scholl reaction to give two isomeric thiophene-fused dibenzo[a, g]corannulenes 3-8 and 3-10 (Scheme) [22].wo benzene rings and four thiophene rings were introduced to conjugated system of corannulene.he larger effective conjugated length of isomer 3-8 and 3-10 led to red-shift absorption compared to that of corannulene.he HOMO levels and optical band gaps were determined to be -5.59 eV/ -5.65 eV and.76 eV/2.87 eV for isomer 3-8/3-10, respectively.he HOMO levels are more closed to the work function of Au (5.2 eV) than LUMO levels, indicating these two compounds may show π-channel transporting properties. We succeeded in obtain-ing the single crystal of isomer 3-8, unlike 3-5, isomer 3-8 exhibited layered packing fashion with two types of packing mode (Fig. 3a).he unique packing mode provided more charge carrier transport channels through different π-π interactions. OFET devices based on micro-ribbons or microwires of isomer 3-8 and 3-10 were fabricated.somer 3-8 displayed a hole mobility of up to 0.06 cm2 V-1 s-1, a threshold voltage of -38 V and a current on/off ratio of 103 in air (Fig. 3b), which represented the highest hole mobility based on corannulene. Compared with isomer 3-8, isomer 3-10 did not show any field-effect properties although many microwires have been tested.he reasons remain unclear due to the lack of single crystal data. Nevertheless, the multi carrier transporting channels in the single crystal of compound 3-8 facilitated the improvement of mobility.

|
Download:
|
| Scheme. 2. Synthetic routes of compounds 3-8 and 3-10. | |

|
Download:
|
| Figure 3. (a) Single-crystal packing of 3-8. (b)ransfer characteristics of 3-8 micro-ribbon tested under ambient conditions. (c) SEM device image of 3-8. Copyright014, Royal Society of Chemistry. | |
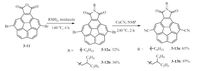
As described before, corannulene has a high LUMO level (-2.65 eV), exhibiting high barrier for injection of electron to electrode.aking both energy levels and arrangement in single crystals into consideration, we introduced imide and cyano groups into corannulene (Scheme 3) to lower its LUMO level and also tune its arrangement in single crystals, thus achieving n-channel transporting properties [23]. Surprisingly, because of the introduction of cyano groups into imide-fused corannulene, not only convex- concave columnar packing was achieved in single crystals, but also electronic couplings between columns were realized, leading to 2D columnar stacking (Fig. 4a).he smallest π-π distances were 3.31- and 3.28 - for 3-13a and 3-13b, and even smaller than those of high- performance air-stable compounds such as dicyanated and fluori- nated PDI [67].he dense packing in single crystals prohibits the penetration of water and oxygen into bulk phase [67, 68].he LUMO levels were also lowered to 〜3.6 eV for two compounds.he dense packing and low LUMO work synergetically to achieve n-channel transporting properties even in air with an electron mobility of 0.07 cm2 V-1 s-1 for 3-13a and 0.04 cm2 V-1 s-1 for 3-13b based on their single-crystal microwires (Fig. 4).
|
Download:
|
| Scheme. 3. Synthetic routes of compounds 3-13a and 3-13b. | |

|
Download:
|
| Figure 4. Single-crystal packing of 3-13a (a) and 3-13b (b). Pathways 1 and are two main charge carrier transporting pathways. t corresponds to electronic couplings. d1 corresponds to the closest π-π distance and d2 corresponds to the -CN…π distance.ransfer and output characteristics of 3-13a (c) and 3-13b (d). (e)evice architecture. (f)Device SEM image. Copyright015, Royal Society of Chemistry. | |
4. Imide-fused corannulene as acceptor units in donor- acceptor polymer
Donor-Acceptor (D-A) conjugated polymers usually have large planar conjugated units, strong π-π interactions, readily tunable frontier orbital energy levels [69, 70].hese features endow them with high carrier mobilities and broad absorption spectra and thereby significantly improved device performances of polymerETs and photovoltaics [54, 71, 72]. Bowl-shaped molecules such as corannulene have seldom been incorporated into conjugated polymers [73-75]. We designed the imide-fused corannulene derivative 4-5 (Scheme 4) [25].he alkyl chains can be easily introduced to imide N position and also the two halogens in 4- and 9-positions provide the coupling polymerization sites. Compound 4-5 copolymerized with 5, 5´-bis(trimethylstannyl)-2, 2´-bithio- phene, giving polymer 4-6 with the number average molecular weights of 40.5 kDa and polydispersity index of 3.5. Calculation based on three repeated units of 4-6 showed LUMO localized on electron-deficient imide-fused corannulene and HOMO delocal-ized along the polymer chain just like typical-A copolymers [76]. HOMO/LUMO levels and optical band gap of 4-6 were determined to be -5.42/-3.31 eV and 1.98 eV.he semiconduct-ing properties of 4-6 were also investigated. 4-6 showed ambipolar transporting properties with a hole mobility of 0.025 cm2 V-1 s-1 and an electron mobility of 7.45 x 10-5 cm2 V-1 s-1 (Fig. 5). Because the bowl-shaped structure of corannulene can accommo-date PCBM through ball-socket interactions [10, 75, 77], corannu- lene-based conjugated polymers may also act as promising donors in organic solar cells.his work paved the way to introduce corannulene into conjugated polymers and significantly extended the applications of corannulene.
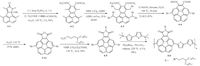
|
Download:
|
| Scheme. 4. Synthetic route of 4-6. | |

|
Download:
|
| Figure 5. (a) Transfer and (b) output characteristics of 4-6. Copyright 2014, Royal Society of Chemistry. | |
5. Corannulenes as non-fullerene acceptors in organic solar cells
Fullerenes and their derivatives are star molecules in organic electronics especially in organic solar cells [78].o our knowledge, their bowl-shaped fragments have never been used in organic solar cells. Compared with fullerenes, corannulene is much easier to be modified, thus more convenient to tune its derivatives’ solubility, energy levels, absorption and charge carrier transporting proper-ties.o lower corannulene’s LUMO level and increase film forming ability, n-hexylphthalimide (5-3) and n-hexylnaphthalimide (5-4) were introduced to corannulene (Scheme 5) by Suzuki reactions [24].he LUMO levels of resulting compounds 5-5 (Cor-PI) and 5-6 (Cor-NI) were significantly decreased to -3.10 eV and -3.24 eV. Blended with P3HT in bulk-heterojunction organic solar cells (BHJ- OSCs), 5-5 and 5-6 showed PCEs of 0.37% and 1.03%, Voc of 0.83 V and 0.82 V (Fig. 6), respectively.he Voc are 0.2 V higher than that of PCBM due to the higher LUMO levels of 5-5 and 5-6 [79].he mobility, from the space charge limited current (SCLC) method, was 1.32 x 10-4 cm2 V-1 s-1 for 5-6/P3HT blend film, which was much higher than that of 5-5 (1.6 x 10-6 cm2 V-1 s-1) and comparable to those of high-performance non-fullerene acceptors [80]. Note that further optimizations such as adding solvent additives, designing novel device structures, and incorporating different donors may increase the devices performances.

|
Download:
|
| Scheme. 5. Synthetic route of 5-5 and 5-6. | |

|
Download:
|
| Figure 6. (a) J-V curves and (b) EQE curves of 5-5 (Cor-PI)/P3HT and 5-6 (Cor-NI)/P3HT blend films. Copyright014, Royal Society of Chemistry. | |
6. Corannulenes as light-emitting materials
Pristine corannulene exhibits a maximum emission peak at 423 nm and a fluorescence quantum yield (Φf) of 0.07 [27, 59, 81]. Siegel et al. found that multiethynyl corannulenes can display tunable emission, depending on the nature of substituent groups as well as the crystalline arrangement [82].hese results were supported by theoretical calculations by Baldridge et al. [83]. Multiethynyl corannulene derivative 6-1 (Fig. 7) even has a fluorescence quantum yield as high as 0.83, indicating its potential as light-emitting materials [82].iederich, Siegel et al. also synthe-sized a series of multi 4-(N, N-dimethylamino)phenylethynyl substituted corannulenes (compounds 6-2 to 6-5). Emission peaks at 533 nm (Φf= 0.98) for compound 6-2, 538 nm (Φf= 0.93) for compound 6-3, 593 nm (Φf = 0.76) for compound 6-4, and 595 nm (Φf = 0.56) for compound 6-5 were observed [63].he fluorescence quantum yields were rather higher than that of pristine corannu- lene. Sutton et al. developed the corannulene-based blue emitters (compounds 6-6 and 6-7) [81]. Compound 6-6 has red-shifted absorption and a higher fluorescence quantum yield than those of 6-7 (Φf= 0.60 for 6-6, Φf= 0.08 for 6-7), resulting from the longer effective conjugated length. Yamada et al. first applied corannulene derivatives to light-emitting active layers in their patent [27].hey fabricated a series of devices based on corannulene derivatives. Compound 6-8 exhibits the highest emission luminance of 2200 cd/ m2, a power efficiency of 6.1 lm/W and a green electrogenerated luminescence peak at 515 nm.t is the first demonstration of using corannulene derivatives in OLED devices, although the performance is moderate [84, 85].urther optimizations could be focused on orbital energy tuning, balanced electron and hole mobilities, stabilities, film-forming properties and so on [86, 87].
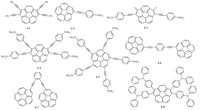
|
Download:
|
| Figure 7. Corannulene derivatives as light-emitting materials. | |
7. Conclusions and outlook
In this mini review, we summarized the recent advance in functionalization and applications of corannulene derivatives in organic electronics.he orbital energy and crystalline arrangement play important roles in their semiconducting properties.he basic results show that corannulene derivatives can be novel materials for organic electronics, although the mobilities or PCEs are moderate. Note that limited applications of corannulene in organic electronics are reported up to date.o advance the applications of corannulenes in organic electronics, more efforts should be spared to enhance their mobilities, PCEs and luminous intensity, which require diversified structures, further device optimizations and an in-depth understanding of the structure-property relationships.
Acknowledgments This work is supported by the 973 Program (No.015CB856500), the National Scienceoundation of China (Nos. 91427304, 21573181, 91227111 and1102120), the Beijing National Labora-tory for Molecular Science (No.0140114), theundamental Researchunds for the Central Universities (No.0720160050) of China and Program for Changjiang Scholars andnnovative Researcheam in University. We thank Prof. Jian Pei, Prof. Jay S. Siegel and Prof. Kim K. Baldridge for the wonderful cooperation in publishing corannulene-based materials for organic electronics.| [1] | W.E. Barth, R.G. Lawton. Dibenzo. J. Am. Chem. Soc. 88 (1966) 380–381. DOI:10.1021/ja00954a049 |
| [2] | A.M. Butterfield, B. Gilomen, J.S. Siegel. Kilogram-scale production of corannulene. Org. Process Res. Dev. 16 (2012) 664–676. DOI:10.1021/op200387s |
| [3] | A. Sygula. Chemistry on a half-shell: synthesis and derivatization of buckybowls. Eur. J. Org. Chem. (2011) 1611–1625. |
| [4] | V.M. Tsefrikas, L.T. Scott. Geodesic polyarenes by flash vacuum pyrolysis. Chem. Rev. 106 (2006) 4868–4884. DOI:10.1021/cr050553y |
| [5] | Y.T. Wu, J.S. Siegel. Aromatic molecular-bowl hydrocarbons: synthetic derivatives, their structures, and physical properties. Chem. Rev. 106 (2006) 4843–4867. DOI:10.1021/cr050554q |
| [6] | B.M. Schmidt, D. Lentz. Syntheses and properties of buckybowls bearing electronwithdrawing groups. Chem. Lett. 43 (2014) 171–177. DOI:10.1246/cl.130984 |
| [7] | J. Kang, D. Miyajima, T. Mori, et al. A rational strategy for the realization of chaingrowth supramolecular polymerization. Science 347 (2015) 646–651. DOI:10.1126/science.aaa4249 |
| [8] | F.G. Klärner, J. Panitzky, D. Preda, L.T. Scott. Modeling of supramolecular properties of molecular tweezers, clips, and bowls. J. Mol. Model. 6 (2000) 318–327. DOI:10.1007/PL00010733 |
| [9] | L. Kobryn, W.P. Henry, F.R. Fronczek, R. Sygula, A. Sygula. Molecular clips and tweezers with corannulene pincers. Tetrahedron Lett. 50 (2009) 7124–7127. DOI:10.1016/j.tetlet.2009.09.177 |
| [10] | A. Sygula, F.R. Fronczek, R. Sygula, P.W. Rabideau, M.M. Olmstead. A double concave hydrocarbon buckycatcher. J. Am. Chem. Soc. 129 (2007) 3842–3843. DOI:10.1021/ja070616p |
| [11] | D. Miyajima, K. Tashiro, F. Araoka, et al. Liquid crystalline corannulene responsive to electric field. J. Am. Chem. Soc. 131 (2009) 44–45. DOI:10.1021/ja808396b |
| [12] | D. Pappo, T. Mejuch, O. Reany, et al. Diverse functionalization of corannulene: easy access to pentagonal superstructure. Org. Lett. 11 (2009) 1063–1066. DOI:10.1021/ol8028127 |
| [13] | J. Kang, D. Miyajima, Y. Itoh, et al. C5-symmetric chiral corannulenes: desymmetrization of bowl inversion equilibrium via "intramolecular" hydrogen-bonding network. J. Am. Chem. Soc. 139 (2014) 10640–10644. |
| [14] | D. Bandera, K.K. Baldridge, A. Linden, R. Dorta, J.S. Siegel. Stereoselective coordination of C5-symmetric corannulene derivatives with an enantiomerically pure. Angew. Chem. Int. Ed. 50 (2011) 865–867. DOI:10.1002/anie.201006877 |
| [15] | L.T. Scott, E.A. Jackson, Q.Y. Zhang, et al. A short, rigid, structurally pure carbon nanotube by stepwise chemical synthesis. J. Am. Chem. Soc. 134 (2012) 107–110. DOI:10.1021/ja209461g |
| [16] | A. Ueda, K. Ogasawara, S. Nishida, et al. Air-stable curved ü-radical based on corannulene: dynamic electronic-spin structure induced by temperature-dependent conformational changes. Aust. J. Chem. 63 (2010) 1627–1633. DOI:10.1071/CH10280 |
| [17] | A. Ueda, K. Ogasawara, S. Nishida, et al. A bowl-shaped ortho-semiquinone radical anion: quantitative evaluation of the dynamic behavior of structural and electronic features. Angew. Chem. Int. Ed. 49 (2010) 6333–6337. DOI:10.1002/anie.201002626 |
| [18] | Y. Morita, A. Ueda, S. Nishida, et al. Curved aromaticity of a corannulene-based neutral radical: crystal structure and 3 D unbalanced delocalization of spin. Angew. Chem. Int. Ed. 47 (2008) 2035–2038. DOI:10.1002/(ISSN)1521-3773 |
| [19] | A. Ueda, S. Nishida, K. Fukui, et al. Three-dimensional intramolecular exchange interaction in a curved and nonalternant ü-conjugated system: corannulene with two phenoxyl radicals. Angew. Chem. Int. Ed. 49 (2010) 1678–1682. DOI:10.1002/anie.200906666 |
| [20] | Y. Morita, S. Nishida, T. Kobayashi, et al. The first bowl-shaped stable neutral radical with a corannulene system: synthesis and characterization of the electronic structure. Org. Lett. 6 (2004) 1397–1400. DOI:10.1021/ol0497786 |
| [21] | A.V. Zabula, A.S. Filatov, S.N. Spisak, A.Y. Rogachev, M.A. Petrukhina. A main group metal sandwich: five lithium cations jammed between two corannulene tetraanion decks. Science 333 (2011) 1008–1011. DOI:10.1126/science.1208686 |
| [22] | R.Q. Lu, Y.N. Zhou, X.Y. Yan, et al. Thiophene-fused bowl-shaped polycyclic aromatics with a dibenzo. Chem. Commun. 51 (2015) 1681–1684. DOI:10.1039/C4CC08451A |
| [23] | R. Chen, R.Q. Lu, K. Shi, et al. Corannulene derivatives with low LUMO levels and dense convex-concave packing for n-channel organic field-effect transistors. Chem. Commun. 51 (2015) 13768–13771. DOI:10.1039/C5CC03550C |
| [24] | R.Q. Lu, Y.Q. Zheng, Y.N. Zhou, et al. Corannulene derivatives as non-fullerene acceptors in solution-processed bulk heterojunction solar cells. J. Mater. Chem. A 2 (2014) 20515–20519. DOI:10.1039/C4TA05310A |
| [25] | R.Q. Lu, W. Xuan, Y.Q. Zheng, et al. A corannulene-based donor-acceptor polymer for organic field-effect transistors. RSC Adv. 4 (2014) 56749–56755. DOI:10.1039/C4RA11824C |
| [26] | K. Shi, T. Lei, X.Y. Wang, J.Y. Wang, J. Pei. A bowl-shaped molecule for organic fieldeffect transistors: crystal engineering and charge transport switching by oxygen doping. Chem. Sci. 5 (2014) 1041–1045. DOI:10.1039/C3SC52701H |
| [27] | N. Yamada, K. Ueno, J. Nishimura, Y. Okada, Corannulene compound and organic light-emitting device, 20070049779. |
| [28] | K.K. Baldridge, J.S. Siegel. Corannulene-based fullerene fragments C20H10-C50H10: when does a buckybowl become a buckytube?. Theor. Chem. Acc. 97 (1997) 67–71. DOI:10.1007/s002140050238 |
| [29] | F.J. Lovas, R.J. McMahon, J.U. Grabow, et al. Interstellar chemistry: a strategy for detecting polycyclicaromatic hydrocarbons in space. J. Am. Chem. Soc. 127 (2005) 4345–4350. DOI:10.1021/ja0426239 |
| [30] | J.C. Hanson, C.E. Nordman. The crystal and molecular structure of corannulene, C20H10. Acta Crystallogr. Sect. B 32 (1976) 1147–1153. DOI:10.1107/S0567740876012430 |
| [31] | C. Bruno, R. Benassi, A. Passalacqua, et al. Electrochemical and theoretical investigation of corannulene reduction processes. J. Phys. Chem. B 113 (2009) 1954–1962. |
| [32] | J. Hölzl, F.K. Schulte. Work function of metals, in: J. Hölzl, F.K. Schulte, H. Wagner (Eds.), Solid Surface Physics,. Springer, Berlin/Heidelberg (1979) 1–150. |
| [33] | Y.T. Wu, J.S. Siegel. Synthesis, structures, physical properties of aromatic molecular-bowl hydrocarbons, in: J.S. Siegel, Y.T. Wu (Eds.), Polyarenes I: Topics in Current Chemistry, 349. Springer, Berlin/Heidelberg (2014) 63–120. |
| [34] | X. Li, F.Y. Kang, M. Inagaki. Buckybowls: corannulene and its derivatives. Small 12 (2016) 3206–3223. DOI:10.1002/smll.v12.24 |
| [35] | L. Zhang, Y. Cao, N.S. Colella, et al. Unconventional, chemically stable, and soluble two-dimensional angular polycyclic aromatic hydrocarbons: from molecular design to device applications. Acc. Chem. Res. 48 (2015) 500–509. DOI:10.1021/ar500278w |
| [36] | J.E. Anthony. Functionalized acenes and heteroacenes for organic electronics. Chem. Rev. 106 (2006) 5028–5048. DOI:10.1021/cr050966z |
| [37] | H. Boedigheimer, G.M. Ferrence, T.D. Lash. Porphyrin on a half-shell. Synthesis and characterization of corannulenoporphyrins. J. Org. Chem. 75 (2010) 2518–2527. DOI:10.1021/jo902592u |
| [38] | K. Ota, T. Tanaka, A. Osuka. meso-ü dibenzo[a,g]corannulene-fused porphyrins. Org. Lett. 16 (2014) 2974–2977. DOI:10.1021/ol501115m |
| [39] | T.C. Wu, H.J. Hsin, M.Y. Kuo, C.H. Li, Y.T. Wu. Synthesis and structural analysis of a highly curved buckybowl containing corannulene and sumanene fragments. J. Am. Chem. Soc. 133 (2011) 16319–16321. DOI:10.1021/ja2067725 |
| [40] | M.K. Chen, H.J. Hsin, T.C. Wu, et al. Highly curved bowl-shaped fragments of fullerenes: synthesis, structural analysis, and physical properties. Chem. Eur. J. 20 (2014) 598–608. DOI:10.1002/chem.v20.2 |
| [41] | A.K. Dutta, A. Linden, L. Zoppi, K.K. Baldridge, J.S. Siegel. Extended corannulenes: aromatic bowl/sheet hybridization. Angew. Chem. Int. Ed. 54 (2015) 10792–10796. DOI:10.1002/anie.201503553 |
| [42] | V. Kapko, D.A. Drabold, M.F. Thorpe. Electronic structure of a realistic model of amorphous graphene. Phys. Status Solidi B 247 (2010) 1197–1200. |
| [43] | A.H. Castro Neto, F. Guinea, N.M.R. Peres, K.S. Novoselov, A.K. Geim. The electronic properties of graphene. Rev. Mod. Phys. 81 (2009) 109–162. DOI:10.1103/RevModPhys.81.109 |
| [44] | M.E. Belowich, J.F. Stoddart. Dynamic imine chemistry. Chem. Soc. Rev. 41 (2012) 2003–2024. DOI:10.1039/c2cs15305j |
| [45] | S.J. Rowan, S.J. Cantrill, G.R.L. Cousins, J.K.M. Sanders, J.F. Stoddart. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41 (2002) 898–952. DOI:10.1002/1521-3773(20020315)41:6<>1.0.CO;2-R |
| [46] | J.M. Lehn. From supramolecular chemistry towards constitutional dynamic chemistry and adaptive chemistry. Chem. Soc. Rev. 36 (2007) 151–160. DOI:10.1039/B616752G |
| [47] | M.N. Eliseeva, L.T. Scott. Pushing the Ir-catalyzed C-H polyborylation of aromatic compounds to maximum capacity by exploiting reversibility. J. Am. Chem. Soc. 134 (2012) 15169–15172. DOI:10.1021/ja307547j |
| [48] | S. Da Ros, A. Linden, K.K. Baldridge, J.S. Siegel. Boronic esters of corannulene: potential building blocks toward icosahedral supramolecules. Org. Chem. Front. 2 (2015) 626–633. DOI:10.1039/C5QO00009B |
| [49] | K. Kawasumi, Q.Y. Zhang, Y. Segawa, et al. A grossly warped nanographene and the consequences of multiple odd-membered-ring defects. Nat. Chem. 5 (2013) 739–744. DOI:10.1038/nchem.1704 |
| [50] | K. Kato, Y. Segawa, L.T. Scott, K. Itami. Synthesis, properties, and packing structures of corannulene-based ü-systems containing heptagons. Chem. Asian J. 10 (2015) 1635–1639. DOI:10.1002/asia.v10.8 |
| [51] | X.W. Wang, G.Z. Sun, P. Routh, et al. Heteroatom-doped graphene materials: syntheses, properties and applications. Chem. Soc. Rev. 43 (2014) 7067–7098. DOI:10.1039/C4CS00141A |
| [52] | M.D. Tzirakis, M. Orfanopoulos. radical reactions of fullerenes: from synthetic organic chemistry to materials science and biology. Chem. Rev. 113 (2013) 5262–5321. DOI:10.1021/cr300475r |
| [53] | O. Vostrowsky, A. Hirsch. Heterofullerenes. Chem. Rev. 106 (2006) 5191–5207. DOI:10.1021/cr050561e |
| [54] | B. Sun, W. Hong, Z.Q. Yan, H. Aziz, Y.N. Li. Record high electron mobility of 6.3 cm2 V-1 s-1 achieved for polymer semiconductors using a new building block. Adv. Mater. 26 (2014) 2636–2642. DOI:10.1002/adma.v26.17 |
| [55] | J.Y. Huang, Z.P. Mao, Z.H. Chen, et al. Diazaisoindigo-based polymers with highperformance charge-transport properties: from computational screening to experimental characterization. Chem. Mater. 28 (2016) 2209–2218. DOI:10.1021/acs.chemmater.6b00154 |
| [56] | U. Purushotham, G.N. Sastry. Conjugate acene fused buckybowls: evaluating their suitability for p-type, ambipolar and n-type air stable organic semiconductors. Phys. Chem. Chem. Phys. 15 (2013) 5039–5048. DOI:10.1039/c3cp44673e |
| [57] | A. Reisi-Vanani, L. Alihoseini. Computational investigation of the adsorption of molecular hydrogen on the nitrogen-doped corannulene as a carbon nano-structure. Surf. Sci. 621 (2014) 146–151. DOI:10.1016/j.susc.2013.11.012 |
| [58] | V.M. Tsefrikas, S. Arns, P.M. Merner, et al. Benzo[a]acecorannulene: surprising formation of a new bowl-shaped aromatic hydrocarbon from an attempted synthesis of 1,2-diazadibenzo[d,m]corannulene. Org. Lett. 8 (2006) 5195–5198. DOI:10.1021/ol061554v |
| [59] | M. Yamaji, K. Takehira, T. Mikoshiba, et al. Photophysical and photochemical properties of corannulenes studied by emission and optoacoustic measurements, laser flash photolysis and pulse radiolysis. Chem. Phys. Lett. 425 (2006) 53–57. DOI:10.1016/j.cplett.2006.04.104 |
| [60] | I.V. Kuvychko, S.N. Spisak, Y.S. Chen, et al. A buckybowl with a lot of potential: C5-C20H5(CF3)5. Angew. Chem. Int. Ed. 51 (2012) 4939–4952. DOI:10.1002/anie.v51.20 |
| [61] | B.M. Schmidt, S. Seki, B. Topolinski, et al. Electronic properties of trifluoromethylated corannulenes. Angew. Chem. Int. Ed. 51 (2012) 11385–11388. DOI:10.1002/anie.201205757 |
| [62] | B.M. Schmidt, B. Topolinski, P. Roesch, D. Lentz. Electron-poor N-substituted imide-fused corannulenes. Chem. Commun. 48 (2012) 6520–6522. DOI:10.1039/c2cc32643d |
| [63] | Y.L. Wu, M.C. Stuparu, C. Boudon, et al. Structural, optical, and electrochemical properties of three-dimensional push-pull corannulenes. J. Org. Chem. 77 (2012) 11014–11026. DOI:10.1021/jo302217n |
| [64] | I.V. Kuvychko, C. Dubceac, S.H.M. Deng, et al. C20H4(C4F8)3: a fluorine-containing annulated corannulene that is a better electron acceptor than C60. Angew. Chem. Int. Ed. 52 (2013) 7505–7508. DOI:10.1002/anie.201300796 |
| [65] | B.M. Schmidt, B. Topolinski, M. Yamada, et al. Fluorinated and trifluoromethylated corannulenes. Chem. Eur. J. 19 (2013) 13872–13880. DOI:10.1002/chem.201301910 |
| [66] | R. Di Pietro, H. Sirringhaus. High resolution optical spectroscopy of air-induced electrical instabilities in n-type polymer semiconductors. Adv. Mater. 24 (2012) 3367–3372. DOI:10.1002/adma.v24.25 |
| [67] | B.A. Jones, A. Facchetti, M.R. Wasielewski, T.J. Marks. Tuning orbital energetics in arylene diimide semiconductors. Materials design for ambient stability of n-type charge transport. J. Am. Chem. Soc. 129 (2007) 15259–15278. DOI:10.1021/ja075242e |
| [68] | J. Zaumseil, H. Sirringhaus. Electron and ambipolar transport in organic fieldeffect transistors. Chem. Rev. 107 (2007) 1296–1323. DOI:10.1021/cr0501543 |
| [69] | A.J. Heeger. Semiconducting polymers: the third generation. Chem. Soc. Rev. 39 (2010) 2354–2371. DOI:10.1039/b914956m |
| [70] | J.D. Yuen, F. Wudl. Strong acceptors in donor-acceptor polymers for high performance thin film transistors. Energy Environ. Sci. 6 (2013) 392–406. DOI:10.1039/c2ee23505f |
| [71] | H. Sirringhaus. 25th anniversary article: organic field-effect transistors: the path beyond amorphous silicon. Adv. Mater. 26 (2014) 1319–1335. DOI:10.1002/adma.201304346 |
| [72] | A.J. Heeger. 25th anniversary article: bulk heterojunction solar cells: understanding the mechanism of operation. Adv. Mater. 26 (2014) 10–28. DOI:10.1002/adma.201304373 |
| [73] | A.R. Mohebbi, J. Yuen, J. Fan, et al. Emeraldicene as an acceptor moiety: balancedmobility,ambipolar,organicthin-filmtransistors. Adv.Mater. 23 (2011) 4644–4648. DOI:10.1002/adma.201102726 |
| [74] | J.L. Jellison, C.H. Lee, X.J. Zhu, et al. Electron acceptors based on an all-carbon donor-acceptor copolymer. Angew. Chem. Int. Ed. 51 (2012) 12321–12324. DOI:10.1002/anie.v51.49 |
| [75] | M.C. Stuparu. Rationally designed polymer hosts of fullerene. Angew. Chem. Int. Ed. 52 (2013) 7786–7790. DOI:10.1002/anie.v52.30 |
| [76] | B. He, A.B. Pun, D. Zherebetskyy, et al. New form of an old natural dye: bayannulated indigo (BAI) as an excellent electron accepting unit for high performance organic semiconductors. J. Am. Chem. Soc. 136 (2014) 15093–15101. DOI:10.1021/ja508807m |
| [77] | M. Yanney, F.R. Fronczek, A. Sygula. A 2:1 receptor/C60 complex as a nanosized universal joint. Angew. Chem. Int. Ed. 54 (2015) 11153–11156. DOI:10.1002/anie.201505327 |
| [78] | J.B. You, L.T. Dou, K. Yoshimura, et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 4 (2013) 1446. DOI:10.1038/ncomms2411 |
| [79] | W. Ma, C. Yang, X. Gong, K. Lee, A.J. Heeger. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 15 (2005) 1617–1622. DOI:10.1002/(ISSN)1616-3028 |
| [80] | W.C. Zhao, D.P. Qian, S.Q. Zhang, et al. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 28 (2016) 4734–4739. DOI:10.1002/adma.v28.23 |
| [81] | J. Mack, P. Vogel, D. Jones, N. Kavala, A. Sutton. The development of corannulenebased blue emitters. Org. Biomol. Chem. 5 (2007) 2448–2452. DOI:10.1039/b705621d |
| [82] | Y.T. Wu, D. Bandera, R. Maag, et al. Multiethynyl corannulenes: synthesis, structure, and properties. J. Am. Chem. Soc. 130 (2008) 10729–10739. DOI:10.1021/ja802334n |
| [83] | L. Zoppi, L. Martin-Samos, K.K. Baldridge. Effect of molecular packing on corannulene-based materials electroluminescence. J. Am. Chem. Soc. 133 (2011) 14002–14009. DOI:10.1021/ja2040688 |
| [84] | W.C. Chen, C.S. Lee, Q.X. Tong. Blue-emitting organic electrofluorescence materials: progress and prospective. J. Mater. Chem. C 3 (2015) 10957–10963. DOI:10.1039/C5TC02420J |
| [85] | P. Kordt, J.J.M. van der Holst, M. Al Helwi, et al. Modeling of organic light emitting diodes: from molecular to device properties. Adv. Funct. Mater. 25 (2015) 1955–1971. DOI:10.1002/adfm.v25.13 |
| [86] | D.M. Sun, Z.J. Ren, M.R. Bryce, S.K. Yan. Arylsilanes and siloxanes as optoelectronic materials for organic light-emitting diodes (OLEDs). J. Mater. Chem. C 3 (2015) 9496–9508. DOI:10.1039/C5TC01638J |
| [87] | S. Scholz, D. Kondakov, B. Lu üssem, K. Leo. Degradation mechanisms and reactions in organic light-emitting devices. Chem. Rev. 115 (2015) 8449–8503. DOI:10.1021/cr400704v |
 2016, Vol. 27
2016, Vol. 27 


