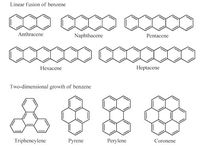
The polycyclic aromatic hydrocarbons, named as PAHs, are generated by linear fusion and two-dimensional growth of benzene as shown in Scheme 1. Last decades have witnessed that PAHs show excellent optical and electrical properties, therefore PAHs have been widely applied to fabricate various organic electronics such as OLED, OFET, and so on [1]. Concerning effective intermolecular π-orbital overlaps, PAHs with planar shape and large p-conjugated system are required. However, the extension of p-conjugation of PAHs may decrease the chemical stability, which would limit the fabrication of organic electronics under the ambient conditions. For example, hexacene [2] and heptacene [3] show diradical feature; therefore, they can react with oxygen to lose the continuous conjugation. In this regard, the modulation of electronic states of PAHs is highly desirable. An effective way toward this purpose is proper lowering the aromaticity of PAHs, which can be achieved by two strategies [4]: (i) introducing heteroatoms into the π-conjugated system; (ii) imbedding nonhexagonal rings into hexagonal sheets. The latter may result in PAHs with non-planar π-surface.
|
Download:
|
| Scheme. 1. Chemical structures of the representative PAHs. | |
While most of attentions have been paid to PAHs having planar p-conjugated system, the non-planar PAHs seem amazing due to their unique physical, chemical, and assembling features [5]. The non-planar PAHs have been created with the geometries of bracelet [6], saddle [7], bowl [8], MÖbius band [9], helicenes [10], and so on. To date, most of the curved PAHs are generated by embedding non-hexagonal rings into the hexagonal sheets. Fullerenes, discovered in 1985 [11], are typical molecules with curved conjugation and they possess the electron-deficient convex surface. Fullerenes have been widely employed as electron acceptor in OPV [12] and conducting component in organic superconductor [13]. The extraordinary properties of fullerenes stimulate intensive studies on PAHs with curved π-electron system. These studies are not only limited to the “closed” system, but also directed to the “open” system. In comparison with the “closed” one, the “open” system shows various structures and properties that can be modulated by chemical functionalization.
The bowl-shaped PAHs, also named as bucky bowls or π-bowls, are representative PAHs with open curved π-surface. Bucky bowls are mainly derived from two representative fragments of C60, corannulene (1) and sumanene (2), as shown in Scheme 2. Bucky bowls have concave and convex surfaces with different distribution of π-electron cloud [14]. Consequently, they show distinct properties and assembly features as compared with fullerenes. Recently, bucky bowls are of growing interest owing to their multidiscipline applications. They have been employed as synthetic intermediates for C60 [15], end-cap of carbon nanotube [16], warped nanographene [17], and so on. They can coordinate with various metal ions [18] and encapsulate fullerenes [19]. Recently, bucky bowls have been applied to fabricate electronic devices, e.g., the ambipolar OFET [20]. While bucky bowls are rich in chemistry and material science, the key issue in this field lies on the development of practical synthetic strategies, because the synthesis of bucky bowls are challenging due to the high ring strain energy. In this short account, we focus on the synthesis of the representative bowl-shaped conjugated polycycles. As for the structure and property of compounds herein and their analogues, one may find in the literatures [8].

|
Download:
|
| Scheme. 2. Schematic depiction for the generation of corannulene (1) and sumanene (2). | |
2. Corannulene and the representative derivatives
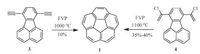
Corannulene (1) is a representative fragment of C60 with C5v symmetry. Chemically speaking, corannulene is constructed by fusion of five benzene rings onto the edges of cyclopentane, therefore shows large ring strain energy. Corannulene was firstly synthesized by Lawton and Barth in 1966 [21], two decades earlier than the discovery of C60. However, this synthesis suffers from the tedious route (17 linear steps) and low yields (overall yield <1%). Consequently, the further research on 1 is limited since then. In 1991, Scott’s group disclosed a much practical synthetic route as shown in Scheme 3. This synthesis is inspired by the early reports of Brown, i.e., the terminal alkynes isomerize reversibly under flash vacuum pyrolysis (FVP) condition by 1, 2-shifts of hydrogen atom and the resulting vinylidenes can be trapped by insertion into nearby C-H bonds [22]. By employing FVP as the key technique, Scott and co-workers successfully made the ring closure of compound 3 at 1000 ℃ to form 1 in 10% yield [23]. However, compound 3 shows strong tendency to polymerization rather than isomerization during heating at high temperature, which leads to the low production yield of 1. To solve this issue, Scott’s group selected various precursors of 1, and they found the best one was 4, a “masked” version of 3. Compound 4 sublimes cleanly without polymerization and loses 2 mol of HCl in the hot zone to generate 3 in situ, which then cyclizes twice to afford 1 in 35%-40% yield [24]. This breakthrough sparks many groups to join in this field.
|
Download:
|
| Scheme. 3. Synthesis of corannulene (1) through the FVP method. | |
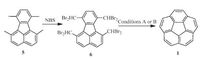
While Scott’s FVP strategy has been successfully employed to create a variety of PAHs with bowl-shaped conjugation [8c], the FVP process cannot be easily handled. To avoid the bimolecular reaction in gas phase, the slow sublimation of precursor in reasonable period (usually 10-30 min) is highly required; thus the reaction is mainly run in small scale. Moreover, the precursor should be sufficiently robust during sublimation into gas phase, say, the FVP strategy shows poor tolerance of substrate. In this regard, the synthesis of 1 under mild synthetic conditions (e.g., in solution phase) became an important goal. In 1996, Siegel and coworkers reported the first solution phase synthesis of corannulene derivative [25]. Inspired by this progress, Siegel [26] and Rabideau [27] groups independently disclosed the non-pyrolytic synthesis of the pristine corannulene as shown in Scheme 4. Compound 5 is tetrabrominated with NBS under forcing condition to produce 6, which undergoes ring closure in the presence of the reduction agents (Condition A) to afford 1 in 70%-80% yield. This synthesis is further refined by Sygula and Rabideau (Condition B), and leads to the large-scale production of 1 [28]. Recently, Siegel’s group has reported the kilogram-scale synthesis of 1 [29], which significantly promotes the application of this peculiar molecule in multiple terminals. To further modulate the electronic structure and explore novel PAHs with curved π-surface, the functionalization of 1 has been performed [8a-c,8f]. In the following, we will introduce several representative examples in this area.
|
Download:
|
| Scheme. 4. Non-pyrolytic synthesis of corannulene (1). Condition A: TiCl4, Zn/Cu, DME (Siegel’s method): 80% yield; or VCl4, LiAlH4, DME (Rabideau’s method): 70%-75% yield; Condition B: (i) NaOH, dioxane, H2O; (ii) Zn, NaI, EtOH: 74% yield. | |
The fusion of 1, 4-benzodithiin rings onto the flanking benzene of 1 is achieved in two steps as shown in Scheme 5. Firstly, corannulene is transformed into decachlorocorannulene (7) in 60% yield [30]. In the second step, a nucleophilic aromatic reaction of compound 7 with sodium benzene-1, 2-dithiolate results in the 1, 4-benzodithiin-fused corannulene (8) in 50% yield [31]. X-ray crystal structure analysis reveals that 8 adopts ‘fly trap’ type conformation. Compound 8 is electron rich (π-donor) owing to the decoration of ten sulfur atoms on the flanking benzenes. On the other hand, the convex surface of C60 is electron deficient (π-acceptor). Thus, compound 8 has the priority, in both electronic states and shape complementary, to encapsulate fullerene molecules. In fact, Scott’s group successfully prepared the donor-acceptor type supramolecular complexes between 8 and C60/C70, and the donor: acceptor ratio was 1: 1 as testified by the 1 HNMR titration [32].

|
Download:
|
| Scheme. 5. Fusion of 1, 4-benzodithiins onto corannulene (8). Reagents and conditions: (a) SO2Cl2, AlCl3, S2Cl2: 60% yield; (b) ortho-C6H4(SNa)2 DMEU: 50%yield. | |
To gain corannulene-based organic semiconductors, Pei’s group synthesized the imide-fused corannulene derivative (13) in four linear steps with an overall yield of 50% (Scheme 6) [20].his modification leads to two substantial effects.irstly, the molecular dipole moment, both the magnitude and direction, is distinctly changed. Consequently, compound 13 forms ordered columnar stack with the smallest π-π stack distance (3.39Å) among corannulene derivatives. Secondly, the HOMO energy level of 13 remains almost the same to that of 1, whereas the LUMO energy level is clearly lowered owing to the decoration of imide group.herefore, compound 13 has a HOMO-LUMO energy gap narrower than that of 1.urther study indicates that 13 shows ambipolar transport property with the hole and electron mobilities of 0.05 cm2 V-1 s-1 and 0.02 cm2 V-1 s-1, respectively [20]. Very recently, Pei and co-workers have synthesized corannulene derivatives 14-18. Compound 14 is a p-type organic semiconductor [33], 16 is an n-type organic semiconductor [34], and 17 and 18 can serve as the non-fullerene organic acceptors to fabricate OPV [35].

|
Download:
|
| Scheme. 6. Fusion of imide onto corannulene (13), along with 14-18 synthesized by Pei and collaborators. Reagents and conditions: (a)CC, MAP, CM, RT, 12 h: 84% yield;(b) acenaphthoquinone, KOH, EtOH, reflux, 15 min: 97% yield; (c) 1-octyl-pyrrole-2, 5-dione, nitrobenzene, 180 ℃, 12 h: 75% yield; (d) Pd(PCy3)2Cl2, BU, MF, 160 ℃, 36 h:82% yield. | |
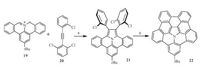
Despite of peripheral functionalization, doping of heteroatoms into backbone of corannulene is promising as the heteroatoms would bring distinct influence on packing motifs and band structures. However, the heteroatom doping of corannulene is very challenging.to, okimaru, and Nozaki reported the first heteracorannulnene by replacing the sp2 carbon on the fivemembered ring with nitrogen atom [36]. However, the simple replacement of any sp2 carbon on the pristine corannulene with nitrogen atom would reduce the chemical stability, i.e., formation of cationic species by oxygen.n this regard, the replacement of sp2 carbon with nitrogen as well as the fusion of benzene rings on the rim of corannulnene is simultaneously carried out as shown in Scheme 7. Employing compound 19 as starting material, the 1, 3- dipolar cycloaddition with diarylethyne (20) results in compound1 21, which then undergoes the palladium-catalyzed intramolecular cyclization to afford compound 22, a nitrogen-doped corannulene with the extended conjugation.his modification brings significant influence on both the structural and electronic features.or example, compound 22 is a much deeper π-bowl and displays distinctly red-shifted and much strong fluorescence, as compared with the pristine corannulene.
|
Download:
|
| Scheme. 7. Synthesis of N-doped corannulene derivative (22). Reagents and conditions: (a) DMSO, reflux: 62% yield; (b) [Pd(PCy3)2Cl2], Cs2CO3, DMA: 14% yield. | |
3. Sumanene and the representative derivatives
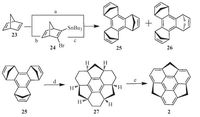
In comparison with corannulene (1), sumanene (2) is a more strained bucky bowl due to embedding three pentagonal rings into the hexagonal sheets. Hence, the synthesis of 2 is highly challenging because of the large strain energy. Mehta and coworkers tried the synthesis of 2 from tris(bromomethyl)triphenylene through FVP, whereas only the mono-cyclized and dicyclized products were obtained [37].he first successful synthesis of was accomplished by Hirao and co-workers in 2003 [38]. As shown in Scheme 8, trimerization of norbornadiene (23) afforded the mixture of 25 (syn-form) and 26 (trans-from) in 7% yield (Condition A). On the other hand, the stepwise transmetallation via an organotin 24 increases the yield of 25/26 mixture to 47% (Conditions B and C). Alkene-bridge exchange of the syn-form 25 affords 27 in 30% yield via Ru-catalyzed tandem metathesis reaction under an ethylene atmosphere.n contrast, the reaction of trans-form6 under the same condition does not give the desired compound 27.inally, the dehydrogenation of 27 afforded in 70% yield.his progress inspires the further studies on sumanene including peripheral functionalization, coordination with metal ions, and application in electronic materials; one may find these topics in a recent personal account of Hirao’s group [8g].
|
Download:
|
| Scheme. 8. Synthesis of sumanene (2). Reagents and conditions: (a) n-BuLi, tBuOK, BrCH2CH2Br, HF, -78 ℃ to-45 ℃ then CuI, RT: 7% yield (syn:anti = 1:3). (b) n-BuLi, tBuOK, BrCH2CH2Br, HF, -78 ℃ to -45 ℃ then Bu3SnCl, RT. (c) Cu(2-C4H3SCO2), -20 ℃ to RT: 47% yield (2 steps; syn:anti = 1:3). (d) Cat (10 mol%). [P(C6H11)3]2RuCl2=CHPh, CH2=CH2, toluene, -78 ℃ to RT, 4 h: 30% yield. (e)DQ, toluene, 110 ℃, 3 h: 70% yield. | |
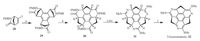
Further modification on sumanene is directed to the introduction of heteroatoms into its backbone, which results in the heterasumanenes [39].he heterasumanenes can be achieved by two strategies: (i) replacing sp2 carbons on the flanking benzenes with heteroatoms and (ii) replacing the benzylic carbons with heteroatoms.In 2013, Sakurai group reported the synthesis of triazasumanene (32) with three flanking benzenes replaced by pyridines as shown in Scheme 9 [40].he Pd-catalyzed cyclotrimerization of enantiopure 28 exclusively afforded the syn-form 29, which was hydrolysed, followed by condensation, to afford bowl-shaped lactam 30. Because the direct transformation of 30 into 32 was unsuccessful, 30 was then converted to thioimidate 31 in three steps.n the final step, 31 was dehydrogenated by employing Ph3CBF4 as oxidant and a nonnucleophilic baseTBMP as acid scavenger.he overall yield of this six-step synthesis for 32 is 16%. Compound 32 has a bowl-tobowl inversion energy (42.2 kcal mol-1)much higher than that of 2(20.3 kcal mol-1), therefore this compound shows very stable chirality.
|
Download:
|
| Scheme. 9. Synthesis of triazasumanene (32). Reagents and conditions: (a) Pd(OAc)2, PPh3, Bu4NOAc, Na2CO3, molecular sieves 4Å, 1, 4-dioxane, 100 ℃: 57% yield; (b) (i) 12 mol/L HCl, AcOH, 60 ℃, 3 h; (ii) C6F5OP(=O)Ph2, N, N-diisopropylethylamine, MF, 0-60 ℃: 59% yield; (c) Lawesson’s reagent, ClCH2CH2Cl, microwave, 160 ℃: 92% yield; (d) CF3CO2H, microwave, 100 ℃: 88% yield; (e) MeI, K2CO3, MF, 30 ℃: 79% yield; (f) Ph3CBF4, TBMP, CH2Cl2, 5 ℃, 8 h: 73% yield. | |
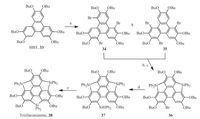
The heterasumanenes derived from 2 by replacing benzylic carbons with heteroatoms are promising, because the electronic structures of the five-membered rings differ from each other in varying heteroatoms. A rational approach toward such heterasumanenes is to build three heteroatom bridges at the bay regions of triphenylene. However, theoretical investigations suggest it would be challenging to synthesize curved heterasumanenes from planar triphenylene due to large strain energy for ring cyclization [41]. On the other hand, heterasumanenes with flat backbone can be accessed through this strategy. Employing a triphenylene analogue HBT (33) as starting material, Kawashima and co-workers synthesized trisilasumanene (38), which is of planar conjugation [42]. As shown in Scheme 10, the ring cyclization was achieved step-wisely, taking the advantage of sila-Friedel-Crafts reaction on the benzene ring developed by the same group. However, this synthesis suffers from the low efficiency of sila-Friedel-Crafts reaction, i.e., the total yield for this five-step synthesis is about 0.7%. Compound 38 shows strong blue emission in both solution and solid state, plausibly ascribable to the unique photophysical nature of the silole rings [43].
|
Download:
|
| Scheme. 10. Synthesis of trisilasumanene (38). Reagents and conditions: (a) Br2, CH2Cl2, 0.5 h: 35% yield; (b) (i) n-BuLi, HF, -78 ℃, (ii) Ph2SiCl2, r.t., (iii) LiAlH4, reflux; (c) Ph3CB(C6H5)4, , 6-lutidine, CH2Cl2, r.t., 2% yield (two steps); (d) (i) t-BuLi, Et2O, -78 ℃, (ii) Ph2SiH2, reflux: 51% yield; (e) Ph3CB(C6F5)4, , 6-lutidine, CH2Cl2, r.t., 18% yield. | |
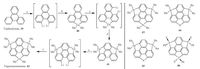
Saito’s group disclosed the synthesis of group 14 elements (Si, Ge, Sn) doped sumanenes without substituents on the flanking benzenes [39b]. Scheme 11 shows the synthesis of trigermasumanene (42) from triphenylene (39), as a representative example for this series. Unlike HBT (33) which can be fully lithiated on the bay regions, the selective and full lithiation on bay positions of 39 is impossible, and the rim positions are partially lithiated simultaneously.herefore, the construction of three germanium bridges at the bay regions of 39 is achieved one by one. While the reagents are same for each step, one should be careful about the amount of n- BuLi in the lithiation step and the reaction temperature for the insertion of germanium. Employing the similar strategy, Saito and co-workers prepared heterasumanenes (43-45) containing multiple heteroatoms [44], whereas an attempt to create tristannosumanene (Sn-doped) failed to afford the desired one. Recently, Saito’s group succeeded the synthesis of compound 46, a phosphorus doped sumanene derivative [45].
|
Download:
|
| Scheme. 11. Synthesis of trigermasumanene (42), along with 43-46 synthesized by Saito and collaborators. Reagents and conditions: (a) n-BuLi (4 equiv.), MEDA (4 equiv.), hexane, 60 ℃ for 3 h; (b) Me2GeCl2 (2 equiv.), HF, -78 ℃ for 12 h; (c) n-BuLi (6 equiv.), MEDA (6 equiv.), hexane, 60 ℃ for 3 h; (d) Me2GeCl2 (6 equiv.), HF, -84 ℃ to RT for 8 h; (e) n-BuLi (10 equiv.), MEDA (10 equiv.), hexane, 60 ℃ for 3 h; (f) Me2GeCl2 (10 equiv.), HF, -88 ℃ to RT for 12 h. | |
Among heterasumanenes, the sulfur-doped sumanene seems amazing, because sulfur-containing conjugated molecules, such as thiophene and tetrathiafulvalene (TTF), show excellent optical and electrical properties. Moreover, sulfur atom can form various van der Waals contacts in solid state and has different valence states that can be chemically modulated.rithiasumanene can be derived from sumanene by replacing benzylic carbons with sulfur. Compared with sumanene, five-membered rings in trithiasumanene are of aromaticity, and the molecule is changed from electron-deficient to electron-rich by replacing Cp ring with thiopehene.n chemcial sense, trithiasumanene can also be derived from coronene by replacing three flanking benzene rings with thiophene rings. Although aromaticity remains, the conjugated system changes from planar to bowl-shaped.rithiasumanene consists of two subunits, benzotrithiophene (47) and triphenylene (39).he retrosynthetic analysis thus provides two plausible synthetic routes: Routes A and B start from compounds 47 and 39, respectively.heoretical study shows that route A is much better than route B, because the latter has large ring strain energy [41].
In 1999, Otsubo’s group successfully synthesized trithiasumanene (54) through route A [46], which was even earlier than the theoretical study. Employing 47 as starting material, Otusbo and co-workers synthesized 54 in four linear steps as shown in Scheme 12.he key step for this synthesis is the ring closure of compounds 52 and 53 under FVP condition (1000 ℃, 0.005 Torr, N2 flow). Consequently, the synthesis suffers from low efficiency (overall yield, 6%) and small scale production (tens of milligrams). Meanwhile, compound 47 is much more expensive than 39.hus, the key issue in trithiasumanene chemistry lies in (i) development of more practical route and (ii) large scale production. Moreover, the synthetic strategy should be applied to synthesize other types of trichalcogenasumanenes.
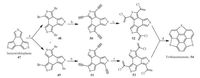
|
Download:
|
| Scheme. 12. Synthesis of trithiasumanene (54) through the FVP method. Reagents and conditions: (a) NBS, DMF, RT; (b) TMSCBBCH, Pd(PPh3)4, CuI, Et3N, reflux (two steps, 53%yield); (c) conc. HCl, AcOH, 80 8C: 33% yield; (d) 1000 8C, 0.005 Torr, N2 flow: 35% yield. | |
In comparison with route A, the route B shows advantage in starting material.he derivatives of 39 are cheaper than those of 47, and can be easily prepared in hundred-gram scale. However, the challenge is that it is thermodynamically unfavorable to construct three sulfur bridges into triphenylene [41]. Only the mono- and di-bridged species are formed in trace amount at around 500 ℃ as reported by Klemm [47]. While difficult to construct three sulfur bridges at bay positions of triphenylene in one time, it is possible to build up multiple less strained 1, 2-dithiin rings in single step. Aiming at this purpose, Shao’s group disclosed a non-pyrolytic two-step synthesis of trithiasumanene (57) from HBT (33) [48].his strategy lies in (i) ring cyclization, fusion of multiple thiophene and 1, 2-dithiin rings at the bay regions of 33, and (ii) ring contraction, desulfurization of 1, 2-dithiin to form the desired 57. As shown in Scheme 13, in the first step, compound 33 was hexalithiated with n-BuLi at 60 ℃, followed by insertion of sulfur.his one-pot reaction almost quantitatively constructs two 1, 2-dithiin rings and one thiophene ring, i.e., compound 55.n the second step, compound 55 was desulfurized by heating with copper nano powder at 200 ℃ to afford 57 in 30% yield, along with 5% of 56 which can be transformed into 57.his protocol shows advantages in cheaper starting material, mild condition, and multigram scale production.nspired by this progress, Shao and coworkers synthesized triselenasumanene (59).n fact, this strategy is more practical to synthesize 59.n the ring cyclization step, compound 58 was produced in yield of 70%, and was then converted to 59 quantitatively.
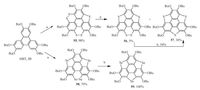
|
Download:
|
| Scheme. 13. Non-pyrolytic two-step synthesis of trithiasumanene (57) and triselenasumanene (59). Reagents and conditions: (a) (i) n-BuLi, MEDA, hexane, 60 ℃, 3 h; (ii) S powder, -78 ℃ to RT; (b) Cu powder (80-100 nm grain size), 00 ℃, h. | |
Theoretical investigation reveals that the chalcogen atoms (O, S, Se) show important contribution on the frontier orbitals of compounds 57 and 58, particularly on the HOMO orbital.herefore, compounds 57 and 58 have two structural features: (i) electron-rich arises from butoxy groups and chacogenole rings; (2) ring strain from the bowl-shaped structure. An oxidation of 57 and 59 by Oxone resulted in the cleavage of one of the flanking benzene rings (Scheme 14), as reported by Shao’s group [49]. On the other hand, HBT (33) is electron-rich without ring strain, and sumanene (2) is a more strained but electron-deficient.he oxidation of 33 and 2 by Oxone did not afford ring-opening products.herefore, this peculiar reaction is driven by the cooperative effect of the electron-rich nature and ring strain. On the basis of the ring-opening products (61 and 62), Shao and coworkers prepared a series of [5-6]-fused system (compounds 64-66) through facile transformations.he central [5-6]-fused frameworks of 64-66 are planar due to ring expansion. Compounds 64-66 form the head-to-tail type columnar stacks with the π-π stack distance of 3.50Å. Compared with 57 and 59, compounds 64-66 display significantly red-shifted absorbance and emission, and the fluorescence quantum yield up to 40%.
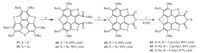
|
Download:
|
| Scheme. 14. Ring reconstruction of 57 and 59. Reagents and conditions: (a) Oxone (2 equiv.), THF/H2O (v/v, 4:1), r.t., 2 h; (b) (i) NaOH, EtOH/H2O (v/v, 10:1), reflux, 2 h; (ii) HCl(3 mol/L, aq.); (iii) Ac2O, reflux; (c) DCC, THF, reflux. | |
4. Concaved conjugated polycycles other than bucky bowls
Apart from the derivation of fullerene fragments (corannulene and sumanene), the bowl-shaped conjugated molecules can also be created by replacing the sp2 carbon on benzene-based twodimensional sheet with multiple heteroatoms.his is because the bond lengths of C=C and C-X (X = N, O, P, S) are different. Additionally, heteroatoms have lone pair electrons on the p-orbitals, therefore they tend to take tetrahedron geometry rather than the flat one even if the p-orbital electrons can involve in the conjugated framework. A typical example is triphenylamine, which adopts a Pyramid-like geometry.n the following, we will introduce several typical examples for this series.
In 1997, Krebs and co-workers reported a concaved heterocycle (TOTPP, 72) based on the triphenyphosphine skeleton [50]. As shown in Scheme 15, they synthesized 72 in four linear steps with the final step being an intramolecular nucleophilic aromatic reaction of compound 71.he phosphorus atom on 72 was further oxidized by H2O2 to form theOTPPO (73) in 63% yield. Crystallographic study shows that 72 takes a bowl-shaped conformation and forms the polarized stacks in solid state.heoretical investigation reveals that compound 72 has a dipole moment of 3.3 ± 0.2 with a direction along the concave face. convex face. Consequently, the crystals of 72 exhibit pyroelectric property with pyroelectric coefficient of -3 µC m-2 K-1 -1 at room temperature.
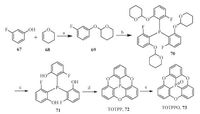
|
Download:
|
| Scheme. 15. Synthesis ofOTPP (72) andOTPPO (73). Reagents and conditions: (a) HCl (37% aq.), -35 ℃ to r.t.: 83% yield; (b) (i) n-BuLi (1 equiv.), HF, -78 ℃, 30 min; (ii) PBr3 (0.3 equiv.), -78 ℃: 65% yield (for one portion reaction); (c) HCl (37% aq.), MeOH, Ar atmosphere, r.t., 4 h: 91% yield; (d) t-BuOK (3 equiv.), NMP, 00 ℃, 1 h: 86% yield; (e) H2O2 (35% aq.), CHCl3, vigorous stirring, overnight: 63% yield. | |
Inspired by Kreb’s work, Yamamura and co-workers have prepared a series of concaved heterocycles based on triphenyphosphine [51]. As a typical example, the synthesis of a sulfur analogue (TSTPP, 76) of 72 is shown in Scheme 16. Employing the same precursor (compound 71) for the synthesis of 72, they synthesized 76 in four linear steps with overall yield of 35% [51a].he key step for this synthesis is the Newman-Kwart rearrangement of compound 75. All these compounds so far synthesized by Yamamura and co-workers adopt the bowl-shaped conformation, whereas the bowl depth can be finely tuned by varying the chalcogen atoms and the convalence state of the central phosphorus atom as well [51a]. Yamamura and co-workers also demonstrated that these compound show strong propensity to encapsulate fullerene molecules [51b-d].
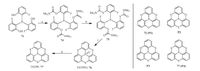
|
Download:
|
| Scheme. 16. Synthesis ofSTPPO (76) andSTPP (77). Reagents and conditions: (a) Me2NC(S)Cl, ABCO, dryMF, 70 ℃: 87% yield; (b) Newman-Kwart rearrangement, 220 ℃: 96% yield; (c) NaOMe, HF, 70 ℃: 61% yield; (d) SiHCl3, Et3N, toluene, 120 ℃: 69% yield. | |
Okada’s group has developed a different analogue of compound 72 by replacing phosphorus atom with nitrogen atom, ., a triphenylamine based concaved heterocyleOTPA [52].OTPA (85) was synthesized in five linear steps with two-fold intramolecular nucleophilic aromatic reaction to construct the desired structure (Scheme 17).OTPA is an excellent electron donor and can form stable cation radical species upon electrochemical oxidation and/or reaction with electron acceptors.heoretical calculation reveals that the spin density of the cation radical is mainly on the central nitrogen atom.OTPA adopts bowl shape in the neutral state, whereas the conformation is changed to flat in its cation radical state.

|
Download:
|
| Scheme. 17. Synthesis ofOTPA (85). Reagents and conditions: (a) NaH/DMSO, 130 ℃: 83% yield; (b) NaH/DMSO, 130 ℃: 86% yield; (c) hydrazine hydrate, Pd/C, pbromophenol/ ethanol, reflux: 85% yield; (d) tBuONa, [Pd-(dba)2], P(t-Bu)3/toluene, reflux: 35% yield; (e) BBr3/CH2Cl2, -78 ℃ to r.t.: 99% yield; (f) K2CO3/DMF, RT: 92% yield. dba = trans, trans-dibenzylideneacetone. | |
Finally, we would like to introduce the bowl-shaped molecules with the bottom opened, for example, chrysaoroles and chrysaorenes developed by Stepien´ group [53]. Chrysaorole differs from typical bucky bowls in that its hub consists of a uniquely large, 18- membered ring [53a]. However, the presence of six hydrogen atoms in the hub makes the remaining opening quite small.he depth of the bowl is 1.96Å, much larger than that of corannulene (0.87Å). Stepien´ group has revealed that geometries of the chrysaorenes depend on the number of the fluorenophane subunit [53b]. When three fluorenophanes fused in sequence to form a large macrocycle, the resulting [3]Chrysaorene is of bowl shape. On the other hand, whereas four fluorenophanes fused one, [4]chrysaorene, is planar.he chrysaorenes show geometry-dependent magnetic properties and are strongly fluorescent.
5. ConclusionIn this short review, we highlight the recent progress on the chemistry of bowl-shaped conjugated polycycles.he bowlshaped molecules are mainly constructed on the basis of two subunits of fullerene C60, the C5v-symmetric corannulnene and the C3v-symmetric sumanene.he breakthrough for the synthesis of these two basic backbones inspires intensive research on the bowlshaped polycycles including peripheral functionalization and heteroatom doping of corannulene and sumanene, as wells as replacing the sp2 carbon on the conjugated two-dimensional sheet with multiple heteroatoms.he achievement of the synthesis polycycles leads to the multiple applications of bowl-shaped conjugated in synthetic chemistry, coordination chemistry, supramolecular chemistry, and materials sciences.
Acknowledgments We are grateful to the National Natural Science Foundation of China (Nos.1522203,1372111, and1190034) and State Key Laboratory of Applied Organic Chemistry for the financial support.| [1] | (a) H.L. Dong, H.F. Zhu, Q. Meng, X. Gong, W.P. Hu, Organic photoresponse materials and devices, Chem. Soc. Rev. 41(2012) 1754-1808; (b) C.L. Wang, H.L. Dong, W.P. Hu, Y.Q. Liu, D.B. Zhu, Semiconducting π-conjugated systems in field-effect transistors: a material Odyssey of organic electronics, Chem. Rev. 112(2012) 2208-2267; (c) W. Jiang, Y. Li, Z.H. Wang, Tailor-made rylene arrays for high performance nchannel semiconductors, Acc. Chem. Res. 47(2014) 3135-3147; (d) A. Narita, X.Y. Wang, X.L. Feng, K. Müllen, New advances in nanographene chemistry, Chem. Soc. Rev. 44(2015) 6616-6643; (e) T.C. Yu, L.L. Liu, Z.Q. Xie, Y.G. Ma, Progress in small-molecule luminescent materials for organic light-emitting diodes, Sci. China Chem. 58(2015) 907-915; (f) Y.Q. Zheng, J.Y. Wang, J. Pei, One-dimensional (1D) micro/nanostructures of organic semiconductors for field-effect transistors, Sci. China Chem. 58(2015) 937-946; (g) X.K. Gao, Z. Zhao, High mobility organic semiconductors for field-effect transistors, Sci. China Chem. 58(2015) 947-968. |
| [2] | M.M. Payne, S.R. Parkin, J.E. Anthony. Functionalized higher acenes: hexacene and heptacene. J. Am. Chem. Soc. 127 (2005) 8028–8029. DOI:10.1021/ja051798v |
| [3] | R. Mondal, B.K. Shah, D.C. Neckers. Photogeneration of heptacene in a polymer matrix. J. Am. Chem. Soc. 128 (2006) 9612–9613. DOI:10.1021/ja063823i |
| [4] | J.G. Mei, Y. Diao, A.L. Appleton, et al. Integrated materials design of organic semiconductors for field-effect transistors. J. Am. Chem. Soc. 135 (2013) 6724–6746. DOI:10.1021/ja400881n |
| [5] | N. Martín, L.T. Scott. Challenges in aromaticity: 150 years after Kekule ′ 's benzene. Chem. Soc. Rev. 44 (2015) 6397–6400. DOI:10.1039/C5CS90085A |
| [6] | H. Omachi, Y. Segawa, K. Itami. Synthesis of cycloparaphenylenes and related carbon nanorings: a step toward the controlled synthesis of carbon nanotubes. Acc. Chem. Res. 45 (2012) 1378–1389. DOI:10.1021/ar300055x |
| [7] | (a) K.Y. Cheung, X.M. Xu, Q. Miao, Aromatic saddles containing two heptagons, J. Am. Chem. Soc. 137(2015) 3910-3914; (b) E. Gońka, P.J. Chmielewski, T. Lis, M. Stępień, Expanded hexapyrrolohexaazacoronenes. Near-infrared absorbing chromophores with interrupted peripheral conjugation, J. Am. Chem. Soc. 136(2014) 16399-16410. |
| [8] | (a) P.W. Rabideau, A. Sygula, Buckybowls: polynuclear aromatic hydrocarbons related to the buckminsterfullerene surface, Acc. Chem. Res. 29(1996) 235-242; (b) Y.T. Wu, J.S. Siegel, Aromatic molecular-bowl hydrocarbons: synthetic derivatives, their structures, and physical properties, Chem. Rev. 106(2006) 4843-4867; (c) V.M. Tsefrikas, L.T. Scott, Geodesic polyarenes by flash vacuum pyrolysis, Chem. Rev. 106(2006) 4868-4884; (d) T. Amaya, T. Hirao, A molecular bowl sumanene, Chem. Commun. 47(2011) 10524-10535; (e) S. Higashibayashi, H. Sakurai, Synthesis of sumanene and related buckybowls, Chem. Lett. 40(2011) 122-128; (f) A. Sygula, Chemistry on a half-shell: synthesis and derivatization of buckybowls, Eur. J. Org. Chem. (2011) 1611-1625; (g) T. Amaya, T. Hirao, Chemistry of sumanene, Chem. Rec. 15(2015) 310-321. |
| [9] | (a) T. Higashino, B.S. Lee, J.M. Lim, D. Kim, A. Osuka, A Möbius antiaromatic complex as a kinetically controlled product in phosphorus insertion to a[32] heptaphyrin, Angew. Chem. Int. Ed. 51(2012) 13105-13108; (b) T. Higashino, J.M. Lim, T. Miura, et al., Möbius antiaromatic bisphosphorus complexes of[30] hexaphyrins, Angew. Chem. Int. Ed. 49(2010) 4950-4954; (c) M. Stępień, N. Sprutta, L. Latos-Grazyński, Figure eights, Möbius bands, and more: conformation and aromaticity of porphyrinoids, Angew. Chem. Int. Ed. 50(2011) 4288-4340; (d) M. Stępień, B. Szyszko, L. Latos-Grazyński, Three-level topology switching in a molecular Möbius band, J. Am. Chem. Soc. 132(2010) 3140-3152. |
| [10] | Y. Shen, C.F. Chen. Helicenes: synthesis and applications. Chem. Rev. 112 (2012) 1463–1535. DOI:10.1021/cr200087r |
| [11] | H.W. Kroto, J.R. Heath, S.C. O'Brien, R.F. Curl, R.E. Smalley. C60: buckminsterfullerene. Nature 318 (1985) 162–163. DOI:10.1038/318162a0 |
| [12] | (a) D.M. Guldi, B.M. Illescas, C.M. Atienza, M. Wielopolski, N. Martín, Fullerene for organic electronics, Chem. Soc. Rev. 38(2009) 1587-1597; (b) G. Li, R. Zhu, Y. Yang, Polymer solar cells, Nat. Photonics 6(2012) 153-161; (c) B.C. Thompson, J.M.J. Fréchet, Polymer-fullerene composite solar cells, Angew. Chem. Int. Ed. 47(2008) 58-77; (d) G. Dennler, M.C. Scharber, C.J. Brabec, Polymer-fullerene bulk-heterojunction solar cells, Adv. Mater. 21(2009) 1323-1338; (e) X. Zhang, X.D. Li, Effect of the position of substitution on the electronic properties of nitrophenyl derivatives of fulleropyrrolidines: fundamental understanding toward raising LUMO energy of fullerene electron-acceptor, Chin. Chem. Lett. 25(2014) 501-504. |
| [13] | (a) R.C. Haddon, A.F. Hebard, M.J. Rosseinsky, et al., Conducting films of C60 and C70 by alkali-metal doping, Nature 350(1991) 320-322; (b) K. Tanigaki, T.W. Ebbesen, S. Saito, et al., Superconductivity at 33 K in CsxRbyC60, Nature 352(1991) 222-223. |
| [14] | U. Purushotham, G.N. Sastry. Conjugate acene fused buckybowls: evaluating their suitability for p-type, ambipolar and n-type air stable organic semiconductors. Phys. Chem. Chem. Phys. 15 (2013) 5039–5048. DOI:10.1039/c3cp44673e |
| [15] | L.A. Scott, M.M. Boorum, B.J. McMahon, et al. A rational chemical synthesis of C60. Science 295 (2002) 1500–1503. DOI:10.1126/science.1068427 |
| [16] | L.T. Scott, E.A. Jackson, Q.Y. Zhang, et al. A short, rigid, structurally pure carbon nanotube by stepwise chemical synthesis. J. Am. Chem. Soc. 134 (2012) 107–110. DOI:10.1021/ja209461g |
| [17] | K. Kawasumi, Q.Y. Zhang, Y. Segawa, L.T. Scott, K. Itami. A grossly warped nanographene and the consequences of multiple odd-membered-ring defects. Nat. Chem. 5 (2013) 739–744. DOI:10.1038/nchem.1704 |
| [18] | (a) T. Amaya, H. Sakane, T. Hirao, A concave-bound CpFe complex of sumanene as a metal in a π bowl, Angew. Chem. Int. Ed. 46(2007) 8376-8379; (b) M.A. Petrukhina, Coordination of buckybowls: the first concave-bound metal complex, Angew. Chem. Int. Ed. 47(2008) 1550-1552; (c) J.S. Siegel, K.K. Baldridge, A. Linden, R. Dorta, d8 Rhodium and Iridium complexes of corannulene, J. Am. Chem. Soc. 128(2006) 10644-10645. |
| [19] | (a) A. Sygula, F.R. Fronczek, R. Sygula, P.W. Rabideau, M.M. Olmstead, A double concave hydrocarbon buckycatcher, J. Am. Chem. Soc. 129(2007) 3842-3843; (b) A.S. Filatov, M.V. Ferguson, S.N. Spisak, et al., Bowl-shaped polyarenes as concave-convex shape complementary hosts for C60- and C70-fullerenes, Cryst. Growth Des. 14(2014) 756-762. |
| [20] | K. Shi, T. Lei, X.Y. Wang, J.Y. Wang, J. Pei. A bowl-shaped molecule for organic fieldeffect transistors: crystal engineering and charge transport switching by oxygen doping. Chem. Sci. 5 (2014) 1041–1045. DOI:10.1039/C3SC52701H |
| [21] | W.E. Barth, R.G. Lawton. Dibenzo. J. Am. Chem. Soc. 88 (1966) 380–381. DOI:10.1021/ja00954a049 |
| [22] | R.F.C. Brown, F.W. Eastwood, G.P. Jackman. Methyleneketenes and methylenecarbenes. IX. Thermal rearrangements of alkyl- and aryl-acetylenes involving alkyl- and aryl-methylenecarbenes. Aust. J. Chem. 30 (1977) 1757–1767. DOI:10.1071/CH9771757 |
| [23] | L.T. Scott, M.M. Hashemi, D.T. Meyer, H.B. Warren. Corannulene. A convenient new synthesis. J. Am. Chem. Soc. 113 (1991) 7082–7084. DOI:10.1021/ja00018a082 |
| [24] | L.T. Scott, P.C. Cheng, M.M. Hashemi, et al. Corannulene. A three-step synthesis. J. Am. Chem. Soc. 119 (1997) 10963–10968. DOI:10.1021/ja972019g |
| [25] | T.J. Seiders, K.K. Baldridge, J.S. Siegel. Synthesis and characterization of the first corannulene cyclophane. J. Am. Chem. Soc. 118 (1996) 2754–2755. DOI:10.1021/ja953734y |
| [26] | T.J. Seiders, E.L. Elliot, G.H. Grube, J.S. Siegel. Synthesis of corannulene and alkyl derivatives of corannulene. J. Am. Chem. Soc. 121 (1999) 7804–7813. DOI:10.1021/ja991310o |
| [27] | A. Sygula, P.W. Rabideau. Non-pyrolytic syntheses of buckybowls: corannulene, cyclopentacorannulene, and a semibuckminsterfullerene. J. Am. Chem. Soc. 121 (1999) 7800–7803. DOI:10.1021/ja991169j |
| [28] | A. Sygula, P.W. Rabideau. A practical, large scale synthesis of the corannulene system. J. Am. Chem. Soc. 122 (2000) 6323–6324. DOI:10.1021/ja0011461 |
| [29] | A.M. Butterfield, B. Gilomen, J.S. Siegel. Kilogram-scale production of corannulene. Org. Process Res. Dev. 16 (2012) 664–676. DOI:10.1021/op200387s |
| [30] | R.B. Huang, W.J. Huang, Y.H. Wang, Z.C. Tang, L.S. Zheng. Preparation of decachlorocorannulene and other perchlorinated fragments of fullerenes by electrical discharge in liquid chloroform. J. Am. Chem. Soc. 119 (1997) 5954–5955. DOI:10.1021/ja970238w |
| [31] | M. Bancu, A.K. Rai, P.C. Cheng, R.D. Gilardi, L.T. Scott. Corannulene polysulfides: molecular bowls with multiple arms and flaps. Synlett (2004) 173–176. |
| [32] | P.E. Georghiou, A.H. Tran, S. Mizyed, M. Bancu, L.T. Scott. Concave polyarenes with sulfide-linked flaps and tentacles: new electron-rich hosts for fullerenes. J. Org. Chem. 70 (2005) 6158–6163. DOI:10.1021/jo0503761 |
| [33] | R.Q. Lu, Y.N. Zhou, X.Y. Yan, et al. Thiophene-fused bowl-shaped polycyclic aromatics with a dibenzo. Chem. Commun. 51 (2015) 1681–1684. DOI:10.1039/C4CC08451A |
| [34] | R. Chen, R.Q. Lu, K. Shi, et al. Corannulene derivatives with low LUMO levels and dense convex-concave packing for n-channel organic field-effect transistors. Chem. Commun. 51 (2015) 13768–13771. DOI:10.1039/C5CC03550C |
| [35] | R.Q. Lu, Y.Q. Zheng, Y.N. Zhou, et al. Corannulene derivatives as non-fullerene acceptors in solution-processed bulk heterojunction solar cells. J. Mater. Chem. A 2 (2014) 20515–20519. |
| [36] | S. Ito, Y. Tokimaru, K. Nozaki. Benzene-fused azacorannulene bearing an internal nitrogen atom. Angew. Chem. Int. Ed. 54 (2015) 7256–7260. DOI:10.1002/anie.v54.25 |
| [37] | G. Mehta, S.R. Shah, K. Ravikumar. Towards the design of tricyclopenta[def, jkl, pqr] triphenylene (‘sumanene’): a ‘bowl-shaped’ hydrocarbon featuring a structural motif present in C60(buckminsterfullerene). J. Chem. Soc. Chem. Commun. (1993) 1006–1008. |
| [38] | H. Sakurai, T. Daiko, T. Hirao. synthesis of sumanene, a fullerene fragment. Science 301 (2003) 1878. DOI:10.1126/science.1088290 |
| [39] | (a) X.X. Li, X.F. Shao, Synthesis of heterasumanene, Synlett 25(2014) 1795-1798; (b) M. Saito, S. Furukawa, J. Kobayashi, T. Kawashima, The chemistry of heterasumanenes, Chem. Rec. 16(2016) 64-72. |
| [40] | Q.T. Tan, S. Higashibayashi, S. Karanjit, H. Sakurai. Enantioselective synthesis of a chiral nitrogen-doped buckybowl. Nat. Commun. 3 (2012) 891–895. DOI:10.1038/ncomms1896 |
| [41] | U.D. Priyakumar, G.N. Sastry. Theory provides a clue to accomplish the synthesis of sumanene, C21H12, the prototypical C3v-buckybowl. Tetrahedron Lett. 42 (2001) 1379–1381. DOI:10.1016/S0040-4039(00)02249-8 |
| [42] | S. Furukawa, J. Kobayashi, T. Kawashima. Development of a sila-Friedel-Crafts reaction and its application to the synthesis of dibenzosilole derivatives. J. Am. Chem. Soc. 131 (2009) 14192–14193. DOI:10.1021/ja906566r |
| [43] | (a) Q. Yang, L.C. Li, S.H. Li, D.M. Yue, C.H. Xu, Synthesis and photophysical properties of poly(aryleneethynylene)s containing dibenzosilole unit, Chin. Chem. Lett. 23(2012) 1303-1306; (b) J. Luo, Z. Xie, J.W.Y. Lam, et al., Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun. (2001) 1740-1741. |
| [44] | (a) M. Saito, T. Tanikawa, T. Tajima, J.D. Guo, S. Nagase, Synthesis and structures of heterasumanenes having different heteroatom functionalities, Tetrahedron Lett. 51(2010) 672-675; (b) T. Tanikawa, M. Saito, J.D. Guo, S. Nagase, Synthesis, structures and optical properties of trisilasumanene and its related compounds, Org. Biomol. Chem. 9(2011) 1731-1735; (c) T. Tanikawa, M. Saito, J.D. Guo, S. Nagase, M. Minoura, Synthesis, structures, and optical properties of heterasumanenes containing group 14 elements and their related compounds, Eur. J. Org. Chem. 2012(2012) 7135-7142. |
| [45] | Y. Suda, S. Furukawa, J. Kobayashi, et al. Abstract of the 2nd International Symposium on π-System Figuration, Saitama, Japan, (2016), p. 53. |
| [46] | K. Imamura, K. Takimiya, T. Otsubo, Y. Aso. Triphenyleno[1,12-bcd:4,5-b'ćd':8,9-b"c"d"] trithiophene: the first bowl-shaped heteroaromatic. Chem. Commun. (1999) 1859–1860. |
| [47] | L.H. Klemm, E. Hall, L. Cousins, C.E. Klopfenstein. The insertion and extrusion of heterosulfur bridges. XIV. Synthesis of nitrotriphenyleno. J. Heterocycl. Chem. 24 (1987) 1749–1755. DOI:10.1002/jhet.v24:6 |
| [48] | X.X. Li, Y.T. Zhu, J.F. Shao, et al. Non-pyrolytic, large-scale synthesis of trichalcogenasumanene: a two-step approach. Angew. Chem. Int. Ed. 53 (2014) 535–538. DOI:10.1002/anie.v53.2 |
| [49] | X.X. Li, Y.T. Zhu, J.F. Shao, et al. Ring reconstruction on a trichalcogenasumanene buckybowl: a facile approach to donor-acceptor-type. Angew. Chem. Int. Ed. 54 (2015) 267–271. DOI:10.1002/anie.201409620 |
| [50] | F.C. Krebs, P.S. Larsen, J. Larsen, et al. Synthesis, structure, and properties of 4,8,12-trioxa-12c-phospha-4,8,12,12c-tetrahydrodibenzo. J. Am. Chem. Soc. 119 (1997) 1208–1216. DOI:10.1021/ja962023c |
| [51] | (a) M. Yamamura, T. Hasegawa, T. Nabeshima, Synthesis of phosphorus-centered and chalcogen-bridged concave molecules: modulation of bowl geometries and packing structures by changing bridging atoms, Org. Lett. 18(2016) 816-819; (b) M. Yamamura, K. Sukegawa, T. Nabeshima, Tuning the depth of bowl-shaped phosphine hosts: capsule and pseudo-cage architectures in host-guest complexes with C60 fullerene, Chem. Commun. 51(2015) 12080-12083; (c) M. Yamamura, D. Hongo, T. Nabeshima, Twofold fused concave hosts containing two phosphorus atoms: modules for the sandwich-type encapsulation of fullerenes in variable cavities, Chem. Sci. 6(2015) 6373-6378; (d) M. Yamamura, T. Saito, T. Nabeshima, Phosphorus-containing chiral molecule for fullerene recognition based on concave/convex interaction, J. Am. Chem. Soc. 136(2014) 14299-14306. |
| [52] | M. Kuratsu, M. Kozaki, K. Okada. 2,2':6',2":6",6-Trioxytriphenylamine: synthesis and properties of the radical cation and neutral species. Angew. Chem. Int. Ed. 44 (2005) 4056–4058. DOI:10.1002/(ISSN)1521-3773 |
| [53] | (a) D. Myśliwiec, M. Stępień, The fold-in approach to bowl-shaped aromatic compounds: synthesis of chrysaoroles, Angew. Chem. 125(2013) 1757-1761; (b) M.A. Majewski, T. Lis, J. Cybińska, M. Stępień, Chrysaorenes: assembling coronoid hydrocarbons via the fold-in synthesis, Chem. Commun. 51(2015) 15094-15097. |
 2016, Vol. 27
2016, Vol. 27 

