Cyclometalated organoplatinum(II) compounds, due to their square-planar geometry, represent an important class of transition-metal coordination complexes [1-8]. The admixing of metal d orbital with the counterparts locating on the conjugated ligands results in a variety of optically electronic transitions, such as ligand-centered (LC) band, metal-to-ligand charge transfer (MLCT) band, and ligand-to-ligand charge transfer (LLCT) band [9]. Moreover, by extensive structural modification of the cyclometa- lating and ancillary ligands, it is possible to tune the photophysical and luminescent characteristics for the resulting complexes [10]. In addition, these compounds possess sufficient stability towards heat and light. Hence, cyclometalated organo- platinum(II) complexes are regarded as one of the promising p- aromatic materials, which could find a wide range of applications in the fields of optoelectronics, sensors, bioimaging materials and anticancer drugs.
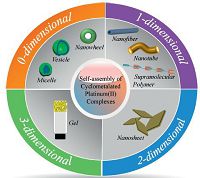
Self-assembly has emerged as an excellent tool to create supramolecular architectures with tunable properties, which underpin the role of solvent-directed approach for the design of functional materials [11]. In this respect, cyclometalated organoplatinum(II) complex is an appealing building block, since it assigns fascinating properties to the resulting assemblies. First, the square planar geometry of the d8 transition metal ions, Pt(II), facilitates the combination of Pt-Pt, π-π, and other non-covalent interactions in a cooperative manner [12]. Second, during the selfassembly process, Pt(II)-Pt(II) orbital overlapping could result in the bathochromic shifts of absorption and emission peaks towards red and NIR regions, respectively, which belong to biologically interesting spectral windows [13]. Third, packingofthe monomers in the fixed assemblies leads to the decrease of non-radiative processes. As a consequence, aggregation induced emission enhancement behaviors could be encountered in some cases [14]. Fourth, on-off switching of the metal center reactivity could be realized, when stimuli-responsiveness is imparted to the resulting supramolecular assemblies [15]. Therefore, self-assembly of cyclometalated platinum(II) complexes into well-ordered supramolecular architectures has been widely explored over the last decades. Depending on the self-assembling morphologies, the assemblies could be divided into 0-D (such as micelles [16, 17], vesicles [18-20]), 1-D (such as fibers [21-26], tubules [27], and linear supramolecular polymers [28-30]), 2-D (such as nanosheets [31, 32]), and 3-D (such as gels [33-48]) structures (Fig. 1). Due to the space limitation, herein we will mainly focus on the recent developments and contributions in the relating fields. Readers could refer to some nice reviews for more detailed information [1-5].
|
Download:
|
| Figure 1. Schematic representation for the self-assembly of cyclometalated platinum(II) complexes into various morphologies. | |
2. 0-D assemblies 2.1. Micelles
Yam and coworkers have designed a variety of triblock poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(- ethylene oxide) (PEO-PPO-PEO) copolymers, which are end- functionalized with alkynylplatinum(II) terpyridine moieties [17]. In light of the fact that PEO-PPO-PEO copolymers display temperature-induced micellization behaviors, the monomers could undergo reversible aggregation/de-aggregation upon varying the temperature. Such a process is accompanied with drastic absorption and emission signal changes. Depending on the difference of electrostatic repulsion forces, the critical micelle temperatures for the synthetic monomers range from 21 ℃ to 30 ℃. For one of the synthetic monomers bearing with CH2NMe2 unit, it exhibits pH-responsive NIR emission behavior, ascribing to the protonation/deprotonation of the CH2NMe2 groups. Hence, it could potentially serve as a probe to sensing pH and temperature changes in the cellular micro-environments.
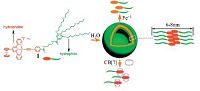
2.2. VesiclesVesicles, representing one of the spherical self-assembled structures, have drawn a lot attention due to their potential applications in drug delivery, nano-reactor, and bionics fields [49]. Wu and coworkers have reported an amphiphilic cationic alkynylplatinum(II) terpyridine complex 1 (Fig. 2), bearing a ferrocene unit on the main ligand, as well as three hydrophilic triethylene glycol (TEG) monomethyl ether chains on the ancillary ligand [18]. In aqueous solution, 1 can self-assemble into bilayer vesicles with the average diameters of 〜100nm, and wall thickness of 〜6-8 nm. Upon adding excess amounts of Fe(ClO4)3 or host molecule CB [7], the vesicles are completely disrupted, leading to the significant spectroscopic and color changes. Alternatively, the reversible assembly/disassembly of vesicles could be realized by in situ electrochemical processes, primarily due to the conversion between ferrocene state and ferrocenium states for 1.
|
Download:
|
| Figure 2. Schematic representation for the reversible assembly process of the cationic platinum(II) amphiphilic molecule 1. Reproduced with permission [18], Copyright 2012, The Royal Society of Chemistry. | |
2.3. Nanowheels
Che and coworkers have reported that neutral monomer 2 and cationic monomer 3 could bind with each other to form a hybrid complex 2/3 (Fig. 3), which further self-assemble into nanowires in the concentrated acetone solution [50]. Upon further replacing the chloride ligand on 2 with acetonitrile, the final morphology of the resulting complex is significantly influenced through a wire-to- wheel metamorphism process [51]. Moreover, higher reaction temperatures (up to 70 ℃) and excess amount of NH4PF6 facilitate to the formation of nano-wheel structures. Hence, it represents an elegant example for morphological evolution of the self-assembled structures, which is conveniently accomplished via ligand- replacement reaction.

|
Download:
|
| Figure 3. (a) Chemical structures of monomers 2 and 3 as well as the resulting hybrid complexes. (b) Diagram illustrating the possible mechanism for the wire-to-wheel metamorphism. Reproduced with permission [51], Copyright 2008, Wiley-VCH. | |
3. 1-D assemblies 3.1. Nanofibers
In 2006, Che and coworkers have reported the formation of micrometer-scale nanowires based on luminescent [Pt(CNtBu)2(CN)2] moiety, which are conveniently obtained by evaporating an acetonitrile solution of [Pt(CNtBu)2(CN)2] on a solid substrate [21]. The resulting 1D nanostructures exhibit high length/diameter aspect ratios (diameter ≈ 25 mm, length > 1000 mm). Injection reprecipitation approach could be further employed to modulate the diameters of the luminescent wires. At room temperature, [Pt(CNtBu)2(CN)2] nanowires exhibit intense emission peaks centered at 560 nm (excitation at 365 nm), which is absent for the acetonitrile solution under the same conditions. Deeper insights into the self-assembly mechanism illustrates that Pt(II)-Pt(II) interactions is not only the main driving force for the formation of 1D nanostructures, but also responsible for the unique green emission signals of the self-assembled nanowires.
Later on, De Cola and coworkers have designed and synthesized a blue-emitting neutral Pt(II) complex, which contains a formally dianionic N-donor tridentate chromophoric ligand [22]. Such a complex displays great tendency to aggregate into micrometerlong and highly crystalline fibers. The aggregates efficiently emit polarized yellow-orange light, with a remarkably high PLQY value of 74%, which is potentially interesting for polarized light-emitting devices applications. On the basis of these results, Manners and coworkers have further gained precise control over the size of the supramolecular nanofibers, with the utilization of a seeded growth approach (Fig. 4) [24]. Briefly, under the sonication condition, the seed fibers are firstly prepared via the living CDSA (crystallizationdriven self-assembly) approach. Further addition of 4 into the seeded fibers (Ln = 60 nm) in acetonitrile leads to the formation of uniform nanofibers with longer length (Ln = 150 nm).

|
Download:
|
| Figure 4. (a) Schematic representation of the formation of seeded fibers by selfassembly of 4. (b) Further formation of elongated nanofibers via the CDSA approach. (c) Linear relationship between the average length (Ln) of elongated fibers and the unimer to seed mass ratio. Reproduced with permission [24], Copyright 2015, The Royal Society of Chemistry. | |
3.2. Nanotubes
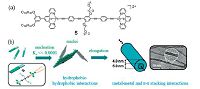
Tubular nanostructures, due to their unique geometry and topological properties, represent a fascinating type of linear assemblies with high degree of complexity [52]. Yam and coworkers have recently reported a dinuclear alkynylplatinum(II) terpyridine complex 5, bearing with two hydrophilic oligo(para- phenylene ethynylene) chains (Fig. 5) [27]. When casting the DMSO solution on copper grid, it tends to aggregate into tubular architectures, with a uniform diameter of 〜13.0 nm and length of 2.0 - 5.5 mm, which represents the first example of tubular nanostructures assembled from square-planar organoplatinum(II) complex. Additionally, the unique optical behaviors of the chromophores have allowed the study of their assembly processes, in which a cooperative supramolecular polymerization mechanism has been elucidated. Hence, it provides an in-depth understanding into the self-assembly process that occurs through non-covalent Pt(II)-Pt(II) and π-π stacking interactions.
|
Download:
|
| Figure 5. (a) Molecular structure of the dinuclear alkynylplatinum(II) complexe 5. (b) Proposed self-assembly model for 5 to form nanotubes by nucleation and elongation processes. Reproduced with permission [27], Copyright 2016, The National Academy of Sciences. | |
3.3. Supramolecular polymer
Supramolecular polymers are defined as polymeric arrays held together by reversible non-covalent interactions, in which the fundamental connecting motif exerts considerable influence on their macroscopic properties [53-58]. An ideal non-covalent recognition motif should concomitantly fulfill high complexation directionality, strong binding affinity and stimuli-sensitive responsiveness. On this account, electron donor-acceptor interactions, which are the intrinsic non-covalent forces between π- conjugated electron-rich and -deficient units, have been seldom exploited for the construction of supramolecular polymers, due to their non-specific and non-directional complexation issues [59].
To address these problems, our research group has recently developed the "tweezering directed self-assembly" strategy for the construction of well-defined donor-acceptor supramolecular polymers [28]. In detail, a heteroditopic AB-type monomer 6 is designed, which bears bis[alkynylplatinum(II)] terpyridine molecular tweezer and pyrene units on both ends (Fig. 6). Considering that the two electron-deficient alkynylplatinum(II) terpyridine pincers on the molecular tweezer facilitates the encapsulation of electron-rich pyrene guest, the resulting heteroditopic monomer 6 could efficiently assemble into linear supramolecular polymers in a head-to-tail fashion. Upon addition of anthracene derivative as the chain stopper, the resulting supramolecular polymers could be disassembled to a large extent. When bis(2-methoxyethyl) dicyanofumarate is successively added, it undergoes quantitative Diels-Alder reaction with the chain-stopper unit, thereby leading to the restore of the supramolecular polymers with a highly controlled manner. Later on, we have also anchored alkynylpla- tinum(II) terpyridine molecular tweezer/pyrene recognition motif on both chain-ends of telechelic polycaprolactone [29]. As a consequence, high-molecular-weight supramolecular polymers could be fabricated vianon-covalent chain extension, which demonstrate fascinating rheological and thermal properties.

|
Download:
|
| Figure 6. Schematic representation for the formation of donor-acceptor-type supramolecular polymers on the basis ofbis[alkynylplatinum(II)] terpyridine molecular tweezer/ pyrene recognition motif (shown in the frame). Reproduced with permission [28], Copyright 2014, Wiley-VCH. | |
Notably, the "tweezering directed self-assembly" strategy displays sufficient compatibility to solvent polarity changes as well as embedded functional units. In another example, we have introduced benzo-21-crown-7/secondary ammonium salt recognition motif to the supramolecular tweezering system [30]. These two non-covalent motifs demonstrate orthogonal recognition behaviors in chloroform/acetonitrile solution, facilitating to the construction of A2B3-type supramolecular hyperbranched polymers. Moreover, stimuli-responsiveness of the resulting polymers could be realized via the addition of KPF6, since the metal ion exhibits a higher affinity toward B21C7 than the ammonium salt unit. Based on the above studies, the precise supramolecular polymerization of p-conjugat- ed donor and acceptor moieties via "tweezering directed selfassembly" strategy demonstrates promising prospects for the applications in nano-sized optoelectronic devices.
The supramolecular polymerization approach could be further utilized for the fabrication of organic-inorganic hybrid materials. For example, Yam and co-workers have recently demonstrated the aggregation of gold nanorods (GNRs) into chain-like nanostructures, which is directed by dimeric alkynylplatinum(II) terpyridine complexation [60]. Depending on the concentration of the Pt(II) complexes, the in situ deprotected complex is preferentially attached at the ends of the gold nanorods (GNRs) and thereby induce the aggregation of GNRs in an "end-to-end" manner, which could be elaborately characterized by electron microscopy, energy dispersed X-ray (EDX) analysis, and UV-vis spectroscopic measurements.
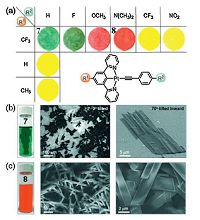
4. 2-D assembliesTwo-dimensional (2-D) nanostructures, denoting the planar structures with a thickness less than 100 nm and lateral dimensions a few orders of magnitude greater than their thickness, have aroused significant interests [61]. Although many 2-D inorganic nanostructures such as graphene and titania nanosheets have been widely reported, much less attempts have been made to well-defined 2-D organometallic nanostructures. Che and coworkers have reported a series of neutral alkynylplatinum(II) diphenylpyridine complexes (Fig. 7), which are capable of forming free-standing and crystalline nanosheets via the reprecipitation method (denoting the addition of excessive amount of apolar solvents such as n-hexane or cyclohexane into CH2Cl2 solution of the organoplatinum(II) complexes) [31]. When CF3 group is located on the main ligand, the resulting pincer-type complexes display a variety of colors such as bright yellow, deep red (compound 8), and dark green (compound 7) in the solid state, depending on the substituents on the ancillary ligand. Such phenomena could be explained by the difference in Pt(II)-Pt(II) interactions, which are significantly influenced by the distinct packing modes in the crystalline structures. Interestingly, these quasi-2D nanosheets display near infrared phosphorescence and light-modulated conductivity properties, which are promising for organometallic- based optoelectronics applications.
|
Download:
|
| Figure 7. (a) Chemical structures and solid-state colors of a series of charge-neutral organoplatinum(II) complexes. (b) and (c) are photographs of dispersions in CH2O2/ n-hexane and SEM images of compounds 7 and 8, respectively. Reproduced with permission [31], Copyright 2009, Wiley-VCH. | |
In another example, Yam and coworkers have designed and synthesized various amphiphilic sulfonate-pendant alkynylplati- num(II) complexes, bearing an anionic bzimpy moiety (bzimpy denotes 2, 6-bis(N-alkylbenzimidazol-2-yl)pyridine) as the tridentate ligand [16]. With the absence of long alkyl chain on the rigid organoplatinum(II) monomer, it tends to assemble into sheet-like nanostructures in aqueous media, accompanying with the appearance of 3MMLCT emission bands in the infrared region. Upon increasing the alkyl chain length on the monomer structure, gradual bathochromic shift of the maximum emission band could be observed, which was attributed to the formation of fibrous nanostructures. The different morphology was rationalized in terms of the packing parameter, which correlates molecular structures very well with the self-assembled morphologies. Therefore, such a study would benefit for understanding the molecular geometric criteria required to pack molecules in the specific shape.
5. 3-D assembliesSelf-assembly of π-organoplatinum molecules into gel materials through weak non-covalent interactions has been a hot topic over the past decades, which shows the capability to convert the information from the molecular level to macroscopic levels. Yam et al. have firstly reported the alkynylplatinum(II) terpyridyl organo-gelators, with the decoration of hydrophobic long alkyl chains on the pheriphery [47]. Due to the cooperative participation of Pt-Pt, π-π, and van der Waals' forces, they are capable of forming stable thermo-reversible metallogels in organic solvents such as dodecance, toluene and DMSO. Based on the electron microscopy measurements, the xerogels show bundling fibrous structures on the micrometer scale, thus indicating the formation of three-dimensional, entangled 3D networks in a hierarchical manner. The counter-anions on the organo-platinum structures exert considerate impacts on the color of the resulting metallogels, since they could govern the degree of aggregation and the extent of Pt-Pt and π-π interactions involved in the gelation process. In a similar manner, the same group has also constructed supramolec- ular gels on the basis of 2, 6-bis(N-alkylbenzimidazol-2'-yl)pyr- idine alkynylplatinum(II) complex 9 (Fig. 8) [36]. Interestingly, such a system shows strong luminescence enhancement upon a gel-to-sol phase transition upon increasing the temperature. This unusual behavior is rarely encountered, which represents a sharp contrast to that exhibited by other typical thermotropic organogels. The unique luminescence enhancement is due to the removal of restricted molecular geometry that favors the formation of aggregate species in the sol form at high temperature. In another study, De Cola et al. have taken full advantage of the high emission properties of the self-assembled organoplatinum complex (photoluminescence quantum yields: up to 87% in thin films and 90% in gel state), which could potentially serve as the dopants for OLEDs applications [14].

|
Download:
|
| Figure 8. Structure of the alkynylplatinum(II) bzimpy complexe 9, and its strong luminescence enhancement upon a gel-to-sol phase transition. Reproduced with permission [36], Copyright 2009, American Chemical Society. | |
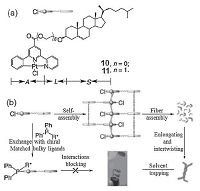
Alternatively, the organogelators could be designed by linking cholesterol units to the organo-platinum(II) complexes. For example, Yi and coworkers have prepared the cholesterol-attached alkynylplatinum(II) terpyridine complex, which can self-assemble into supramolecular gels in DMSO [62]. They found that the steric hindrance on the terpyridine unit would affect the gelation capabilities and emission properties. Tu et al. have also designed such ALS (aromatic-linker-steroid)-type gelators 10-11 with a chloro alkynylplatinum(II) core (Fig. 9), which could efficiently encage a variety of aprotic solvents such as chloroform, dichloroethane, dimethylacetamide, and benzonitrile [37]. The chloride unit coordinated to platinum pincer complexes could undergo ligand exchange by bulky phosphine ligands, which leads to huge steric hindrance and thereby induces the collapse of the organo-gels. More excitingly, chiral discrimination of (R)- and (S)- binap enantiomers could be realized, through a visually enantio- controlled breakup of the resulting metallogels.
|
Download:
|
| Figure 9. (a) Structures of the ALS-type Pt(II) pincer complexes. (b) Suggested gel assembly and gel collapsing process through interactions hampered by coordination of bulky phosphine ligands. Reproduced with permission [37], Copyright 2011, Wiley-VCH. | |
On the other hand, when hydrophilic PEG chain, in replace of the aformentioned hydrophobic alkyl chains or steroids, are attached on the periphery of the Pt(II) complex, it is prone to form hydrogels under certain conditions. For example, De Cola etal.have synthesized a neutral, water-soluble Pt(II) complex [42]. It shows the tendency to form supramolecular aggregate in aqueous solutions, the luminescence of which is not quenched by molecular oxygen. They can further obtain phosphorescent hydrogels utilizing host-guest interactions between cyclodextrins and the tetraethylene glycol tails of the Pt(II) complex, which features with host-dependent emission properties.
Che and coworkers have reported that organoplatinum(II) salts could form 1-D higher-order liquid crystalline supramolecular polyelectrolytes in water [63]. Based on these findings, they have further synthesized dinuclear cyclometalated platinum(II) complexes, which were covalently connected by flexible oligo(ox- yethylene) chain with variable length [34]. When adding small amounts of the dinuclear complexes into the mononuclear assemblies, the former ones could act as the supramolecular cross-linkers, facilitating to the formation of two-component hydrogels. Moreover, upon changing the ratio of the mono- and dinuclear complexes, it could induce the reversible sol-gel transition with distinct color and luminescent changes.
Stimuli-responsive gels undergo reversible and controlled changes such as fluidity, viscoelasticity and luminescence in response to external stimuli [64]. Naota and co-workers have reported a chiral, clothespin-shaped binuclear trans-bis(salicylal- diminato) Pt(II) complex 12 connected by flexible methylene spacers (Fig. 10) [65]. Upon irradiation with ultrasound for a few seconds, hetero-chiral, interpenetrative dimer structure favors to form and thereby leads to the further supramolecular polymerization process. As a result, sol-to-gel conversation for 12 occurs immediately. Moreover, the stable gels were converted back to the solution states after heating above Tgel. This example provides a new and non-chemical approach for instant, remote, and precise control of properties of gels.

|
Download:
|
| Figure 10. Chemical structure of clothespin-shaped molecular 12 and its ultrasoundinduced sol-to-gel phase transition behaviors. Reproduced with permission [65], Copyright 2011,American Chemical Society. | |
6. Interconversion among different morphologies
The elaborate manipulation of multiple, competing selfassembly pathways remains an unsolved challenge. Yam and coworkers have demonstrated an interesting example for morphology conversion between vesicles and nanofibers induced by solvent composition (Fig. 11) [19]. Specifically, for the amphiphilic alkynylplatinum(II) monomer 13 bearing a bzimpy moiety, bilayered vesicles structures have been observed at high water content. A plausible explanation is that charged sulfonate groups are pointing outward in water, whilst the hydrophobic Pt(bzimpy) moieties would be forced to pack together to avoid unfavorable contact with water. Upon adding equivalent amount of acetone into water, it is expected to disperse Pt(bzimpy) moieties in the monomeric state. When acetone content is further increased (9:1 acetone:water), the sulfonate groups are no longer well solvated by the organic solvent. As a consequence, the sulfonate ionic heads start to aggregate and pull the complexes into close proximity, leading to their aggregation into nanofibers. More interestingly, the morphological transformation was accompanied with a neat variation for the optical signals, which could be directly monitored by the naked eye. Specifically, it shows red color in aqueous solution, whilst blue color is visualized 9:1 acetone:water, both of which are distinct from the monomeric state (yellow color in 1:1 acetone:water). In another example, the same group has synthesized an amphiphilic alkynylplatinum(II) terpyridine complex functionalized with polyhedral oligomeric silsesquioxanes (POSS) moieties, which could also self-assemble into various distinguishable nanostructures with interesting morphological transformation from rings to rods under different solvent conditions [66].

|
Download:
|
| Figure 11. UV-vis absorption and color changes of 13 upon increasing the acetone content in water. The morphological transformation from vesicles to nanofibers has been demonstrated by TEM. Reproduced with permission [19], Copyright 2011, American Chemical Society. | |
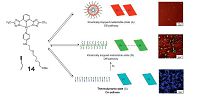
Besides the precise tuning and directing of such morphology transitions, another challenge lies in that how to monitor the interchanges of the self-assembling structures in real time. Very recently, De Cola and coworkers have addressed these challenges in an elegant manner, by exploring the self-assembly pathways of an amphiphilic platinum(II) coordination compound 14 into different morphologies (Fig. 12) [23]. In detail, 14 first aggregates into the kinetically metastable state A (soft nanoparticle), as manifested by the orange phosphorescence emission due to the formation of directional metallophilic (Pt-Pt) interactions. Over a few weeks, it gradually converts to the thermodynamically stable state C, which shows micrometer long ribbon-like structures as well as blue-emitting properties. The conversion process from kinetic to thermodynamic states is rather slow, which can be sped up by increasing the relative volume of the organic solvent in water. Detailed kinetic studies illustrate the presence of a transient state B, which represents a second off-pathway isoform. With the confocal fluorescence microscopic imaging technique, it is possible to visualize these three assemblies in real time, by taking advantage of the photo-physical and morphological differences for each state. These findings demonstrate that chemists are gaining better understanding of the complexed self-assembly equilibria, and progressing in the ability to control or fine-tune them through external inputs.
|
Download:
|
| Figure 12. Illustration of the landscape for supramolecular assembly of 14,together with the corresponding snapshots of the time-dependent morphologies. Reproduced with permission [23],Copyright 2015,Macmillan Publishers Limited. | |
7. Conclusion
In summary, cyclometalated organoplatinum(II) complexes are demonstrated to be efficient building blocks for the construction of various types of supramolecular assemblies. During the selfassembly processes, non-covalent Pt-Pt and π-π interactions are the main driving forces and play a crucial role in controlling the structures and functions of the resulting assemblies. Although a large number of supramolecular assemblies have been constructed, there are still many uncharted terrains for chemists to exploit. For example, much more attention should be paid to the stimuli-responsive properties of the resulting organoplatinum(II) assemblies, especially via a non-chemical way. Moreover, the establishment of structure-morphology relationships for the resulting supramolecular assemblies should be emphasized. Finally, deeper insights into the thermodynamics and kinetics of self-assembly are still required, which will enable chemists to generate supramolecular structures and materials through a specific, chosen self-assembly pathway.
Acknowledgments This work was supported by the National Natural Science Foundation of China (No. 21274139)and the Fundamental Research Funds for the Central Universities (Nos. WK3450000001, WK2060200012).| [1] | I. Eryazici, C.N. Moorefield, G.R. Newkome. Square-planar Pd(II), Pt(II), and Au(III) terpyridine complexes: their syntheses, physical properties, supramolecular constructs, and biomedical activities. Chem. Rev. 108 (2008) 1834–1895. DOI:10.1021/cr0781059 |
| [2] | K.M.C. Wong, V.W.W. Yam. Self-assembly of luminescent alkynylplatinum(II) terpyridyl complexes: modulation of photophysical properties through aggregation behavior. Acc. Chem. Res. 44 (2011) 424–434. DOI:10.1021/ar100130j |
| [3] | A. Aliprandi, D. Genovese, M. Mauro, L. De Cola. Recent advances in phosphorescent Pt(II) complexes featuring metallophilic interactions: properties and applications. Chem. Lett. 44 (2015) 1152–1169. DOI:10.1246/cl.150592 |
| [4] | K.M.C. Wong, M.M.Y. Chan, V.W.W. Yam. Supramolecular assembly of metalligand chromophores for sensing and phosphorescent OLED applications. Adv. Mater. 26 (2014) 5558–5568. DOI:10.1002/adma.201306327 |
| [5] | V.W.W. Yam, V.K.M. Au, S.Y.L. Leung. Light-emitting self-assembled materials based on d8 and d10 transition metal complexes. Chem. Rev. 115 (2015) 7589–7728. DOI:10.1021/acs.chemrev.5b00074 |
| [6] | T.C. Johnstone, K. Suntharalingam, S.J. Lippard. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 116 (2016) 3436–3486. DOI:10.1021/acs.chemrev.5b00597 |
| [7] | J.J. Wilson, S.J. Lippard. Synthetic methods for the preparation of platinum anticancer complexes. Chem. Rev. 114 (2014) 4470–4495. DOI:10.1021/cr4004314 |
| [8] | J.A.G. Williams, S. Develay, D.L. Rochester, L. Murphy. Optimising the luminescence of platinum(II) complexes and their application in organic light emitting devices (OLEDs). Coord. Chem. Rev. 252 (2008) 2596–2611. DOI:10.1016/j.ccr.2008.03.014 |
| [9] | K.M.C. Wong, V.W.W. Yam. Luminescence platinum(II) terpyridyl complexes-from fundamental studies to sensory functions. Coord. Chem. Rev. 251 (2007) 2477–2488. DOI:10.1016/j.ccr.2007.02.003 |
| [10] | Y. Li, L. Zhao, A.Y.Y. Tam, et al. Luminescent amphiphilic 2,6-Bis (1, 2, 3-triazol-4-yl) pyridine-platinum (II) complexes: synthesis, characterization, electrochemical, photophysical, and Langmuir-Blodgett film-formation studies. Chem. Eur. J. 19 (2013) 14496–14505. DOI:10.1002/chem.v19.43 |
| [11] | M.D. Ward, P.R. Raithby. Functional behaviour from controlled self-assembly: challenges and prospects. Chem. Soc. Rev. 42 (2013) 1619–1636. DOI:10.1039/C2CS35123D |
| [12] | Y.J. Tian, E.W. Meijer, F. Wang. Cooperative self-assembly of platinum(II) acetylide complexes. Chem. Commun. 49 (2013) 9197–9199. DOI:10.1039/c3cc44997a |
| [13] | C.Y.S. Chung, S.P.Y. Li, M.W. Louie, K.K.W. Lob, V.W.W. Yam. Induced self-assembly and disassembly of water-soluble alkynylplatinum(II) terpyridyl complexes with "switchable" near-infrared (NIR) emission modulated by metal-metal interactions over physiological pH: demonstration of pH-responsive NIR luminescent probes in cell-imaging studies. Chem. Sci. 4 (2013) 2453–2462. DOI:10.1039/c3sc50196e |
| [14] | C.A. Strassert, C.H. Chien, M.D.G. Lopez, et al. Switching on luminescence by the self-assembly of a platinum(II) complex into gelating nanofibers and electroluminescent films. Angew. Chem. Int. Ed. 50 (2011) 946–950. DOI:10.1002/anie.201003818 |
| [15] | M. Mauro, A. Aliprandi, D. Septiadi, N.S. Kehr, L. De Cola. When self-assembly meets biology: luminescent platinum complexes for imaging applications. Chem. Soc. Rev. 43 (2014) 4144–4166. DOI:10.1039/c3cs60453e |
| [16] | C. Po, A.Y.Y. Tam, V.W.W. Yam. Tuning of spectroscopic properties via variation of the alkyl chain length: a systematic study of molecular structural changes on selfassembly of amphiphilic sulfonate-pendant platinum(II) bzimpy complexes in aqueous medium. Chem. Sci. 5 (2014) 2688–2695. DOI:10.1039/c4sc00411f |
| [17] | C.Y.S. Chung, V.W.W. Yam. Dual pH- and temperature-responsive metallosupramolecular block copolymers with tunable critical micelle temperature by modulation of the self-assembly of NIR-emissive alkynylplatinum(II) complexes induced by changes in hydrophilicity and electrostatic effects. Chem. Eur. J. 19 (2013) 13182–13192. DOI:10.1002/chem.v19.39 |
| [18] | L.B. Xing, S. Yu, X.J. Wang, et al. Reversible multistimuli-responsive vesicles formed by an amphiphilic cationic platinum(II) terpyridyl complex with a ferrocene unit in water. Chem. Commun. 48 (2012) 10886–10888. DOI:10.1039/c2cc35960j |
| [19] | C. Po, A.Y.Y. Tam, K.M.C. Wong, V.W.W. Yam. Supramolecular self-assembly of amphiphilic anionic platinum(II) complexes: a correlation between spectroscopic and morphological properties. J. Am. Chem. Soc. 133 (2011) 12136–12143. DOI:10.1021/ja203920w |
| [20] | K.Y. Liu, L.Y. Meng, S.L. Mo, et al. Colour change and luminescence enhancement in a cholesterol-based terpyridyl platinum metallogel via sonication. J. Mater. Chem. C 1 (2013) 1753–1762. DOI:10.1039/c2tc00643j |
| [21] | Y. Sun, K. Ye, H. Zhang, et al. Luminescent one-dimensional nanoscale materials with PtII…PtII interactions. Angew. Chem. Int. Ed. 45 (2006) 5610–5613. DOI:10.1002/(ISSN)1521-3773 |
| [22] | M. Mauro, A. Aliprandi, C. Cebrián, et al. Self-assembly of a neutral platinum(II) complex into highly emitting microcrystalline fibers through metallophilic interactions. Chem. Commun. 50 (2014) 7269–7272. DOI:10.1039/c4cc01045k |
| [23] | A. Aliprandi, M. Mauro, L. De Cola. Controlling and imaging biomimetic selfassembly. Nat. Chem. 8 (2016) 10–15. |
| [24] | M.E. Robinson, D.J. Lunn, A. Nazemi, et al. Length control of supramolecular polymeric nanofibers based on stacked planar platinum(II) complexes by seededgrowth. Chem. Commun. 51 (2015) 15921–15924. DOI:10.1039/C5CC06606A |
| [25] | M.Y. Yuen, V.A.L. Roy, W. Lu, et al. Semiconducting and electroluminescent nanowires self-assembled from organoplatinum(II) complexes. Angew. Chem. Int. Ed. 47 (2008) 9895–9899. DOI:10.1002/anie.v47:51 |
| [26] | X.S. Xiao, W.L. Kwong, X.G. Guan, et al. Platinum(II) and Gold(III) allenylidene complexes: phosphorescence, self-assembled nanostructures and cytotoxicity. Chem. Eur. J. 19 (2013) 9457–9462. DOI:10.1002/chem.201301481 |
| [27] | S.Y.L. Leung, K.M.C. Wong, V.W.W. Yam. Self-assembly of alkynylplatinum(II) terpyridine amphiphiles into nanostructures via steric control and metal-metal interactions. Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 2845–2850. DOI:10.1073/pnas.1601673113 |
| [28] | Y.K. Tian, Y.G. Shi, Z.S. Yang, F. Wang. Responsive supramolecular polymers based on the bis[alkynylplatinum(II)] terpyridine molecular tweezer/arene recognition motif. Angew. Chem. Int. Ed. 53 (2014) 6090–6094. DOI:10.1002/anie.201402192 |
| [29] | H.Q. Liu, X.H. Han, Z.C. Gao, Z. Gao, F. Wang. Linear supramolecular polymers via connecting telechelic polycaprolactone through alkynylplatinum(ii) terpyridine molecular tweezer/pyrene recognition motif. Macromol. Rapid Commun. 37 (2016) 718–724. DOI:10.1002/marc.v37.8 |
| [30] | Y.K. Tian, Z.S. Yang, X.Q. Lv, R.S. Yao, F. Wang. Construction of supramolecular hyperbranched polymers via the "tweezering directed self-assembly" strategy. Chem. Commun. 50 (2014) 9477–9480. DOI:10.1039/C4CC03158J |
| [31] | Y. Chen, K. Li, W. Lu, et al. Photoresponsive supramolecular organometallic nanosheets induced by PtII…PtII and C-H…π interactions. Angew. Chem. Int. Ed. 48 (2009) 9909–9913. DOI:10.1002/anie.v48:52 |
| [32] | C.M. Che, C.F. Chow, M.Y. Yuen, et al. Single microcrystals of organoplatinum(II) complexes with high charge-carrier mobility. Chem. Sci. 2 (2011) 216–220. DOI:10.1039/C0SC00479K |
| [33] | F. Camerel, R. Ziessel, B. Donnio, et al. Formation of gels and liquid crystals induced by PtI…Pt and π-π* interactions in luminescent σ-alkynyl platinum(II) terpyridine complexes. Angew. Chem. Int. Ed. 46 (2007) 2659–2662. DOI:10.1002/(ISSN)1521-3773 |
| [34] | X.S. Xiao, W. Lu, C.M. Che. Phosphorescent nematic hydrogels and chromonic mesophases driven by intra- and intermolecular interactions of bridged dinuclear cyclometalated platinum(II) complexes. Chem. Sci. 5 (2014) 2482–2488. DOI:10.1039/c4sc00143e |
| [35] | M. Shirakawa, N. Fujita, T. Tani, et al. Organogels of 8-quinolinol/metal(II)-chelate derivatives that show electron- and light-emitting properties. Chem. Eur. J. 13 (2007) 4155–4162. DOI:10.1002/(ISSN)1521-3765 |
| [36] | A.Y.Y. Tam, K.M.C. Wong, V.W.W. Yam. Unusual luminescence enhancement of metallogels of alkynylplatinum(II) 2,6-bis(N-alkylbenzimidazol-2'-yl) pyridine complexes upon a gel-to-sol phase transition at elevated temperatures. J. Am. Chem. Soc. 131 (2009) 6253–6260. DOI:10.1021/ja900895x |
| [37] | T. Tu, W.W. Fang, X.L. Bao, X.B. Li, K.H. Dötz. Visual chiral recognition through enantioselective metallogel collapsing: synthesis, characterization, and application of platinum-steroid low-molecular-mass gelators. Angew. Chem. Int. Ed. 50 (2011) 6601–6605. DOI:10.1002/anie.v50.29 |
| [38] | A.Y.Y. Tam, V.W.W. Yam. Recent advances in metallogels. Chem. Soc. Rev. 42 (2013) 1540–1567. DOI:10.1039/c2cs35354g |
| [39] | M. Shirakawa, N. Fujita, T. Tani, K. Kaneko, S. Shinkai. Organogel of an 8-quinolinol platinum(II) chelate derivative and its efficient phosphorescence emission effected by inhibition of dioxygen quenching. Chem. Commun. 33 (2005) 4149–4151. |
| [40] | D. Kumaresan, K. Lebkowsky, R.H. Schmehl. Photoinduced charge separation and recombination in solution and in gels of a Pt(II) terpyridyl-naphthalene diimide complex. J. Photochem. Photobio. A Chem. 207 (2009) 86–93. DOI:10.1016/j.jphotochem.2009.03.013 |
| [41] | W. Lu, Y.C. Law, J. Han, et al. A dicationic organoplatinum(II) complex containing a bridging 2,5-Bis-(4-ethynylphenyl)-[1,3,4] oxadiazole ligand behaves as a phosphorescent gelator for organic solvents. Chem. Asian J. 3 (2008) 59–69. DOI:10.1002/(ISSN)1861-471X |
| [42] | N.K. Allampally, M. Bredol, C.A. Strassert, L. De Cola. Highly phosphorescent supramolecular hydrogels based on platinum emitters. Chem. Eur. J. 20 (2014) 16863–16868. DOI:10.1002/chem.201403772 |
| [43] | K.C. Chang, J.L. Lin, Y.T. Shen, et al. Synthesis and photophysical properties of self-assembled metallogels of platinum(II) acetylide complexes with elaborate long-chain pyridine-2,6-dicarboxamides. Chem. Eur. J. 18 (2012) 1312–1321. DOI:10.1002/chem.v18.5 |
| [44] | J.L.L. Tsai, T.T. Zou, J. Liu, et al. Luminescent platinum(II) complexes with selfassembly and anti-cancer properties: hydrogel, pH dependent emission color and sustained-release properties under physiological conditions. Chem. Sci. 6 (2015) 3823–3830. DOI:10.1039/C4SC03635B |
| [45] | A.Y. Tam, K.C. Wong, N.Y. Zhu, G.X. Wang, V.W. Yam. Luminescent alkynylplatinum(II) terpyridyl metallogels stabilized by Pt…Pt, π-π, and hydrophobic-hydrophobic interactions. Langmuir 25 (2009) 8685–8695. DOI:10.1021/la804326c |
| [46] | N.K. Allampally, C.A. Strassert, L. De Cola. Luminescent gels by self-assembling platinum complexes. Dalton Trans. 41 (2012) 13132–13137. DOI:10.1039/c2dt30369h |
| [47] | A.Y.Y. Tam, K.M.C. Wong, G.X. Wang, V.W.W. Yam. Luminescent metallogels of platinum(II) terpyridyl complexes: interplay of metal…metal, π-π and hydrophobic-hydrophobic interactions on gel formation. Chem. Commun. 20 (2007) 2028–2030. |
| [48] | Y. Chen, W. Lu, C.M. Che. Luminescent pincer-type cyclometalated platinum(II) complexes with auxiliary isocyanide ligands: phase-transfer preparation, solvatomorphism, and self-aggregation. Organometallics 32 (2013) 350–353. DOI:10.1021/om300965b |
| [49] | Z. Al-Ahmady, K. Kostarelos. Chemical components for the design of temperatureresponsive vesicles as cancer therapeutics. Chem. Rev. 116 (2016) 3883–3918. DOI:10.1021/acs.chemrev.5b00578 |
| [50] | W. Lu, V.A.L. Roy, C.M. Che. Self-assembled nanostructures with tridentate cyclometalated platinum(II) complexes. Chem. Commun. 38 (2006) 3972–3974. |
| [51] | W. Lu, S.S.Y. Chui, K.M. Ng, C.M. Che. A submicrometer wire-to-wheel metamorphism of hybrid tridentate cyclometalated platinum(II) complexes. Angew. Chem. Int. Ed. 47 (2008) 4568–4572. DOI:10.1002/(ISSN)1521-3773 |
| [52] | G.T. Barclay, K. Constantopoulos, J. Matisons. Nanotubes self-assembled from amphiphilic molecules via helical intermediates. Chem. Rev. 114 (2014) 10217–10291. DOI:10.1021/cr400085m |
| [53] | T.F.A. De Greef, M.M.J. Smulders, M. Wolffs, et al. Supramolecular polymerization. Chem. Rev. 109 (2009) 5687–5754. DOI:10.1021/cr900181u |
| [54] | S.Y. Dong, B. Zheng, F. Wang, F.H. Huang. Supramolecular polymers constructed from macrocycle-based host-guest molecular recognition motifs. Acc. Chem. Res. 47 (2014) 1982–1994. DOI:10.1021/ar5000456 |
| [55] | L.L. Yang, X.X. Tan, Z.Q. Wang, X. Zhang. Supramolecular polymers: historical development, preparation, characterization, and functions. Chem. Rev. 115 (2015) 7196–7239. DOI:10.1021/cr500633b |
| [56] | J.F. Xu, L.H. Chen, X. Zhang. How to make weak noncovalent interactions stronger. Chem. Eur. J. 21 (2015) 11938–11946. DOI:10.1002/chem.v21.34 |
| [57] | X. Yan, F. Wang, B. Zheng, F. Huang. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 41 (2012) 6042–6065. DOI:10.1039/c2cs35091b |
| [58] | P.F. Wei, X.Z. Yan, F.H. Huang. Supramolecular polymers constructed by orthogonal self-assembly based on host-guest and metal-ligand interactions. Chem. Soc. Rev. 44 (2015) 815–832. DOI:10.1039/C4CS00327F |
| [59] | A. Das, S. Ghosh. Supramolecular assemblies by charge-transfer interactions between donor and acceptor chromophores. Angew. Chem. Int. Ed. 53 (2014) 2038–2054. DOI:10.1002/anie.201307756 |
| [60] | F.C.M. Leung, S.Y.L. Leung, C.Y.S. Chung, V.W.W. Yam. Metal-metal and π-π interactions directed end-to-end assembly of gold nanorods. J. Am. Chem. Soc. 138 (2016) 2989–2992. DOI:10.1021/jacs.6b01382 |
| [61] | M.S. Xu, T. Liang, M.M. Shi, H.Z. Chen. Graphene-like two-dimensional materials. Chem. Rev. 113 (2013) 3766–3798. DOI:10.1021/cr300263a |
| [62] | Y.Y. Mao, K.Y. Liu, L.Y. Meng, et al. Solvent induced helical aggregation in the self-assembly of cholesterol tailed platinum complexes. Soft Matter 10 (2014) 7615–7622. DOI:10.1039/C4SM01213E |
| [63] | W. Lu, Y. Chen, V.A.L. Roy, S.S.Y. Chui, C.M. Che. Supramolecular polymers and chromonic mesophases self-organized from phosphorescent cationic organoplatinum(II) complexes in water. Angew. Chem. Int. Ed. 48 (2009) 7621–7625. DOI:10.1002/anie.v48:41 |
| [64] | S.K. Ahn, R.M. Kasi, S.C. Kim, N. Sharma, Y.X. Zhou. Stimuli-responsive polymer gels. Soft Matter 4 (2008) 1151–1157. DOI:10.1039/b714376a |
| [65] | N. Komiya, T. Muraoka, M. Iida, et al. Ultrasound-induced emission enhancement based on structure-dependent homo-and heterochiral aggregations of chiral binuclear platinum complexes. J. Am. Chem. Soc. 133 (2011) 16054–16061. DOI:10.1021/ja2039369 |
| [66] | H.L. Au-Yeung, S.Y.L. Leung, A.Y.Y. Tam, V.W.W. Yam. Transformable nanostructures of platinum-containing organosilane hybrids: non-covalent self-assembly of polyhedral oligomeric silsesquioxanes assisted by Pt…Pt and π-π stacking interactions of alkynylplatinum(II) terpyridine moieties. J. Am. Chem. Soc. 136 (2014) 17910–17913. DOI:10.1021/ja510048b |
 2016, Vol. 27
2016, Vol. 27 


