Organic conjugated materials have attracted intense attention due to their potential application in low cost,large area,lightweight and flexible optoelectronic devices such as organic field-effect transistors (OFETs),organic light-emitting diodes (OLEDs),organic photovoltaics (OPVs),and sensors [1]. Introduction of heteroatoms such as sulfur,nitrogen and oxygen into the conjugated carbon skeleton endows those conjugated molecules withdiversephotophysicalandelectronicproperties [2]. Moreover,heteroatoms in aromatics provide various intra- and/or intermolecular interactions such as hydrogen bonding,sulfur-sulfur interaction,and dipole-dipole interaction,which afford great effect on molecular packing structures in solid state,leading to improved device performance [3].
Pioneering work on BN-substituted polycyclic aromatic hydrocarbons (PAHs) by Dewar and co-workers in 1958 opened the gate of azaborine chemistry [4]. Since then,a large number of BN- embedded aromatics have been synthesized. Due to the challenges in synthesis and purification of these azaborine compounds,great efforts in the early stage were mainly devoted to the synthetic methods and characterization of chemical properties [5]. As the development of azaborine chemistry,applications ofBN embedded aromatics in biological systems,coordination chemistry and materials science have been exploited [6],which raised more concern about this new family of molecules. Especially,incorporation of BN units renders PAHs to possess new optical and electronic properties,which offers more promise for their application in optoelectronic devices [7]. However,optoelectronic application of BN-embedded aromatics is still in its infancy,which has aroused increasing interests of both chemists and material scientists.
This mini-review article will not discuss much about the history and chemistry of azaborine compounds,which have been already summarized in several recent reviews on azaborine chemistry by Piers et al.,Liu et al. and Pei et al. [8]. It is mainly focused on the recent progresses of BN embedded aromatics for optoelectronic applications in the areas of OFETs,OLEDs,OPVs and chemical sensors. Organized by application fields,the article has summarized three types of BN heterocycles,including BN covalent bond based,BN dative bond based,and disconnected BN substitution based molecules,in which the BN pairs are incorporated into aromatic six-membered rings in different ways (Fig. 1). Properties and applications of other types of BN heterocycles,such as boron dipyrrole (BODIPY) [9],and boron and nitrogen atoms substituted on PAHs separately as electron- withdrawing and donating groups [10],are not discussed in this review.

|
Download:
|
| Figure 1. Three types of azaborine compounds as discussed in this review. | |
2. BN-embedded aromatics for organic field-effect transistors
Replacement of C=C unit in common organic PAHs with its isoelectronic structure B-N unit will create new types of organic semiconductors with different electronic structures and properties. In 2011,Nakamura and co-workers applied Dewar's strategy of Friedel-Crafts-type electrophilic cyclization for the synthesis of BN- substituted PAHs and developed a tandem intramolecular electrophilic arene borylation method (Scheme 1) [11]. Single crystal X-ray analysis revealed that compound 1 adopted a twisted conformation with a torsion angle of 38.87° and exhibited enantiomeric helical structures (P- and M-helix) in the crystal. Time-resolved microwave conductivity (TRMC) measurements showed that compound 1 had an intrinsic hole mobility of 0.07 cm2 V-1 s-1,which is one order of magnitude higher than that of its carbon analog dibenzo[g,p]chry- sene (0.007 cm2 V-1 s-1). The higher hole mobility of compound 1 was thought to be caused by the partial localization of the frontier orbitals induced by BN substitution. An extended framework 2 was also synthesized following the same method yet its carrier mobility was not reported.

|
Download:
|
| Scheme. 1. BN-substituted PAHs 1 and 2 synthesized through tandem electrophilic borylation. | |
In 2012,Nakamura and co-workers used the same strategy to synthesize azaboradibenzo[6]helicene 3,which was obtained as a racemic mixture (Scheme 2) [12]. Compared to the previously reported compound 1,the two more fused benzene rings made the P-and M-enantiomers isolable by chiral HPLC. The packing structures of racemic 3 (rac-3) and enantiopure P-3 were determined by X-ray crystallography. The charge transport properties of the thin films of these compounds were evaluated by the time-of-flight (TOF) method. A hole mobility of 4.6 × 10-4 cm2 V-1 s-1 was measured for rac-3,whereas the enantiopure P-3 showed a higher electron mobility of 4.5 × 10-3 cm2 V-1 s-1 than the hole mobility (7.9 × 10-4 cm2 V-1 s-1). This carrier inversion could be attributed to the different packing structures of the hetero- and homochiral crystals of 3,which resulted in different intermolecular electronic couplings.

|
Download:
|
| Scheme. 2. The synthetic route of BN-embedded compound 3. | |
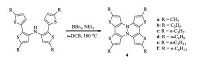
The first OFET device based on azaborine compound was demonstrated by Pei and co-workers in 2013 [13],which was also the first electronic device based on BN-substituted polycyclic aromatics. A systematic study on the influence of alkyl side chain length on the solid-state properties and device performance was thoroughly carried out later [14]. A series of BN-substituted tetrathienonaphthalene derivatives 4 with different alkyl side chain lengths were designed and synthesized (Scheme 3). These compounds were very stable against ambient oxygen and moisture and could be purified by column chromatography on silica gel. They also showed excellent thermal stability,as evidenced by thermogravimetric analysis (TGA) with decomposition temperatures (5% weight loss) in the range of 340-400 ℃. Such remarkable stability was considered essential for device applications. Single crystal analysis of the six compounds revealed that the B-N bond lengths are around 1.46-1.48 Æ,which are the typical values of delocalized BN double bonds in aromatic systems. Nucleus-independent chemical shift (NICS) analysis indicated a moderate aromaticity of central C4BN rings in 4,which is greater than that of BN-substituted dibenzo[g,p]chrysene 1. This increased aromaticity may account for the remarkably high chemical and thermal stability of these compounds. Sig nificantly,OFETs based on 4 were successfully fabricated with a bottom-gate/top-contact (BG/TC) device configuration. The organic layers were thermally evaporated onto the CYTOP-modified Si/SiO2 substrates. Then gold was patterned through a shadow mask as the source/drain electrodes. In the initial study,compound 4c with propyl side chains showed the highest hole mobility of up to 0.15 cm2 V-1 s-1,whereas compound 4f with hexyl side chains only showed the highest hole mobility of 0.03cm2V-1 s-1. The distinct device performance was ascribed to their different packing structures in solid state. The extensive characterization of compounds 4 with methyl to hexyl side chains showed that different alkyl chain length did not change the intrinsic optoelectronic properties of the π-conjugated backbone,but significantly influenced the solid-state properties,including intermolecular packing,energy levels and thin film morphologies,resulting in great difference in device performance. Interestingly,an odd-even effect of alkyl side chains on the thin film morphologies was observed (Fig. 2).
|
Download:
|
| Scheme. 3. Synthesis of BN-embedded compound 4 with different alkyl chain lengths. | |

|
Download:
|
| Figure 2. Surface roughness (square) and average hole mobilities (circle) of the thin films of compounds 4 plotted as a function of alkyl chain length. Reprinted from Ref. [14] with permission from The Royal Society of Chemistry. | |
Thin films of compound 4 with even-numbered alkyl chains generally displayed high surface roughness (RMS) and large grain boundaries,which made the films discontinuous. As a result,4c showed the highest mobility of 0.15 cm2V-1 s-1,but 4b and 4d did not show any FET characteristics. This work opened up the application of BN-substituted polycyclic aromatics in electronic devices,and demonstrated the importance of synergetic consideration of conjugated backbone and side chains in developing novel organic semiconductors.
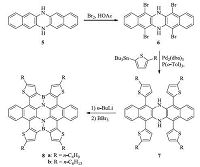
In 2014,Pei and co-workers applied a straightforward strategy to construct large BN-embedded π-systems simply from azaacenes [15]. As shown in Scheme 4,bromination of 5 with Br2 gave compound 6,which was subjected to a Stille coupling reaction to afford 7. Finally,electrophilic borylation reaction produced compounds 8 as yellow solids. This strategy explored azaacenes as a platform to construct BN-doped nanographenes,which is very simple and straightforward. The resulted BN heterosuperbenzene derivatives 8 are the largest BN-embedded polycyclic aromatics up to now.
|
Download:
|
| Scheme. 4. Synthesis of p-extended BN heterosuperbenzene derivatives 8. | |
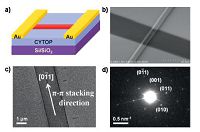
Compounds 8 also showed excellent stability with thermal decomposition temperatures (5% weight loss) of over 450 ℃. The structure of 8b was determined by single crystal X-ray analysis. Due to the steric hindrance among the peripheral rings,the aromatic core of 8b showed two different contorted conformations,which were stacked in a 2:1 ratio to form a columnar packing structure. Concentration-dependent 1H NMR spectroscopy indicated a strong aggregation tendency of compound 8b in solution. One-dimensional (1D) micro-ribbons were readily obtained through a solution process. Micro-ribbon FET devices were fabricated to evaluate the charge transport properties (Fig. 3a and b). The devices exhibited the highest hole mobility of 0.23cm2V-1s-1,with a low threshold of -3V and a current on/off ratio over 104,which is quite impressive for curved aromatics. Transmission electron microscopy (TEM) (Fig. 3c) and selected area electron diffraction (SAED) (Fig. 3d) were performed to in vestigate the packing structure inside the micro-ribbons. It was found that the micro-ribbons were grown along the columnar π-π stacking direction,which is favorable for charge transport. The micro-ribbons also showed photoresponsive electrical conductivity as measured in the same device configuration in a two- wire resistor mode. The current was enhanced significantly under light compared to that under dark. The observed photoconductivity indicated the potential applications of BN-embedded polycyclic aromatics in organic photovoltaics.
|
Download:
|
| Figure 3. (a) Graphic illustration of the micro-ribbon FET device of 8b. (b) SEM image of the micro-ribbon device. (c) TEM image of the micro-ribbon and (d) its corresponding SAED pattern. Reprinted with permission from Ref. [15] . Copyright 2014 American Chemical Society. | |
Besides the small molecule devices,OFET devices based on BN- embedded conjugated polymers were also developed by Pei and coworkers in 2015 [16]. A BN-substituted tetrathienonaphthalene (BNTTN) monomer was designed and synthesized to construct the azaborine-based conjugated polymers P9 and P10 for OFETs through Stille coupling reactions with 2,5-bis(trimethylstannyl)thiophene or 5,5'-bis(trimethylstannyl)-2,2'-bithiophene (Fig. 4). Both polymers exhibited excellent thermal stability with high decomposition temperatures around 400 ℃. OFET devices with a top-gate/bottom- contact (TG/BC) configuration were fabricated based on these polymers,and a hole mobility of up to 0.38cm2V-1s-1 was achieved for P10.

|
Download:
|
| Figure 4. The structures of BN-embedded conjugated polymers P9 and P10. | |
3. BN-embedded aromatics for organic light-emitting diodes
Many BN-embedded aromatics show strong emission,making them good candidates applied in OLED devices. Feng and co-workers developed a series of ladder-type BN-embedded heteroacenes (Fig. 5) [17]. Suzuki coupling reactions between 2,5-dibromo-1,4-phenylenediamine and furan- or thiophene-based boronic esters gave the intermediates 2,5-bis-heterocycle-substituted phenylene- diamines,which further reacted with an excess amount of PhBCl2 using trimethylamine as base to yield the final BN-embedded heteroacenes 11-13. These azaborine aromatics showed good chemical and thermal stability. Compared with their all-carbon analogs,these BN-embedded heteroacenes showed similar geometries but completely different UV-vis absorption features. Moreover,compounds 11-13 exhibited blue luminescence with emission maxima from 393 nm to 418 nm,which makes them good blue emitters in OLEDs. Blue OLED devices based on these BN-embedded heteroacenes were fabricated for the first time,with the maximum external quantum efficiencies (EQEs) around 1.3% to 1.4%,which are comparable to those of devices fabricated on the basis ofheteroatom (e.g.,B and S) containing π-conjugated molecules.
|
Download:
|
| Figure 5. The structures of ladder-type BN-embedded heteroacenes 11-13. | |
Using similar synthetic strategy,1,5,9-triaza-2,6,10-triphenyl- boracoronene (14),a BN-embedded analogue of coronene,was synthesized independently by Pei et al. and Zhang et al. (Fig. 6) [18]. The BN heterocoronene backbone exhibited a fully planar geometry from the single crystal structure analysis. DFT calculations indicated that the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) of both 14 and its carbon analogue are fully delocalized over the whole backbone,however,with different orbital distributions, implying that BN substitution changed the electronic structure significantly. Moreover,the BN substitution resulted in a lowered HOMO level and a raised LUMO level,leading to a bandgap opening up to 0.32 eV. Interestingly,compound 14 showed strong blue fluorescence in chloroform solution with a fluorescence quantum yield of 0.42,whereas its coronene counterpart emitted in the UV region with a much lower quantum yield (Φf = 0.098 in chloroform). The blue emission property of 14 makes it a possible candidate for light-emitting materials in OLEDs.

|
Download:
|
| Figure 6. The structure of compound 14 and its absorption and fluorescence spectra in chloroform. Insert pictures: left,in solution; right,fluorescence. Reprinted with permission from Ref. [18b]. Copyright 2014 American Chemical Society. | |
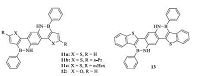
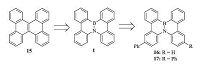
Besides the light-emitting materials in OLEDs,BN-embedded aromatics were also explored as host materials for phosphorescent OLEDs. In 2014,Nakamura and co-workers fabricated phosphorescent OLEDs using 4b-aza-12b-boradibenzo[g,p]chrysene 1 and its phenylated derivatives 16 and 17 as host materials (Fig. 7) [19]. Experimental and computational results indicated that the replacement of C=C unit with the B-N unit hardly changed singlet- singlet excitation energy (ES),but significantly increased singlet- triplet excitation energy (ET) through suppression of the exchange interaction between SOMOs. The carrier-transport properties of compounds 1,15,16,17 were evaluated by TOF measurement. All the compounds showed ambipolar carrier transport characteristic. In particular,compound 1 showed the highest hole (4.1 × 10-4cm2 V-1 s-1) and electron (2.3 × 10-3cm2V-1 s-1) mobilities. The large ET value and ambipolar carrier transportabilities make these BN-embedded aromatics good host materials for phosphorescent OLEDs. As a result,the phosphorescent OLEDs with BN-embedded aromatics as host materials exhibited a superior performance over the 4,4'-bis(N-carbazolyl)-1,1'-biphenyl (CBP,a reference host material) device in terms of the driving voltage (V1000),current efficiency (ηc,1000),power efficiency (ηp,1000),and the external quantum efficiency (EQE1000). In addition,the introduction of one or two phenyl groups (16,17) significantly improved the device lifetime,which could be attributed to their high Tg.
|
Download:
|
| Figure 7. The structures of compounds 15-17 for phosphorescent organic light-emitting diodes. | |
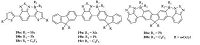
In 2015,Turner and co-workers reported a synthetic strategy for modifying a series of donor-acceptor (D-A) molecules by directed C-H electrophilic borylation using BCl3 and benzothiadiazole (BT) (Fig. 8) [20]. Though the borylated halide species were stable to non-protic Lewis bases,they still underwent slow hydrolysis. In situ functionalization of boron in the borylated halide species using zinc and aluminum organometallic nucleophiles to install exocyclic aryl and alkyl groups could further improve the stability of these compounds. The resulted fused borylated benzothiadiazole containing D-A materials exhibited significantly lowered LUMO levels and reduced bandgaps compared to the unborylated precursors. In contrast to the thiophene derivatives 18,19 and 20 were proved to be significantly more emissive. OLED devices were fabricated using these BN-embedded aromatics as emitters,and those with 19b as emitters showed the highest maximum EQE values (0.46% and 0.48%),which are among the highest reported for solution processed OLED devices emitting in the red/NIR region of the spectra.
|
Download:
|
| Figure 8. The structures of compounds 18-20. | |
Recently,thermally activated delayed fluorescence (TADF) has been proved to be an efficient strategy to achieve an internal quantum efficiency (IQE) of ≈100% in OLEDs,where a small energy gap (△EST) between S1 and T1 is required. In 2016,Hatakeyama and co-workers designed and synthesized two BN-embedded heterocycles 21 and 22 (Fig. 9) [21]. The synthetic strategy involved a coupling reaction between 1-bromo-2,3-dichlorobenzene and aromatic amines and a one-pot electrophilic borylation. The para-substitution of B and N atoms could significantly separate the HOMO and LUMO,leading to small △Est that was suitable for TADF-based OLEDs. Consequently,OLED devices based on these BN-embedded aromatics displayed ultrapure blue emission with an IQE of ≈100% and an external quantum efficiency(EQE) of 20.2% for 22 was achieved,which represents record-setting performance for blue OLEDs.

|
Download:
|
| Figure 9. The structures of compounds 21 and 22 with disconnected BN substitution. | |
4. BN-embedded aromatics for organic solar cells
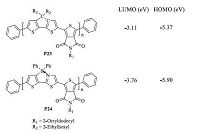
Replacing the C-C single bond in organic conjugated materials with B N dative bond could effectively tune the HOMO and LUMO energy levels of materials. In 2015,Liu and co-workers developed a D-A conjugated polymer P24,in which a C-C unit was replaced with aB ← N unit (Fig. 10) [22]. Compared with those of its carbon analog P23,introduction of B ← N dative bond significantly lowered both the HOMO and LUMO energy levels,from -3.11/-5.37eV for LUMO/HOMO to -3.76/-5.90eV. As a result,the resulting D-A conjugated polymer P24 changed from electron donor to electron acceptor,which was confirmed by fluorescence quenching experiments and the photovoltaic response. Polymer solar cell with active layer containing P24 as acceptor and its carbon analog P23 as donor exhibited a power conversion efficiency (PCE) of 0.085% with an open-circuit voltage of 1.08 V,a short-circuit current density of 0.35 mA cm-2,and a fill factor of 0.23. When the donor material was changed to poly(3- hexylthiophene) (P3HT),a little higher PCE of 0.14% was achieved. The poor device performance was attributed to the large phase separation size of the blend films.
|
Download:
|
| Figure 10. The structures of polymers P23 and P24 and their HOMO/LUMO energy levels. | |
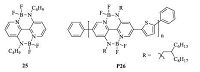
Using the same design concept,in 2016,Liu and co-workers synthesized another novel electron-deficient building block,a double B ← N bridged bipyridyl (25),which showed low LUMO/HOMO energy levels of-3.19/-5.63 eV [23]. Conjugated polymer P26 based on 25 and thiophene was constructed with high thermal stability and low LUMO/HOMO energy levels of -3.50/-5.77 eV (Fig. 11). An electron mobility of 6.9 × 10-5 cm2 V-1 s-1 was measured by space- charge-limited current (sCLC) method. All polymer solar cell (PsC) device with polymer P26 as the electron acceptor and poly[[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl][3- fluoro-2-[(2-ethylhexy)carbonyl]thieno[3,4-b]-thiophenediyl]] (PTB7) as the electron donor exhibited a PCE of 3.38%.
|
Download:
|
| Figure 11. The structures of compound 25 containing two BN dative bonds and polymer P26 based on it. | |
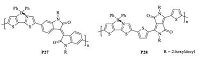
The previously developed D-A polymer containing B ← N dative bond showed low electron mobility. In order to increase the electron mobility and further improve the solar cell performance,Liu and coworkers designed two new D-A conjugated polymers P27 and P28 containing larger acceptor units,isoindigo and dithienyldiketopyr- rolopyrrole (DPP) (Fig. 12) [24]. The larger acceptor units could alleviate the steric hindrance effect of the pendant phenyl groups on the B atoms,leading to a decreased π-stacking distance. Consequently,the electron mobility was enhanced by nearly two orders of magnitude to be 2.8 × 10-5 cm2V-1 s-1. The PCE ofall polymer solar cells with polymer P27 acting as the electron acceptor and poly[4,8- bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene- co-3-fluorothieno[3,4-b]thiophene-2-carboxylate] (PTB7-Th) as the donor was thus greatly improved from 0.12% to 5.04%.
|
Download:
|
| Figure 12. The structures of D–A polymers P27 and P28. | |
5. BN-embedded aromatics for stimuli-responsive luminescent devices
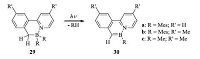
Usually,the synthesis of azaborine compounds involves multistep reactions or use of transition metal catalysts,which is in general very challenging. In 2013,Wang and coworkers reported a new photoelimination method for the synthesis of BN-substituted polycyclic aromatics [25]. This unprecedented reaction involved the breaking of a C-H bond and a B-C bond of the BN-heterocyclic compounds 29,leading to the formation of polycyclic azaborine compounds 30 by eliminating an R-H molecule from a BR2-CH2 unit (Scheme 5). Unlike the precursor compounds which were nonemissive,the resulting azaborine compounds exhibited bright green or yellow-green fluorescence. Interestingly,the unique photoelimination reaction could also take place in a polymer substrate,which provides the possibility of creating patterned fluorescent polymer film by light due to the high contrast of the non-emissive precursors and the highly emissive polycyclic azaborine compounds.
|
Download:
|
| Scheme. 5. Synthesis of polycyclic azaborine compounds 30 by photoelimination. | |
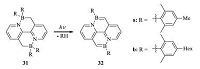
Very recently,using the developed photoelimination reaction,Wang and co-workers successfully synthesized rare yellow fluorescent BN-pyrenes (32) that contain two BN units (Scheme 6) [26]. Photophysical and electrochemical properties of the fluorescent BN-pyrenes were carefully studied,indicating that these BN-pyrenes are promising candidates used as yellow emitters for electroluminescent (EL) devices. However,the inefficient photoelimination reaction and air-sensitivity of BN-pyrenes impeded their application in EL through photochemical procedure. Significantly,the double arene elimination can also be driven by excitons generated electrically within electroluminescent (EL) devices,which enabled the in situ solid-state conversion of BN-heterocycles to BN-pyrenes and the use of BN-pyrenes as emitters for EL devices.
|
Download:
|
| Scheme. 6. The in situ exciton-driven elimination reaction of 31 to form 32. | |
Wang and co-workers also discovered a series of photo- and thermal-induced transformations and isomerizations of BN- embedded compounds accompanied by distinct color change [27]. These transformations stimulated by light,heator excitons may be applied to stimuli-responsive electronic devices.
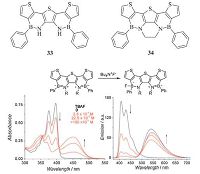
6. BN-embedded aromatics for chemical sensorsThe strong Lewis acidity of boron atoms due to their vacant 2p orbitals makes azaborine compounds good sensors for anions,especially for fluoride. In 2010,Perepichka and coworkers synthesized two azaborine-fused oligothiophenes 33 and 34 (Fig. 13) [28]. Considering the acidity of the N-H protons in 33 which might be problematic for applications and further functionalization,they incorporated an ethylene bridge into 34 to protect these positions. Both 33 and 34 displayed almost identical absorption and deep- blue emission features. Interestingly,adding n-Bu4NF to solution of compound 34 resulted in obvious change in both absorption and emission spectra. The emergence of long-wavelength absorption/ emission band and decrease of the original absorption/emission band were observed. Although the fluoride sensing by boron- containing conjugated materials is well known,most of these systems exhibited a blueshift of the absorption spectra and quenching of the fluorescence. The pronounced red-shifted absorption and emission in this system,which could be easily observed with a naked eye,made these molecules suitable for an "off-on" sensor of F-.
|
Download:
|
| Figure 13. The structures of compounds 33 and 34,and the absorption (bottom left) and emission (bottom right) spectra of 34 in CH2Cl2 upon addition of Bu4NF. Reprinted from Ref. [28] with permission from The Royal Society of Chemistry. | |
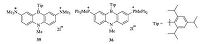
|
Download:
|
| Figure 14. The structures of compounds 35 and 36. | |
Kawashima and co-workers synthesized a series of 1,4- azaborine-fused linear acenes which were exploredas efficient fluorescent sensors for fluoride and cyanide anions [29]. For example,in 2009,Kawashima and co-workers synthesized two 1,4-azaborine compounds 35 and 36 bearing ammonio or phosphonio groups on the periphery (Fig. 14) [30]. The introduction of cationic groups enhanced the Lewis acidity of B atoms and improved the water solubility of the molecules,making these 1,4-azaborine compounds good sensors for fluoride and cyanide ions in aqueous media,which could be monitored by optical spectroscopy.
7. ConclusionIn this review article,we summarized the recent development of BN-embedded aromatic compounds for optoelectronic applications such as OFET,OLED and OPV. Replacing the CC unit with its isoelectronic BN unit in organic π-systems has produced a new type of organic semiconductors with intriguing properties. BN substitution has shown to be effective in modulating the photophysical and redox properties as well as intermolecular interactions of conjugated molecules,which promotes the research on the applications of BN-embedded polycyclic aromatics in optoelectronic devices. Though some promising applications of azaborine compouds have been demonstrated,applications of BN- embedded polycyclic aromatics in optoelectronic devices is still in its infancy. For example,their device performance is still not satisfying compared to their carbon counterparts. Poor materials accessibility due to the synthetic challenges,as well as the thermal and chemical instability limit the fast advance of the field. Therefore,efficient synthetic strategies are needed to enrich the chemical diversity of BN-containing compounds. In addition,relationship between BN-substituted aromatic structures and the device performance needs to be investigated deeply. It is expected that rationally designed BN-substituted polycyclic aromatics will provide new opportunities for organic electronics with high performance and unique functionality.
Acknowledgments This work was supported by the Major State Basic Research Development Program (Nos. 2013CB933501 and 2015CB856505) from the Ministry ofScience and Technology, and National Natural Science Foundation of China.| [1] | (a) K. Takimiya, I. Osaka, M. Nakano, π-Building blocks for organic electronics: revaluation of "inductive" and "resonance" effects of π-electron deficient units, Chem. Mater. 26(2014) 587-593; (b) W.P. Wu, Y.Q. Liu, D.B. Zhu, π-Conjugated molecules with fused rings for organic field-effect transistors: design, synthesis and applications, Chem. Soc. Rev. 39(2010) 1489-1502; (c) L.T. Dou, J.B. You, Z.R. Hong, et al., 25th Anniversary article: a decade of organic/polymeric photovoltaic research, Adv. Mater. 25(2013) 6642-6671; (d) H. Usta, A. Facchetti, T.J. Marks, n-Channel semiconductor materials design for organic complementary circuits, Acc. Chem. Res. 44(2011) 501-510; (e) J.G. Mei, Y. Diao, A.L. Appleton, L. Fang, Z.N. Bao, Integrated materials design of organic semiconductors for field-effect transistors, J. Am. Chem. Soc. 135(2013) 6724-6746; (f) P.M. Beaujuge, J.M.J. Fréchet, Molecular design and ordering effects in pfunctional materials for transistor and solar cell applications, J. Am. Chem. Soc. 133(2011) 20009-20029; (g) C.L. Wang, H.L. Dong, W.P. Hu, Y.Q. Liu, D.B. Zhu, Semiconducting π-conjugated systems in field-effect transistors: a material odyssey of organic electronics, Chem. Rev. 112(2012) 2208-2267; (h) Z.T. Huang, C.C. Fan, G.B. Xue, et al., Solution-grown aligned crystals of diketopyrrolopyrroles (DPP)-based small molecules: rough surfaces and relatively low charge mobility, Chin. Chem. Lett. 27(2016) 523-526. |
| [2] | (a) W. Jiang, Y. Li, Z.H. Wang, Heteroarenes as high performance organic semiconductors, Chem. Soc. Rev. 42(2013) 6113-6127; (b) E.J. Wang, C.L. Wang, Q. Meng, et al., Syntheses of molecular wires containing redox center: reversible redox property and good energy level matching with Au electrode, Chin. Chem. Lett. 19(2008) 1285-1289; (c) B. Gao, Y. Li, H. Tian, Synthesis and near-infrared characteristics of novel perylenebisimide dyes bay-functionalized with naphthalimide chromophores, Chin. Chem. Lett. 18(2007) 283-286. |
| [3] | (a) M. Mas-Torrent, M. Durkut, P. Hadley, X. Ribas, C. Rovira, High mobility of dithiophene-tetrathiafulvalene single-crystal organic field effect transistors, J. Am. Chem. Soc. 126(2004) 984-985; (b) H. Ebata, T. Izawa, E. Miyazaki, et al., Highly soluble[1] benzothieno[32-b]benzothiophene (BTBT) derivatives for high-performance, solution-processed organic field-effect transistors, J. Am. Chem. Soc. 129(2007) 15732-15733. |
| [4] | M.J.S. Dewar, V.P. Kubba, R. Pettit. 624. New heteroaromatic compounds. Part I. 9-Aza-10-boraphenanthrene. J. Chem. Soc (1958) 3073–3076. DOI:10.1039/jr9580003073 |
| [5] | (a) M.J.S. Dewar, W.H. Poesche, New heteroaromatic compounds. XXI. Some tetracyclic systems 2, J. Org. Chem. 29(1964) 1757-1762; (b) M.J.S. Dewar, W.H. Poesche, New heteroaromaticcompounds. XVIII. Boron-containing analogs of benz[a]anthracene, J. Am. Chem. Soc. 85(1963) 2253-2256; (c) D.J.H. Emslie, W.E. Piers, M. Parvez, 2,2'-Diborabiphenyl: a Lewis acid analogue of 2,20-bipyridine, Angew. Chem. Int. Ed. 42(2003) 1252-1255; (d) C.A. Jaska, D.J.H. Emslie, M.J.D. Bosdet, et al., Triphenylene analogues with B2N2C2 cores: synthesis, structure, redox behavior, and photophysical properties, J. Am. Chem. Soc. 128(2006) 10885-10896. |
| [6] | (a) D.H. Knack, J.L. Marshall, G.P. Harlow, et al., BN/CC isosteric compounds as enzyme inhibitors: N- and B-ethyl-1,2-azaborine inhibit ethylbenzene hydroxylation as nonconvertible substrate analogues, Angew. Chem. Int. Ed. 52(2013) 2599-2601; (b) A.J. Ashe III, Aromatic borataheterocycles: surrogates for cyclopentadienyl in transition-metal complexes, Organometallics 28(2009) 4236-4248; (c) W. Luo, P.G. Campbell, L.N. Zakharov, S.Y. Liu, A single-component liquid-phase hydrogen storage material, J. Am. Chem. Soc. 133(2011) 19326-19329; (d) P.G. Campbell, L.N. Zakharov, D.J. Grant, D.A. Dixon, S.Y. Liu, Hydrogen storage by boron-nitrogen heterocycles: a simple route for spent fuel regeneration, J. Am. Chem. Soc. 132(2010) 3289-3291. |
| [7] | (a) M.J.D. Bosdet, W.E. Piers, T.S. Sorensen, M. Parvez, 10a-Aza-10b-borapyrenes: heterocyclic analogues of pyrene with internalized BN moieties, Angew. Chem. Int. Ed. 46(2007) 4940-4943; (b) X.Y. Wang, D.C. Yang, F.D. Zhuang, et al., Postfunctionalization of BN-embedded polycyclic aromatic compounds for fine-tuning of their molecular properties, Chem. Eur. J. 21(2015) 8867-8873. |
| [8] | (a) M.J.D. Bosdet, W.E. Piers, B-N as a C-C substitute in aromatic systems, Can. J. Chem. 87(2009) 8-29; (b) P.G. Campbell, A.J.V. Marwitz, S.Y. Liu, Recent advances in azaborinechemistry, Angew. Chem. Int. Ed. 51(2012) 6074-6092; (c) X.Y. Wang, J.Y. Wang, J. Pei, BN heterosuperbenzenes: synthesis and properties, Chem. Eur. J. 21(2015) 3528-3539. |
| [9] | G. Ulrich, R. Ziessel, A. Harriman. The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew. Chem. Int. Ed. 47 (2008) 1184–1201. DOI:10.1002/(ISSN)1521-3773 |
| [10] | (a) D.M. Chen, Q. Qin, Z.B. Sun, Q. Peng, C.H. Zhao. Synthesis and properties of B,Nbridged p-terphenyls, Chem. Commun., 2014,50: 782-784; (b) D.M. Chen, S. Wang, H.X. Li, X.Z. Zhu, C.H. Zhao, Solid-state emissive B,Sbridged p-terphenyls: synthesis, properties, and utility as bifunctional fluorescent sensor for Hg2+ and F- ions. Inorg. Chem. 53 (2014) 12532–12539. DOI:10.1021/ic502088k |
| [11] | T. Hatakeyama, S. Hashimoto, S. Seki, M. Nakamura. Synthesis of BN-fused polycyclic aromatics via tandemintramolecular electrophilic areneborylation. J. Am. Chem. Soc. 133 (2011) 18614–18617. DOI:10.1021/ja208950c |
| [12] | T. Hatakeyama, S. Hashimoto, T. Oba, M. Nakamura. Azaboradibenzo[6] helicene: carrier inversion induced by helical homochirality. J. Am. Chem. Soc. 134 (2012) 19600–19603. DOI:10.1021/ja310372f |
| [13] | X.Y. Wang, H.R. Lin, T. Lei, et al. Azaborine compounds for organic field-effect transistors: efficient synthesis, remarkable stability, and BN dipole interactions. Angew. Chem. Int. Ed. 52 (2013) 3117–3120. DOI:10.1002/anie.201209706 |
| [14] | X.Y. Wang, F.D. Zhuang, X. Zhou, et al. Influence of alkyl chain length on the solidstate properties and transistor performance of BN-substituted tetrathienonaphthalenes. J. Mater. Chem. C 2 (2014) 8152–8161. DOI:10.1039/C4TC01369G |
| [15] | X.Y. Wang, F.D. Zhuang, R.B. Wang, et al. A straightforward strategy toward large BN-embedded π-systems: synthesis, structure, and optoelectronic properties of extended BN heterosuperbenzenes. J. Am. Chem. Soc. 136 (2014) 3764–3767. DOI:10.1021/ja500117z |
| [16] | X.Y. Wang, F.D. Zhuang, J.Y. Wang, J. Pei. Incorporation of polycyclic azaborine compounds into polythiophene-type conjugated polymers for organic field-effect transistors. Chem. Commun. 51 (2015) 17532–17535. DOI:10.1039/C5CC06927K |
| [17] | X.Y. Wang, F. Zhang, J. Liu, et al. Ladder-type BN-embedded heteroacenes with blue emission. Org. Lett. 15 (2013) 5714–5717. DOI:10.1021/ol402745r |
| [18] | (a) X.Y. Wang, F.D. Zhuang, X.C. Wang, et al., Synthesis, structure and properties of C3-symmetric heterosuperbenzene with three BN units, Chem. Commun. 51(2015) 4368-4371; (b) G. Li, W.W. Xiong, P.Y. Gu, et al., 15,9-Triaza-2,6,10-triphenylboracoronene: BN-embedded analogue of coronene, Org. Lett. 17(2015) 560-563. |
| [19] | S. Hashimoto, T. Ikuta, K. Shiren, et al. Triplet-energy control of polycyclic aromatic hydrocarbons by BN replacement: development of ambipolar host materials for phosphorescent organic light-emitting diodes. Chem. Mater. 26 (2014) 6265–6271. DOI:10.1021/cm503102d |
| [20] | D.L. Crossley, I.A. Cade, E.R. Clark, et al. Enhancing electron affinity and tuning band gap in donor-acceptor organic semiconductors bybenzothiadiazole directed C-H borylation. Chem. Sci. 6 (2015) 5144–5151. DOI:10.1039/C5SC01800E |
| [21] | T. Hatakeyama, K. Shiren, K. Nakajima, et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect. Adv. Mater. 28 (2016) 2777–2781. DOI:10.1002/adma.v28.14 |
| [22] | C.D. Dou, Z.C. Ding, Z.J. Zhang, et al. Developing conjugated polymers with high electron affinity by replacing a C-C unit with a B←N unit. Angew. Chem. Int. Ed. 54 (2015) 3648–3652. DOI:10.1002/anie.201411973 |
| [23] | C.D. Dou, X.J. Long, Z.C. Ding, et al. An electron-deficient building block based on the B←N unit: an electron acceptor for all-polymer solar cells. Angew. Chem. Int. Ed. 55 (2016) 1436–1440. DOI:10.1002/anie.201508482 |
| [24] | R.Y. Zhao, C.D. Dou, Z.Y. Xie, J. Liu, L.X. Wang. Polymer acceptor based on B←N units with enhanced electron mobility for efficient all-polymer solar cells. Angew. Chem. Int. Ed. 55 (2016) 5313–5317. DOI:10.1002/anie.201601305 |
| [25] | (a) J.S. Lu, S.B. Ko, N.R. Walters, et al., Formation of azaborines by photoelimination of B,N-heterocyclic compounds, Angew. Chem. Int. Ed. 52(2013) 4544-4548; (b) D.T. Yang, S.K. Mellerup, X. Wang, J.S. Lu, S.N. Wang, Reversible 1,1-hydroboration: boryl insertion into a C-N bond and competitive elimination of HBR2 or R-H, Angew. Chem. Int. Ed. 54(2015) 5498-5501; (c) Y.G. Shi, D.T. Yang, S.K. Mellerup, et al., 1,1-Hydroboration of fused azole-isoindole analogues as an approach for construction of B,N-heterocycles and azole-fused B,N-naphthalenes, Org. Lett. 18(2016) 1626-1629. |
| [26] | S.N. Wang, D.T. Yang, J.S. Lu, et al. In situ solid-state generation of (BN)2-pyrenes and electroluminescent devices. Angew. Chem. Int. Ed. 54 (2015) 15074–15078. DOI:10.1002/anie.201507770 |
| [27] | (a) Y.L. Rao, H. Amarne, L.D. Chen, et al., Photo- and thermal-induced multistructural transformation of 2-phenylazolyl chelate boron compounds, J. Am. Chem. Soc. 135(2013) 3407-3410;(b) Y.L. Rao, C. Hörl, H. Braunschweig, S.N. Wang, Reversible photochemical and thermal isomerization of azaboratabisnorcaradiene to azaborabenzotropilidene, Angew. Chem. Int. Ed. 53(2014) 9086-9089. |
| [28] | M. Lepeltier, O. Lukoyanova, A. Jacobson, S. Jeeva, D.F. Perepichka. New azaborinethiopheneheteroacenes. Chem. Commun. 46 (2010) 7007–7009. DOI:10.1039/c0cc01963a |
| [29] | (a) T. Agou, M. Sekine, J. Kobayashi, T. Kawashima, Multi-step detection of cyanide ion by a bis(dimesitylboryl)dibenzoazaborine, J. Organomet. Chem. 694(2009) 3833-3836; (b) T. Agou, M. Sekine, J. Kobayashi, T. Kawashima, Synthesis and reactivity of a bis(dimesitylboryl)azaborine and its fluoride sensing ability, Chem. Commun. (2009) 1894-1896. |
| [30] | T. Agou, M. Sekine, J. Kobayashi, T. Kawashima. Detection of biologically important anions in aqueous media by dicationic azaborines bearing ammonio or phosphonio groups. Chem. Eur. J. 15 (2009) 5056–5062. DOI:10.1002/chem.v15:20 |
 2016, Vol. 27
2016, Vol. 27 


