b Department of Chemistry, The Hong Kong University of Science and Technology, Hong Kong, China ;
c Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, Hong Kong, China
To develop advanced organic materials, the design and synthesis of conjugated molecules are essential. The conjugated molecules can be broadly grouped into two categories: through- bond and through-space conjugated molecules [1-3]. Compared to the widely studied through-bond conjugated molecules, the studies on those with through-space conjugation character are at the infant stage, for the lack of sufficient well-established model molecules. As a matter of fact, a better understanding of the properties and functionalities of through-space conjugated molecules is conducive to many research areas. For instance, the electronic communication between antennae and reaction centers in the field of photosynthesis of various natural substances involves a through-space pathway [4, 5]. Moreover, the transport of charges along duplex DNA is also thought to proceed through through-space conjugated bases [6-8]. To further get into all of these interesting subjects, in-depth researches on the exploration of through-space conjugated molecules and the mechanism of how they conduct electricity and energy are absolutely of high importance.
Inspired by the long-range electron transport in DNA, recently, great endeavors have been paid on the artificially synthesized molecules with almost parallel-arranged aromatic rings. These molecules feature the character of through-space conjugation, which can serve as nice models for the research of carrier and energy transport via a through-space pattern. So far, there are some reports about the development of new through-space conjugated oligomers and polymers [9], but most of them are built based on the paracyclophanes (pCp) and its derivatives [10]. The pCp consists of two face-to-face located and overlapped benzene rings, which are linked by two alkyl groups in the para- positions of benzene rings. The distance between the two benzene planes in pCp is shorter than 3.5Å (the typical distance for π-π stacking interaction), being close enough to generate through- space conjugation.
With the help of experimental investigation and theoretical calculation, researchers have gained much information about pCp and its derivatives in many aspects, such as the geometrical conformations [11, 12], electronic structures [13, 14], photophysical properties [15], NMR spectral features [16], intramolecular charge transfer (ICT) process [17], two-photon absorption ability [18], and so forth. Not only the structure-property relationship, but also the potential applications in different frontiers, including solar cell [19], luminescent materials [20], single molecular wires [2], etc., of pCp derivatives are extensively studied. Besides the pCp system, other molecules with through-space conjugation also show promising applications in organic electronics [21], DNA molecular wires [22], and redox active biocomplexes [23]. Although much progress has been made in studying through-space conjugated molecules in past decades, the through-space conjugated systems are still limited. Therefore, in order to further understand the nature and application of through-space conjugation, exploration of new through-space conjugated molecules is of high significance.
On the other side, materials chemists have been dedicated in developing advanced organic luminescent materials, and many great achievements have been made. But most luminophores show good emissions only in dilute solutions, and become almost non-fluorescent in the aggregated state, presenting an undesired aggregation-caused quenching (ACQ) effect. This ACQ problem is turned out to be a thorny obstacle to the evolution of many optoelectronic devices based on organic luminescent materials, particularly organic light-emitting diodes (OLEDs). Recently, a unique phenomenon of aggregation-induced emission (AIE) draws much attention because the luminogens with AIE characteristic (AIEgens) behavior oppositely to the ACQ chromophores [24]. Hence, the AIE phenomenon provides a high opportunity to solve the ACQ problem. To date, a great many of AIEgens have been developed, amongst which tetraphenylethene (TPE) is the most popular one. TPE has the merits of facile synthesis and excellent AIE characteristic, and numerous TPE-based functional materials have been used in OLED [25], biological probes [26], chemical sensors, [27] and so on [28].
In our recent studies on the synthesis and functionalization of TPE derivatives, a series of folded molecules were obtained. The crystallography analysis and theoretical calculation revealed that these distinctive folded TPE derivatives possess interesting conjugation pattern. They coinstantaneously combine the characters of through-bond and through-space conjugations, and exhibit good photoluminescence (PL) properties and large Stokes shifts. They are also good new models for the deciphering of AIE mechanism. In addition, they show unique bipolar carrier transporting ability in OLED devices and multichannel conductance in single-molecule wires, indicating their great potential in optoelectronics. In this review, the synthesis, characterization, crystal and electronic structures, photophysical properties as well as applications of these folded TPE derivatives are reviewed.
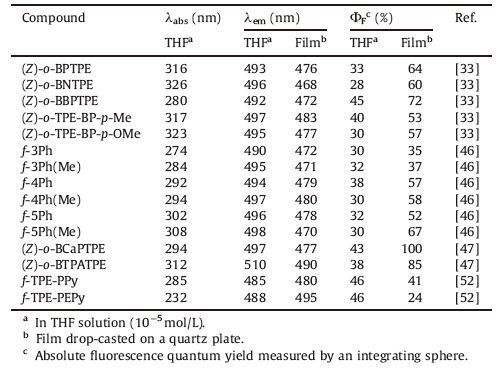
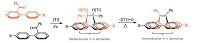
2. Synthesis and property of prototypal folded moleculesMcMurry reaction is an effective method to build C=C double bonds from ketones, and has been widely used to prepare TPE and its derivatives [29-32]. However, the products of McMurry reactions are usually stereo-random, namely, when unsymmetrically modified benzophenones are adopted as substrates of the reaction, nearly equal amounts of cis/trans-isomers will be generated (Fig. 1A). Interestingly, we found that this reaction, in certain situations, can also be used in stereoselective synthesis of TPE derivatives [33]. For instance, McMurry reaction of benzo- phenones modified by aromatic groups at the 2-position can yield stereoregular TPE derivatives with a folded cis-conformation. Due to their distinctive structures, folded TPE derivatives have some special properties, and thus, endow them with interesting applications.
|
Download:
|
| Figure 1. Syntheses of (A) linear and (B) folded TPE derivatives. The Ha protons in (Z)-o-BPTPE are indicated as an example. (C) The X-ray determined molecular structure of (Z)-o- BPTPE (CCDC 955718). The shortest distances between φ1 and φ2 and distances from Has to the center of φ1s are indicated. (D) Molecular orbitals ranging from HOMO - 1 to LUMO + 1 of (Z)-o-BPTPE. Reproduced with permission from Ref. [33]. Copyright (2013) Royal Society of Chemistry. | |
The archetypal folded TPE derivative, (Z)-o-BPTPE, was synthesized from 2-phenylbenzophenone, as illustrated in Fig. 1B. In order to make description more convenient and clearer, we label three kinds of aromatic rings in (Z)-o-BPTPE as φ1, φ2 and φ3. For folded molecules which are mentioned in the paper also applies the labelling. Under the common condition of McMurry reaction (Zn and TiCl4), (Z)-o-BPTPE is generated solely in a yield of around 50%, without other TPE derivatives. Unlike the situation in other McMurry reaction that cis/trans-isomers of the products can hardly be isolated by common purification methods, the product in this reaction is able to be isolated and purified easily by column chromatography. In the mixture of THF and ethanol, the single crystal of (Z)-o-BPTPE is successfully obtained and the analysis results of X-ray diffraction crystallography confirm that (Z)-o- BPTPE is indeed a cis-isomer with a folded conformation (Fig. 1C). The φ1 and φ2 are almost parallel, and the overlap of surface φ1 and φ2 is about 50%. The shortest distance between φ1 and φ2 reaches 3.264 Å, which is shorter than the typical distance for π-π stacking interaction (3.5 Å) [15, 34, 35]. Such a parallel, stacked, and offset geometry is favorable of the π-π stacking interaction between φ1 and φ2 [36]. The 1H NMR spectrum of (Z)-o-BPTPE also suggests that the existence of π-π stacking interaction between φ1 and φ2. In the 1H NMR spectrum, distinctive peaks appears at ~5.7 ppm, which is supposed to be the Ha protons. Since the Has in φ2 overlies f1, and the distance between Ha and the center of f1 is less than 3.2 Å, the distinctive chemical shift of Ha is hence easy to understand: Has locate within the shielding zone of φ1, and feel the sheilding effect of φ1, resulting in peak movements towards upfield [37].
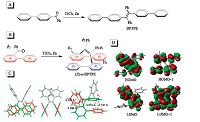
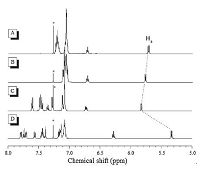
In addition to (Z)-o-BPTPE, other folded TPE derivatives can also be obtained. When φ2s are taken place by larger aromatic moieties, naphthyls and biphenylyls, (Z)-o-BNTPE and (Z)-o-BBPTPE were attained from the McMurry reaction, respectively (Fig. 2). Their 1H NMR spectra are shown in Fig. 3. Compared to (Z)-o-BPTPE, the distinctive signals from Has of (Z)-o-BNTPE are shifted towards upfield, while the Has' peak positions of (Z)-o-BBPTPE are almost the same as those of (Z)-o-BPTPE. This is persumably due to that naphthalenyl rings have more p-electrons than phenyl rings. The folded structures of (Z)-o-BNTPE and (Z)-o-BBPTPE reveal that it is possible to replace the phenyl rings (φ1) with other larger aromatic rings systems without causing damage to the folded conformation. Moreover, it is also noteworthy that the positions of substituent groups on φ1 will obviously impact the formation of the folded structure. 2-Phenylbenzophenone derivatives with methyl or methoxyl groups at the different positions of φ1 were tried as substrates in McMurry reaction to produce the folded molecules. As a result, reactions of 2-phenylbenzophenone derivatives with the substituents at the para- and meta-positions of f1proceeded successfully just like other examples mentioned above. The folded molecular structures of the products were also confirmed by X-ray single-crystal diffraction analysis. On the contrary, the reaction of substrates which possess the substituents at the ortho-position of φ1 were failed to yield the target molecules, which should be ascribed to severe steric hindrance.
|
Download:
|
| Figure 2. Examples of (Z)-o-BPTPE derivatives. | |
|
Download:
|
| Figure 3. 1H NMR spectra of (A) (Z)-o-BPTPE, (B) (Z)-o-TPE-BP-p-OMe, (C) (Z)-o-BBPTPE and (D) (Z)-o-BNPTPE in chloroform-d, where asterisk denotes solvent peak. Reproduced with permission from Ref. [33]. Copyright (2013) Royal Society of Chemistry. | |
Apparently, it is very critical to find the working mechanism of the formation of folded cis-products. It was proposed that repulsive force from φ3 and intramolecular π-π stacking interaction are two important factors (Fig. 4). When 1-(biphenyl-2-yl)ethanone, an analogue of 2-phenylbenzophenone with a methyl group rather than φ3 was used as substrate for the McMurry reaction, the product was an unfolded trans-isomer [33]. This result evidenced that φ3 is important to the formation of folded conformation. Therefore, it was rationalized that when two 2-phenylbenzophe- nones formed the intermediate complex of titanium species, the repulsive force from φ3 prevented the formation of trans-isomer. When φ13 was replaced by methyl group, molecules became more flexible and less crowded. The trans-conformation could lower total energy of the molecule, and thus the reaction leaded to transproducts.
|
Download:
|
| Figure 4. Proposed mechanism for the formation of folded TPE derivatives. Reproduced with permission from Ref. [47]. Copyright (2011) Wiley-VCH. | |
To further investigate through-space conjugation of the folded TPE derivatives, theoretical calculation was carried out. Taking (Z)- o-BPTPE as example, it was found that its HOMO was dominated by the orbitals of two φ2s as well as the vinyl group (Fig. 1D). With regard to its LUMO + 1, whereas the vinyl group contributed little, φ1 and φ2 showed major contributions. Apparent bonding character could be observed in the inter-ring region of φ1 and φ2, which could actually be referred as through-space conjugation. Therefore, the spatial repulsion from φ3 is a crucial driving force of the formation of folded cis-products, and the intramolecular p-p stacking interaction, or through-space conjugation bring about the stable parallel arrangement of f i and φ2. These two factors work collectively, leading to the stereoselectivity of the reaction and the folded cis-products.
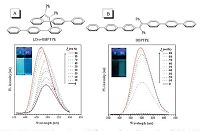
Most reported linear TPE derivatives are AIEgens, namely they are non-fluorescent in dilute solutions but become strong emitters when they form aggregates in poor solvents or in the solid state. The folded TPE derivatives like (Z)-o-BPTPE and (Z)-o-BBPTPE, however, showed quite different emission behaviors. They exhibited good emissions in both in dilute solutions and in the solid state. Taking (Z)-o-BBPTPE as example, in dilute solution, its fluorescence quantum yield (ΦF) is 45%, being much higher than that of its linear analogue BBTPE (Φf = 0.62%). In general, the Φf values of the folded molecules in the aggregated state can be further enhanced relative to those in dilute solution. Fig. 5 displays the PL spectra of (Z)-o-BBPTPE and BBTPE in a series of THF-water mixtures. It can be seen that the PL intensity of BBTPE is very low when water fraction is low, but the PL intensity is boosted swiftly as the increase of water fraction, presenting typical AIE feature. (Z)- o-BBPTPE, however, shows good PL intensity in pure THF solution, and with the addition of water, the PL intensity is further enhanced, behavioring an aggregation-enhanced emission (AEE) characteristic.
|
Download:
|
| Figure 5. PL spectra of (A) (Z)-o-BBPTPE and (B) BBPTPE in THF/water mixtures with different water fractions (fw). Inset: photos of (A) (Z)-o-BBPTPE and (B) BBPTPE in THF/water mixtures (fw = 0 and 90%),taken under the illumination of a UV lamp. Reproduced with permission from Ref. [33]. Copyright (2013) Royal Society of Chemistry. | |
The working mechanism of different PL behaviors of folded TPE derivatives in comparison to linear TPE derivatives should be the molecular structural rigidification. As discussed above, in the folded molecules, a pair of phenyl rings is located closely with efficient through-space conjugation. In this case, the molecular structures become rigid, and the rotation of φ1 and φ2 is restricted greatly (Fig. 6). Therefore, the nonradiative decay of the excited state is thus reduced, rendering good PL emission of the folded TPE derivatives in the solution state. On the other hand, φ3 is still able to rotate freely in dilute solutions, and this rotation will consume part of excited-state energy. Therefore, when the rotation of φ3 is suppressed by aggregate formation, the PL intensity can be further enhanced. Concerning linear TPE derivatives, there are many rotatable phenyl rings deactivating the excited-state efficiently, and making them non-fluorescent in solutions, but in the aggregated state, the restriction of intramolecular rotation (RIR) is activated, allowing the molecules emit strongly. These results could serve as direct evidences to support that restriction of intramolecular rotation (RIR) mechanism is reasonable for the AIE effect of most TPE derivatives.

|
Download:
|
| Figure 6. Potential energy curves along the torsion angles (C1-C2-C3-C4 and C1-C2-C5-C6) of the ground state for the isolated (Z)-o-BPTPE molecule. Reproduced with permission from Ref. [33]. Copyright (2013) Royal Society of Chemistry. | |
3. Folded molecules with intramolecular energy transfer process
Materials with high fluorescence efficiency and large Stokes shift are highly desired in many research fields such as fluorescent probes, OLEDs and organic lasers. But unfortunately, in most cases, high fluorescence efficiency and large Stokes shifts are not compatible. For conventional fluorescent dyes, like BODIPY [38, 39], coumarin [40, 41] and fluorescein [42, 43], they suffer from ACQ effect, which should be partially owing to the severe self-absorption caused by their small Stokes shifts. To enlarge Stokes shifts, people usually think about molecules with certain special photophysical processes including excited state intramolecular proton transfer (ESIPT) and intramolecular charge transfer (ICT). But both ESIPT and ICT processes will depress the fluorescence of the molecules [44, 45] in high polar conditions.
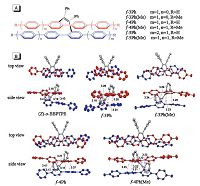
Recently, we found a new approach to overcome the challenge of preparing pure hydrocarbon materials with high fluorescence efficiency and large Stokes shift at the same time [46]. We stereoselectively synthesized a series of tailored folded molecules comprising a pair of vinyl-bridged, π-stacked oligo-p-phenylenes (Fig. 7A) through McMurry coupling of oligo-p-phenylenes bearing a benzoyl group. The distinctive doublet peaks at 〜6.0 ppm were also observed in the 1H NMR spectra of these folded molecules. Single crystals of (Z)-o-BBPTPE, f-3Ph, f-3Ph(Me) and f-4Ph, f- 4Ph(Me) were analyzed by X-ray diffraction crystallography, and the results revealed that all of these molecules had a folded cis- conformation as displayed in Fig. 7B. Two oligo-p-phenylene chains linked by a vinyl unit were located closely in a face-to-face parallel manner. The shortest distance between the nearest phenyl rings were shorter than 3.5 Å, indicating that they were through- space conjugated.
|
Download:
|
| Figure 7. (A) Molecular and (B) crystal structures of (Z)-o-BBPTPE (CCDC 1059693),f-3Ph (CCDC 1059694),f-3Ph(Me) (CCDC 1059695),f-4Ph (CCDC 1059696) and f-4Ph(Me) (CCDC1059697) with indicated distances (Á) between π-stacked phenyl rings. Hydrogen atoms are omitted for clarity. Reproduced with permission from Ref. [46]. Copyright (2015). American Chemistry Society. | |
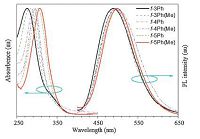
Fig. 8 shows the absorption and PL spectra in THF solutions, and Table 1 provides some related optical data of these folded molecules. The main absorption peaks of theses folded molecules were in the range of 278-308 nm, and as the elongation of oligo-p- phenylene chain, the peaks moved to long-wavelength region progressively. These absorption bands were demonstrated to be associated with the absorption of linear individual oligo-p- phenylene chain, while the absorption of vinyl-bridged entire molecular backbones can be negligible. For instance, the absorption peak off-4Ph is located at 292 nm, which is close to that of p- quaterphenyl (299 nm). The PL maxima of these folded molecules were much stable, varying slightly in the range of 489-498 nm, which were close to the PL peak of (Z)-o-BPTPE (493 nm). Notably, the polarity of solvents had almost no impact on the emission wavelengths of these molecules, illustrating the absence of ICT effect. Hence, it was believed that PL emissions were actually originated from the central folded part rather than the entire molecules or the oligo-p- phenylene moieties. These folded molecules exhibited strong fluorescence, with ΦF values of 3038% in THF solutions and enhanced ΦF values of up to 67% in solid films. In addition, very large Stokes shifts of 190-214 nm were found in these folded molecules (Fig. 8), which were rarely reported for pure hydrocarbon fluorophores. An intramolecular energy transfer process from photon-absorbing oligo-p- phenylene chains to the light-emitting central TPE fragment is considered to be responsive for the large Stoke shifts as well as good fluorescence of these folded molecules.
|
Download:
|
| Figure 8. Absorption and PL spectra of folded fluorophores in THF solution (10-5 mol/L). Reproduced with permission from Ref. [46]. Copyright (2015). American Chemistry Society. | |
|
|
Table 1 Photophysical properties of folded TPE derivatives. |
4. Folded molecules with bipolar carrier transport ability
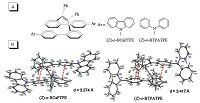
Luminescent materials with high charge mobility are important for the fabrication of efficient optoelectronic devices. It's no wonder that folded TPE derivatives that possess distinctive molecular structures and optical properties will inspire people to exploit their potential applications. By incorporating carbazole or triphenylamine groups in the folded (Z)-o-BPTPE core (Fig. 9A), we gained two novel materials with interesting bipolar carrier transport ability [47]. The new functionalized folded TPE derivatives, (Z)-o-BCaPTPE and (Z)-o-BTPATPE, were synthesized facilely by McMurry reaction, and no trans-isomers were generated. The structures of two molecules were confirmed by single crystal X-ray diffraction (Fig. 9B). Both molecules showed perfectly folded conformations. The distances between two p-p stacked phenyl rings were 3.274 AA for (Z)-o-BCaPTPE and 3.417 AA for (Z)-o- BCaPTPE, respectively, indicative of through-space conjugation nature. The folded conformation enhanced the rigidity of the molecules, and reduced the intramolecular rotations, rendering the good fluorescence both in dilute solutions and aggregated state. They showed AEE characteristics. In dilute solution, the ΦF values of (Z)-o-BCaPTPE and (Z)-o-BTPATPE are 43 and 38%, while in solid films, the ΦF values went up to 100 and 85%, respetively (Table 1). What's more, these rigid molecules also had excellent thermal stability. Their decomposition temperature (Td) were 427 ℃ for (Z)-o-BCaPTPE and 421 ℃ for (Z)-o-BTPATPE; their glass- transition temperatures (Tg) were detected at 222 and 217 ℃ for (Z)-o-BCaPTPE and 421 ℃ for (Z)-o-BTPATPE, respectively, clearly disclosing they are thermally and morphologically stable.
|
Download:
|
| Figure 9. (A) Molecular structures and (B) ORTEP drawings of functionalized folded TPE derivatives, (Z)-o-BCaPTPE (CCDC 825621) and (Z)-o-BTPATPE (CCDC 825622). Reproduced with permission from Ref. [47]. Copyright (2011) Wiley-VCH. | |
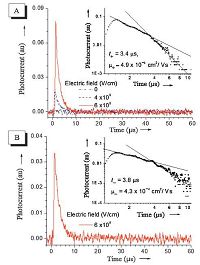
What made (Z)-o-BCaPTPE and (Z)-o-BTPATPE different from some other organic materials was their bipolar charge transfer ability, which means that the electrons and holes transport ability of both molecules reach a certain balance. Time-of-flight (TOF) transient photocurrent technique was used to investigate the carrier mobility of (Z)-o-BCaPTPE and (Z)-o-BTPATPE (Fig. 10) [47]. A hole mobility of (Z)-o-BCaPTPE (4.9 × 10-4cm2/V s) was determined, and the value was almost five-holds than that of typical hole-transport material N, N'-diphenyl-N, N'-bis(3-methyl- phenyl)-1, 1'-biphenyl-4, 4'-diamine (TPD) under the same measurement conditions. Furthermore, when the polarity of the applied bias was switched, photocurrent for electrons was also detected and it was surprising that the electron mobility (4.3 × 10-4 cm-2 V-1 s-1) of (Z)-o-BCaPTPE was very close to the hole mobility of its own. Compared with tris(8-hydroxyquinoli- nolato)aluminium (Alq3), a widely used electron-transporting material, the electron mobility of (Z)-o-BCaPTPE was even one- order higher at the same condition. Similarly, (Z)-o-BTPATPE also showed bipolar carrier transfer ability, but its hole mobility and electron mobility were not as good as those of (Z)-o-BCaPTPE. A possible reason was that the more rigid conformation and stronger intramolecular π-π stacking interaction endow (Z)-o-BCaPTPE with better carrier mobility.
|
Download:
|
| Figure 10. Transient photocurrents in (A) hole transport and (B) electron transport configurations for film (10 mm) of (Z)-o-BCaPTPE:PS:C60 (50:48.5:1.5 wt%) composite. Inset: log-log plot of the photocurrent as a function of time. Reproduced with permission from Ref. [47]. Copyright (2011) Wiley-VCH. | |
Drawing inspiration from the high PL efficiency and bipolar carrier transport ability of (Z)-o-BCaPTPE and (Z)-o-BTPAPTPE, we designed three kinds of OLED devices based on them. Devices with configurations of a) ITO/(Z)-o-BCaPTPE or (Z)-o-BTPATPE (80 nm)/ TPBi (10 nm)/Alq3 (30 nm)/LiF (1 nm)/Al (100 nm), in which (Z)-o- BCaPTPE and (Z)-o-BTPATPE served as light-emitting layers and electron-transporting layers; b) ITO/NPB (60 nm)/(Z)-o-BCaPTPE or (Z)-o-BTPATPE (60nm)/LiF (1 nm)/Al (100 nm), in which (Z)-o- BCaPTPE and (Z)-o-BTPATPE served as light-emitting layers and hole-transporting layers, and c) ITO/NPB (60 nm)/(Z)-o-BCaPTPE or (Z)-o-BTPATPE (20nm)/TPBi (10nm)/Alq3 (30nm)/LiF (1 nm)/Al (100 nm), in which (Z)-o-BCaPTPE and (Z)-o-BCaPTPE served as light-emitting layers only were fabricated. According to the experimental data, the devices without hole-transporting layer (NPB) or electron-transporting layer (Alq3) could afford good EL efficiencies. Taking (Z)-o-BCaPTPE as an example, the device without TPBi/Alq3 showed the best performance, and its maximum current efficiency (ηc,max) reached 7.9 cd A-1 and the external quantum efficiency (ηext, max) reached 3.1%, which were even better than those of the device containing both NPB and TPBi/Alq3. The device without NPB also performed well, giving ηcmax and ηext, max of 4.8cdA-1 and 2.1%, respectively. (Z)-o-BTPATPE also showed the ability of being a mutifunctional layer in OLED devices. These results in turn further validated the bipolar carrier transport ability of both molecules. Given that there were no typical electron-transporting groups in both molecules, the good electron-transporting ability should result from the through-space conjugated folded conformation. The excellent emission efficiency, high thermal stability and good bipolar carrier transport ability of (Z)-o-BCaPTPE and (Z)-o-BTPATPE sufficiently proved that they were surely a new kind of promising materials for optoelectronic device.
5. Folded molecules in single-molecule wires with multichannel conductanceIn past decades much attention had been paid in the field of molecular electronics. More and more people realize that organic materials are prospective candidates for next-generation electronic devices due to their various advantages, such as sufficient variety, low cost, regulated molecular structure, solution-processability and so on. Single-molecule wires which can link with the electrodes and transport current are the most basic components for molecular devices, and thus are of great importance. Currently, the artificial single-molecule wires can be roughly divided into two categories according to the different conjugation pattern of the molecules, one is through-bond conjugated molecular wires, and the other is through-space conjugated ones. For instance, oligophenylenes [48, 49] belong to the first category, while molecular wires based on pCp derivatives belong to the second category [1, 2, 50, 51]. In most cases, single-molecule wires are of single-channel conductance, namely they realize electric conduction by the way of either through-bond or through-space. The appearance of folded molecules like (Z)-o-BPTPE provides researchers with a new design strategy of single-molecule wires with multichannel conductance. In (Z)-o-BPTPE, a pair of well p- stacked phenyl rings are linked together by a vinyl group. Such a structrue makes it possible to conduct current via the channels of through-bond and through-space, that is mutichannel conductance [52].
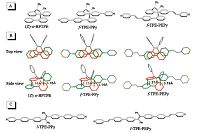
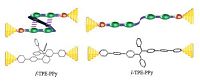
We prepared novel folded single-molecule wires by grafting pyridine groups at two ends of (Z)-o-BPTPE core as the terminal anchors, which enable the molecules to attach to the metal electrode (like gold for example) efficiently, and form metal- molecule-metal junction. For comparison, linear counterparts were also prepared and investigated (Fig. 11). The addtion of pyridine anchors did not affect the folded conformation of molecules f-TPE-PPy and f-TPE-PEPy, which were confirmed by crystallographic analysis. Not only the crystal structure, but also the spectroscopy and theoretical calculation results suggested that through-space conjugation exists in both two folded molecules. The conductivity of the folded and linear single-molecule wires was measured by STM-based break-junction (STM-BJ) technique, and the models of electron conductance in linear and folded molecules are shown in Fig. 12. Experimental results revealed that f-TPE-PPy and f-TPE-PEPy had conductance of 1.40 and 0.50 nS, respectively, which were close to those of l-TPE-PPy (1.50 nS) and l-TPE-PEPy (0.55 nS) [52]. In general, a better π-conjugation [53] and closer energy level to the Fermi energy level of Au usually lead to better conductance. Apparently, with a more twisted molecular backbone, folded molecules should have worse p-conjugation than linear conunterparts. Meanwhile, the differences between HOMO energy levels of the folded molecules (-5.80 and -5.66eV for f- TPE-PPy and f-TPE-PEPy, respectively) and Fermi energy level of Au (-5.3 eV) are larger than the linear ones (-5.70 and -5.59 eV for l- TPE-PPy and l-TPE-PEPy, respectively). In view of these, the conductance of the folded molecules should be one or two order magnitude lower than that of linear counterparts [54], but the fact is that the practical conductance of folded molecules is comparable to that of linear molecules. A reasonable explanation for the close conductance was that folded molecules f-TPE-PPy and f-TPE-PEPy had more than one channels for conductance that is the current could be conducted via through-bond and through-space channels. The through-space conductance can compensate the loss of through-bond one. Such kind of multichannel conductance could help to increase conductivity and stability of single-molecule wires, indicative of a promising and conceptual new strategy to design robust single-molecule wires.
|
Download:
|
| Figure 11. (A) Molecular structures and (B) top and side views of crystal structures of (Z)-o-BPTPE (CCDC 955718),f-TPE-PPy (CCDC 1030245) and f-TPE-PEPy (CCDC 1030246). Hydrogens are omitted for clarity. (C) Molecular structures of l-TPE-PPy and l-TPE-PEPy. Reproduced with permission from Ref. [52]. Copyright (2015) Wiley-VCH. | |
|
Download:
|
| Figure 12. Schematic representation of circuits of f-TPE-PPy and l-TPE-PPy anchored onto gold electrodes. Reproduced with permission from Ref. [52]. Copyright (2015) Wiley-VCH. | |
6. Conclusion
In this review, we have systematically introduced a new kind of TPE derivatives with a folded conformation and through-space conjugation. These folded molecules have two aromatic chains which are closely parallel-aligned with the distances between them shorter than 3.5 A, and through-space conjugation feature appears in the inter-chain regions. They are AEE-active because their rigidified molecular structures have suppressed the nonradiative decay of the excited state in some degree. Hence, they have good fluorescence in dilute solutions. In the aggregated state, the intramolecular rotation is further restricted, leading to enhanced fluorescence, with high ΦF values of up to unit. What's more, large Stokes shifts are also observed, which is believed to be caused by the intramolecular energy transfer. The functionalized folded TPE derivatives have showed interesting bipolar carrier transport ability and multichannel conductance in single-molecule wires. Owing to all of these brilliant features, these folded TPE derivatives are of great potential to become a new kind of optoelectronic materials. As a newly-developing through-space conjugated system, there is still much room for further improvement, and in-depth systematical theoretical researches and application exploration are currently in progress.
Acknowledgments We acknowledge the financial support from the National Natural Science Foundation of China (No. 51273053), the National Basic Research Program of China (973 Program, Nos. 2015CB655004 and 2013CB834702), the Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2014A030306035), the Guangdong Innovative Research Team Program of China (No. 201101C0105067115), the Science and Technology Project of Guangdong Province (No. 2016B090907001), the ITC-CNERC14S01, the Fundamental Research Funds for the Central Universities (Nos. 2015PT020 and 2015ZY013), the Natural Science Foundation of Guangdong Province (No. 2016A030312002) and the National Undergraduate Innovative and Entrepreneurial Training Program (No. 201410561032).| [1] | A. Molina-Ontoria, M. Wielopolski, J. Gebhardt, et al. [2,2']Paracyclophane-based p-conjugated molecular wires reveal molecular-junction behavior. J. Am. Chem. Soc. 133 (2011) 2370–2373. DOI:10.1021/ja109745a |
| [2] | S.T. Schneebeli, M. Kamenetska, Z. Cheng, et al. Single-molecule conductance through multiple p-p-stacked benzene rings determined with direct electrode-to-benzene ring connections. J. Am. Chem. Soc. 133 (2011) 2136–2139. DOI:10.1021/ja111320n |
| [3] | G.P. Bartholomew, G.C. Bazan. Bichromophoric paracyclophanes: models for interchromophore delocalization. Acc. Chem. Res. 34 (2001) 30–39. DOI:10.1021/ar9901568 |
| [4] | J. Linnanto, V.M. Helenius, J.A.I. Oksanen, et al. Exciton interactions and femtosecond relaxation in chlorophyll a water and chlorophyll a dioxane aggregates. J. Phys. Chem. A 102 (1998) 4337–4349. |
| [5] | T. Gensch, S.E. Braslavsky. Volume changes related to triplet formation of watersoluble porphyrins. A laser-induced optoacoustic spectroscopy (LIOAS) study. J. Phys. Chem. B 101 (1997) 101–108. DOI:10.1021/jp960643u |
| [6] | G.B. Schuster. Long-range charge transfer in DNA: transient structural distortions control the distance dependence. Acc. Chem. Res. 33 (2000) 253–260. DOI:10.1021/ar980059z |
| [7] | F.D. Lewis, T.F. Wu, X.Y. Liu, et al. Dynamics of photoinduced charge separation and charge recombination in synthetic DNA hairpins with stilbenedicarboxamide linkers. J. Am. Chem. Soc. 122 (2000) 2889–2902. DOI:10.1021/ja993689k |
| [8] | E. Meggers, M.E. Michel-Beyerle, B. Giese. Sequence dependent long range hole transport in DNA. J. Am. Chem. Soc. 120 (1998) 12950–12955. DOI:10.1021/ja983092p |
| [9] | Y. Morisaki, T. Sawamura, T. Murakami, et al. Synthesis of anthracene-stacked oligomers and polymer. Org. Lett. 12 (2010) 3118–3191. |
| [10] | R. Hoffmann. Interaction of orbitals through space and through bonds. Acc. Chem. Res. 4 (1971) 1–9. DOI:10.1021/ar50037a001 |
| [11] | S.M. Bachrach. DFT study of[2.2]-,[3.3]-, and[4.4] paracyclophanes: strain energy, conformations, and rotational barriers. J. Phys. Chem. A 115 (2011) 2396–2401. DOI:10.1021/jp111523u |
| [12] | H. Wolf, D. Leusser, R.V.J. Mads, et al. Phase transition of. Chem. Eur. J. 20 (2014) 7048–7053. DOI:10.1002/chem.201304972 |
| [13] | J. Poater, P. Alemany, M. Sola. Role of electron density and magnetic couplings on the nucleus-independent chemical shift (NICS) profiles of [2.2] paracyclophane and related species. J. Org. Chem. 71 (2006) 1700–1702. DOI:10.1021/jo052095z |
| [14] | G.F. Caramori, S.E. Galembeck, K.K. Laali. A computational study of[2.2] cyclophanes. J. Org. Chem. 70 (2005) 242–3250. |
| [15] | S. Mukhopadhyay, S.P. Jagtap, V. Coropceanu, et al. p-Stacked oligo(phenylene vinylene)s based on pseudo-geminal substituted[2.2] paracyclophanes: impact of interchain geometry and interactions on the electronic properties. Angew. Chem. Int. Ed. 51 (2012) 11629–11632. DOI:10.1002/anie.201205738 |
| [16] | H. Dodziuk, S. Szymanski, J. Jazwinski, et al. Structure and NMR spectra of some [2.2] paracyclophanes. The dilemma of[2.2] paracyclophane symmetry. J. Phys. Chem. A 115 (2011) 10638–10649. DOI:10.1021/jp205693a |
| [17] | G.P. Bartholomew, G.C. Bazan. Synthesis, characterization, and spectroscopy of 4,7,12,15-[2.2] paracyclophane containing donor and acceptor groups: impact of substitution patterns on through-space charge transfer. J. Am. Chem. Soc. 124 (2002) 5183–5196. DOI:10.1021/ja0121383 |
| [18] | L. Ferrighi, L. Frediani, E. Fossgaard, et al. Two-photon absorption of [2.2] paracyclophane derivatives in solution: a theoretical investigation. J. Chem. Phys. 127 (2007) 244103. DOI:10.1063/1.2814168 |
| [19] | S. Park, J.H. Heo, C.H. Cheon, et al. A[2,2] paracyclophane triarylamine-based hole-transporting material for high performance perovskite solar cells. J. Mater. Chem. A 3 (2015) 24215–24220. DOI:10.1039/C5TA08417B |
| [20] | M. Gon, Y. Morisaki, Y. Chujo. Optically active cyclic compounds based on planar chiral [2.2] paracyclophane: extension of the conjugated systems and chiroptical properties. J. Mater. Chem. C 3 (2015) 521–529. DOI:10.1039/C4TC02339K |
| [21] | G.D. Scholes, G. Rumbles. Excitons in nanoscale systems. Nat. Mater. 5 (2006) 683–696. DOI:10.1038/nmat1710 |
| [22] | J.D. Slinker, N.B. Muren, S.E. Renfrew, et al. DNA charge transport over 34 nm. Nat. Chem. 3 (2011) 228–233. |
| [23] | T. Hirao. Conjugated systems composed of transition metals and redox-active pconjugated ligands. Coord. Chem. Rev. 226 (2002) 81–91. DOI:10.1016/S0010-8545(01)00436-2 |
| [24] | J. Luo, Z. Xie, J.W.Y. Lam, et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. (2001) 1740–1741. |
| [25] | Z. Zhao, J.W.Y. Lam, B.Z. Tang. Tetraphenylethene: a versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 22 (2012) 23726–23740. DOI:10.1039/c2jm31949g |
| [26] | X. Wang, J. Hu, G. Zhang, S. Liu. Highly selective fluorogenic multianalyte biosensors constructed via enzyme-catalyzed coupling and aggregation-induced emission. J. Am. Chem. Soc. 136 (2014) 9890–9893. DOI:10.1021/ja505278w |
| [27] | L. Liu, G. Zhang, J. Xiang, et al. Fluorescence "turn on" chemosensors for Ag+ and Hg2+ based on tetraphenylethylene motif featuring adenine and thymine moieties. Org. Lett. 10 (2008) 4581–4584. DOI:10.1021/ol801855s |
| [28] | J. Mei, N.L. Leung, R.T. Kwok, et al. Aggregation-induced emission: together we shine. United We Soar. Chem. Rev. 115 (2015) 11718–11940. DOI:10.1021/acs.chemrev.5b00263 |
| [29] | J.E. McMurry. Carbonyl-coupling reactions using low-valent titanium. Chem. Rev. 89 (1989) 1513–1524. DOI:10.1021/cr00097a007 |
| [30] | Z. Zhao, J.W.Y. Lam, B.Z. Tang. Self-assembly of organic luminophores with gelation-enhanced emission characteristics. Soft Matter 9 (2013) 4564–4579. DOI:10.1039/c3sm27969c |
| [31] | Z. Zhao, S. Chen, J.W.Y. Lam, et al. Pyrene-substituted ethenes: aggregationenhanced excimer emission and highly efficient electroluminescence. J. Mater. Chem. 21 (2011) 7210. DOI:10.1039/c0jm04449k |
| [32] | J. Zhou, Z. Chang, Y. Jiang, et al. From tetraphenylethene to tetranaphthylethene: structural evolution in AIE luminogen continues. Chem. Commun. 49 (2013) 2491–2493. DOI:10.1039/c3cc00010a |
| [33] | Z. Zhao, B. He, H. Nie, et al. Stereoselective synthesis of folded luminogens with arene-arene stacking interactions and aggregation-enhanced emission. Chem. Commun. 50 (2014) 1131–1133. DOI:10.1039/C3CC47696K |
| [34] | D. Sun, S.V. Rosokha, J.K. Kochi. Through-space (cofacial) p-delocalization among multiple aromatic centers: toroidal conjugation in hexaphenylbenzene-like radical cations. Angew. Chem. Int. Ed. 44 (2005) 5133–5136. DOI:10.1002/(ISSN)1521-3773 |
| [35] | Y. Morisaki, N. Kawakami, T. Nakano, et al. Energy-transfer properties of a [2.2] paracyclophane-based through-space dimer. Chem. Eur. J. 19 (2013) 17715–17718. DOI:10.1002/chem.201303108 |
| [36] | S.M. Mathew, J.T. Engle, C.J. Ziegler, et al. The role of arene-arene interactions in the folding of ortho-phenylenes. J. Am. Chem. Soc. 135 (2013) 6714–6722. DOI:10.1021/ja4026006 |
| [37] | C.A. Hunter, J.K.M. Sanders. The nature of p-p interactions. J. Am. Chem. Soc. 112 (1990) 5525–5534. DOI:10.1021/ja00170a016 |
| [38] | H. Lu, J. Mack, Y. Yang, et al. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 43 (2014) 4778–4823. DOI:10.1039/c4cs00030g |
| [39] | A. Loudet, K. Burgess. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem. Rev. 107 (2007) 4891–4932. DOI:10.1021/cr078381n |
| [40] | S.R. Trenor, A.R. Shultz, B.J. Love, et al. Coumarins in polymers: from light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 104 (2004) 3059–3078. DOI:10.1021/cr030037c |
| [41] | S. Maiti, N. Park, J.H. Han, et al. Gemcitabine-coumarin-biotin conjugates: a target specific theranostic anticancer prodrug. J. Am. Chem. Soc. 135 (2013) 4567–4572. DOI:10.1021/ja401350x |
| [42] | M. Beija, C.A. Afonso, J.M. Martinho. Synthesis and applications of rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 38 (2009) 2410–2433. DOI:10.1039/b901612k |
| [43] | Y.Q. Sun, J. Liu, X. Lv, et al. Rhodamine-inspired far-red to near-infrared dyes and their application as fluorescence probes. Angew. Chem. Int. Ed. 51 (2012) 7634–7636. DOI:10.1002/anie.201202264 |
| [44] | Y. Zhou, Y. Xiao, S. Chi, et al. Isomeric boron-fluorine complexes with donoracceptor architecture: strong solid/liquid fluorescence and large stokes shift. Org. Lett. 10 (2008) 633–636. DOI:10.1021/ol702963w |
| [45] | M. Jia, X. Ma, L. Yan, et al. Photophysical properties of intramolecular charge transfer in two newly synthesized tribranched donor-p-acceptor chromophores. J. Phys. Chem. A 114 (2010) 7345–7352. DOI:10.1021/jp1032355 |
| [46] | B. He, H. Nie, L. Chen, et al. High fluorescence efficiencies and large stokes shifts of folded fluorophores consisting of a pair of alkenyl-tethered, p-stacked oligo-pphenylenes. Org. Lett. 17 (2015) 6174–6177. DOI:10.1021/acs.orglett.5b03152 |
| [47] | Z. Zhao, J.W.Y. Lam, C.Y. Chan, et al. Stereoselective synthesis, efficient light emission, and high bipolar charge mobility of chiasmatic luminogens. Adv. Mater. 23 (2011) 5430–5435. DOI:10.1002/adma.201102804 |
| [48] | Y. Xing, T.H. Park, R. Venkatramani, et al. Optimizing single-molecule conductivity of conjugated organic oligomers with carbodithioate linkers. J. Am. Chem. Soc. 132 (2010) 7946–7956. DOI:10.1021/ja909559m |
| [49] | C.R. Arroyo, S. Tarkuc, R. Frisenda, et al. Signatures of quantum interference effects on charge transport through a single benzene ring. Angew. Chem. Int. Ed. 52 (2013) 3152–3155. DOI:10.1002/anie.201207667 |
| [50] | A. Batra, G. Kladnik, H. Vázquez, et al. Quantifying through-space charge transfer dynamics in p-coupled molecular systems. Nat. Commun. 3 (2012) 1086. DOI:10.1038/ncomms2083 |
| [51] | D.S. Seferos, A.S. Blum, J.G. Kushmerick, et al. Single-molecule charge-transport measurements that reveal technique-dependent perturbations. J. Am. Chem. Soc. 128 (2006) 11260–11267. DOI:10.1021/ja062898j |
| [52] | L. Chen, Y.H. Wang, B. He, et al. Multichannel conductance of folded singlemolecule wires aided by through-space conjugation. Angew. Chem. Int. Ed. 54 (2015) 4231–4235. DOI:10.1002/anie.201411909 |
| [53] | J.R. Quinn, F.W. Foss Jr., L. Venkataraman, et al. Oxidation potentials correlate with conductivities of aromatic molecular wires. J. Am. Chem. Soc. 129 (2007) 12376–12377. DOI:10.1021/ja0745097 |
| [54] | A. Nitzan. Electron transmission through molecules and molecular interfaces. Annu. Rev. Phys. Chem. 52 (2001) 681–750. DOI:10.1146/annurev.physchem.52.1.681 |
 2016, Vol. 27
2016, Vol. 27