b University of Chinese Academy of Sciences, Beijing 100049, China
Electrochromic materials display reversible absorption spectral changes in response to electrochemical stimulus [1, 2]. Common electrochromic materials include inorganic metal oxides, organic polymers, metal complexes and coordination metallopolymers [3-9]. They have received much attention because of their potential applications in information storage, smart windows, and optical telecommunications [10, 11]. Redox-active ruthenium complexes exhibit well-defined redox events and rich charge- transfer absorptions in the visible and near-infrared (NIR) spectral region [12-15]. More importantly, their absorption spectra are largely dependent on the redox state of the complex [16]. These features make ruthenium complexes very attractive for electro- chromism studies and other applications [17, 18].
In order to become practically useful, metal complexes need to be deposited onto electrode surfaces as thin films. Routine film formation methods include layer-by-layer assembly [19], drop-casting and spin-coating [20, 21], self-assembled monolayer formation [22], doctor blading [23], and electropolymerization [24]. Among these methods, electropolymerization are advantageous in a few aspects [25, 26]. Firstly, polymer formation and film deposition are achieved simultaneously, which shortens the experimental time. Secondly, the solubility problem often met in other methods is avoided during electropolymerization, which only needs the good solubility ofthe monomer. Thirdly, the surface coverage or film thickness of electropolymerized films can be readily controlled by varying the polymerization duration and thin films of mixed components can be obtained by co-polymerization. In addition, electropolymerized films are adhesive, stable and electrochemically active. Because of these features, electropolymerization has become an important method for making thin films for applications in various optoelectronic applications.
According to the difference of polymerization mechanism, reductive or oxidative electropolymerization can be distinguished. In the case of reductive electropolymerization, metal complexes functionalized with vinyl groups are usually synthesized as monomers [27]. The mechanism is believed to be anionically initiated, followed by radical-radical chain propagation [28]. In general, at least two vinyl groups are needed for highly efficient polymerization, reflecting the importance of cross-linking affordedz by multiple vinyl groups. The metal coordination sphzere remains essentially intact upon polymerization. In addition to vinyl groups, other functional groups such as chloro or pyridinium group has been reported to initiate reduction electropolymerization [29, 30]. In comparison, many polymerizable groups are available for oxidative electropolymerization, such as thiophene [31], pyrrole [32], carbazole [33], or triphenylamine [34]. These groups are readily oxidized at low potentials to give corresponding radical cations, followed by radical-radical chain propagation to afford polymers. One distinct feature of oxidative electropolymerization is that new electrochemically active units are generated during the polymerization, such as bithiophene or biphenyl-bridged bis-amine units when thiophene or triphenylamine is used as the polymerizable group. In this review, we summarize our recent works on the preparation of electropolymerized coordination films from vinyl- or triphenylamine-functionalized ruthenium monomers and applications of the resulting thin films in multistate NIR electrochromism, ion sensing, and information storage.
2. Reductively electropolymerized filmsThe reductive electropolymerization of vinyl-substituted complexes was pioneered by Murray, Meyer, Abruna, and co-workers in the early 1980s [27, 35]. Since then, the reductive electropolymerization of vinyl-substituted polypyridine complexes has become a convenient method for the in situ preparation and deposition of metallopolymeric films on electrode surfaces [25, 36-38]. Scheme 1 shows the schematic representation of the mechanism of the reductive electropolymerization of [Ru(dvb- py) (bpy)2]2+ (dvbpy = 5, 5'-divinyl-2, 2'-bipyridine; bpy = 2, 2'- bipyridine). The reduction of [Ru(dvbpy)(bpy)2]2+ gives rise to a radical anion intermediate. The dimerization of this intermediate via radical-radical coupling, followed by subtraction of two protons from the solvent, affords a diruthenium complex connected by a saturated four-carbon chain. Upon repetitive reaction, metallopolymers are obtained, which have very limited solubility and form in situ as a thin film coated on the electrode surface.

|
Download:
|
| Scheme. 1. Schematic representation of the mechanism of the reductive electropolymerization [Ru(dvbpy)(bpy)2]2+. | |
The most popular vinyl-substituted polypyridine ligands used for reductive electropolymerization include 4-vinylpyridine, 4- methyl-40-vinyl-2, 2'-bipyridine, and 4'-vinyl-2, 2':6', 2''-terpyridine (vtpy). One classical method for the synthesis of these vinyl- substituted ligands is via a Wittig reaction from corresponding aldehydes [39-41]. A more general and practical method through the Suzuki-Miyauru cross-coupling of bromopolypyridines with potassium vinyltrifluoroborate was reported recently [42]. For example, vtpy can be synthesized by the reaction of commercially available 4'-bromo-2, 2':6', 2''-terpyridine with potassium vinyltrifluoroborate in excellent yield.
Several years ago, we began a project on the electronic coupling studies of diruthenium complexes connected by a bis-cyclometa- lating bridging ligand [43]. In addition to the relevance to the fundamental photoinduced electron transfer processes, these complexes are interesting electro-active molecular materials with switchable physical properties. For instance, biscyclo- metalated ruthenium complexes [Ru2 (tpb) (tpy)2]2+ [44] and [Ru2(tppyr)(tpy)2]2+ [45] (tpb = 1, 2, 4, 5-tetra (pyrid-2-yl) benzene; tppyr = 1, 3, 6, 8-tetra (pyrid-2-yl) pyrene; tpy = 2, 2':6', 2〃-terpyri-dine) show two consecutive Ru(III/II) processes at low potentials. One-electron oxidation of these complexes affords [Ru2(tpb)(t- py)2]3+ and [Ru2(tppyr)(tpy)2]3+, respectively, which exhibit intense intervalence charge-transfer (IVCT) transitions in the NIR region. These NIR absorptions are totally absent in the +2 and +4 redox states. We considered that NIR electrochromic devices could be made if these complexes were deposited on electrode surfaces as polymeric films. Therefore, vinyl-substituted cyclometalated diruthenium complexes [Ru2(tpb)(vtpy)2]2+ (12+) [46] and [Ru2(tp-pyr) (vtpy)2]2+(22+) [47] were prepared and studied (Figs. 1 and 2).

|
Download:
|
| Figure 1. (a) Schematic representation of the reductive electropolymerization of 12+. (b) CV recorded during the reductive electropolymerization of 12+ at a glass ITO electrode. (c) CV of poly -12+/ITO film at 100 mV/s. (d,e) Absorption spectral changes of poly -12+/ITO film upon stepwise oxidation. The potential is referenced vs. Ag/AgCl. See details in ref [46]. | |

|
Download:
|
| Figure 2. (a) Schematic representation of the reductive electropolymerization of 22+. (b) CV recorded during the reductive electropolymerization of 22+ at a glass ITO electrode. (c) CV of poly -22+/ITO film at 100 mV/s. (d,e) Absorption spectral changes of poly -22+/ITO film upon stepwise oxidation. The potential is referenced vs. Ag/AgCl. See details in ref [47]. | |
Complexes 12+ and 22+ were successfully deposited on electrode surfaces by reductive electropolymerization. The resulting metal- lopolymeric films possess two stepwise redox couples. The redox potentials and the potential separation between two waves of the polymeric film are similar to those observed for compounds without two vinyl groups. This indicates that the basic electrochemical properties of the dimetallic unit are retained after electropolymerization. These films exhibited good electrochemical stability. Well-defined redox couples were retained when the potential was scanned for over 1000 cycles.
The absorption spectral changes of the obtained films were monitored upon stepwise oxidative electrolysis. These metallo- polymeric films exhibited three-step electrochromism in the NIR region. For the poly -12+ film, when the applied potential was gradually increased to +0.4 V (the first one-electron oxidation), the IVCT transitions at 1165 nm increased and the deep blue color of the original film changed to pink. Upon further increasing the potential to +1.0 V, the IVCT band decreased. As a result, the film changed from pink to green. These two-step processes are totally reversible. The polymeric film showed appealing electrochromic behavior in the NIR region, including multicolor switching, good contrast ratio (around 40% at 1165 nm), short response time (around 5 s), low switching voltage, and remarkably long memory time, which may make them useful in applications such as smart windows.
The poly -22+ film showed similar two-step electrochromic processes (Fig. 2). The poly -22+ film on ITO electrode displays two Ru(III/II) waves at +0.47 and +0.67 V vs. Ag/AgCl. The IVCT band in the mixed-valence state showed absorption at 1900 nm. The appearance and disappearance of the IVCT band can be observed by applying potentials to stepwise oxidize two ruthenium sites. The color of the film changed from blue to brown and then to orange during this two-step process.
The above polymeric films displayed NIR electrochromic behavior at 1160 nm and 1900 nm, respectively. It is known that common glass fiber has the lowest energy loss at 1310 and 1550 nm [7, 10]. To optimize the NIR electrochromism wavelength for potential applications in optic telecommunications, another related polymeric film was prepared from the vinyl-containing biscyclometalated ruthenium complex [Ru2(dpb)(vbpy)4]2+ (32+, dpb is 1, 4-di(pyrid-2-yl)benzene; vbpy is 5-vinyl-2, 2'-bipyridine; structure not shown) [48]. This film displays two redox couples at +0.16 and +0.60 V vs. Ag/AgCl and NIR electrochromism at 1300 nm with a contrast ratio of 41%. It is potentially useful as variable optical attenuators for fiber telecommunications.
In addition to the above diruthenium complexes, we recently found that a simple cyclometalated monoruthenium complex conjugated with a diarylamine unit could give rise to interesting electrochromism at two wavelengths [49]. A related vinyl- substituted ruthenium-amine conjugated complex 4+ was thus prepared (Fig. 3) [50]. This complex was deposited on ITO electrodes by reductive electropolymerization. The obtained poly -4+ film displays two well-defined redox couples at +0.32 and +0.68 V vs. Ag/AgCl, corresponding to the oxidations of the amine and ruthenium components, respectively. It shows electro- chromism at two wavelengths (1070 and 700 nm). In the first- electron oxidation step, an intense charge transfer absorption band at 1070 nm increased. In the second oxidation step, this band disappeared, and a more intense band at 700 nm appeared. The latter band is due to the N+-localized π→π* transitions. The color of the film is purple, brown, and sky blue at three different redox states (-0.2, +0.55, and +1.05 V). These two steps are totally reversible. When the applied potential was switched stepwise between -0.20 and +0.55 V, a transmittance change (△T%) of 52% at 1070 nm was achieved. When the potential was switched between +0.55 and +1.05 V, a best contrast ratio of 76% at 700 nm was achieved.

|
Download:
|
| Figure 3. (a) Schematic representation of the reductive electropolymerization of 4+. (b) CV recorded during the reductive electropolymerization of 4+ at a glass ITO electrode. (c) CV of poly -42+/ITO film at 100 mV/s. (d,e) Absorption spectral changes of poly -42+/ITO film upon stepwise oxidation. The potential is referenced vs. Ag/AgCl. See details in ref [50]. | |
The above electrochromism is featured by long retention time at all three stages (infinity at -0.20 V, 4 h at +0.55 V, and 30 min at +1.05 V, respectively). This suggests that this film is potentially useful for information storage with electrochemical inputs and optical outputs. For instance, the special spectroelectrochemical response and long retention time of the poly -4+ film make it useful for the demonstrations of a set/reset flip-flop logic gate (Fig. 4), which is a binary sequential logic circuit constructed from a pair of cross-coupled NOR logic gates [51]. The singly- and doubly- oxidized states of a film about 10 nm thick were used to build such a surface-confined Set/Reset flip-flop logic gate. Two electrochemical potentials at +0.55, and +1.05 V were treated as In1, and In2, respectively, and the optical absorbance at 700, and 1070 nm were treated as Out1, and Out2, respectively. By using another intermediate state between the singly- and doubly-oxidized states, a multi-valued logic system has also been demonstrated [50].
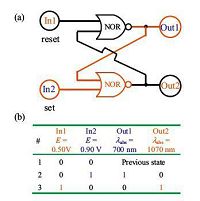
|
Download:
|
| Figure 4. Logic circuit (a) and truth table (b) for a mimicking flip-flop logic based on poly -42+ film. See details in ref [50]. | |
To demonstrate a more complicated logic gate function, poly - 52+ film featuring a triarylamine-bridged diruthenium structure was prepared by reductive electropolymerization (Fig. 5) [52]. This film displayed three consecutive redox couples at +0.20, +0.41, and +1.01 V vs. Ag/AgCl. Upon stepwise oxidation from poly -52+ through poly -55+, the film shows three-step spectral changes. When the potential was gradually applied from -0.10 V to +0.35 V vs.Ag/AgCl, a distinct absorption band centered on 1680 nm appeared. When the potential was further increased from +0.35 V to +0.75 V, the NIR band at 1680 nm gradually decreased and a new peak at 1170 nm appeared. In a third step oxidation (from +0.75 V to +1.30 V), the new peak at 1170 nm decreased again and the appearance of a band at 750 nm was observed. These three step spectral changes are fully reversible when the potential was decreased from +1.30V to -0.10 V. In addition, this film also has long retention time at different redox states. The poly -52+ and poly -53+ are essentially stable states. The retention time of poly - 54+ and poly -55+ states are six 6 h and 20 min, respectively.

|
Download:
|
| Figure 5. (a) Schematic representation of the reductive electropolymerization of 52+. (b 52+ film at different redox states. (d,e) Logic circuit and truth table of the flip-flap logic gate based on the poly -52+ film. See details in ref [52]. | |
Three higher redox states have three exclusive and well- separated absorption bands (1680 nm for 53+; 1170 nm for 54+; 750 nm for 55+) and these states can be triggered by well-separated electrochemical potentials. This feature makes it useful in a more complicated logic gate system. Three electrochemical potentials are treated as three input signals (+0.35 V for In1, +0.75 V for In2, and +1.30 V for In3, respectively). The optical absorption at 750, 1170, and 1070 nm are treated as three output signals, Out1, Out2, and Out3, respectively. A flip-flap-flop logic circuit was mimicked by using the poly -52+ film (around 15-20 nm thick). When In1 is applied, Out 3 is high with an output string of 001. When In2 is applied, the input and output strings are both 010. When In3 is applied, Out1 is high with an output string of 100 [52]. When no potential is applied, the output displays the absorption signal memorized in the previous state.
In addition to the applications in electrochromism, electro- polymerized films are useful as solid-supported sensors [53-55]. Compared to the solution-based technology, ion sensing with a thin film is advantageous for a number of reasons. Ion sensing with thin films is very easy to operate and the detection process is instantaneous. In addition, thin films can be readily separated from the analytes and recycled for repeated uses. We recently reported that poly -6, a ruthenium copolymer modified with a dipicolyla- mine (DPA) motif, could be used for the selective detection of Cu2+ (Fig. 6) [56]. This polymer was obtained by the reductive copolymerization of two ruthenium monomers containing vinyl functional groups. The DPA motif functions as the ion recognition site. The copolymer structure is beneficial for decreasing the solubility of the polymer and thus enhancing the adhesion of the polymer to electrode surfaces. In addition, the copolymeric structure is expected to decrease the density of the DPA unit and expand the free space for the incorporation of Cu2+. Poly-6/ITO film is able to selectively detect Cu2+ via optical and electrochemical readouts at the solid-liquid interface. After being soaked in the solution of Cu(ClO4)2, the film turned brown from purple, as a result of the decrease of the metal-to-ligand charge transfer (MLCT) transitions and increase of a new ligand-to-metal charge transfer (LMCT) absorption at 720 nm. When the polymeric film was soaked in solutions containing other ions (Ag+, Cd2+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Zn2+, Mn2+, Ca2+, Mg2+, Na+, or K+), the color did not change at all. In addition, a new redox peak at +0.20 V appeared when poly -6/ITO film was treated with Cu2+. However, this peak could not be observed in the absence of Cu2+ or in the presence of other transition-metal ions examined. This redox peak is corresponding to the CuII/I process when the Cu2+ ion binds to the DPA unit in the film [57]. By using EDTA to remove the incorporated Cu2+, the film can repeatedly detect Cu2+ with good reversibility and film stability, suggesting of the potential use of the film in sensory devices.

|
Download:
|
| Figure 6. (a) poly -6. (b) Absorption spectral changes of the poly -6/ITO film before and after being soaked in Cu(ClO4)2 solution for 5 min. See details in ref [56]. | |
3. Oxidatively electropolymerized films
Compared with the reductive electropolymerization, preparation of metallopolymeric films by oxidative electropolymerization has received much more attention [58-60]. Metal complexes functionalized with appropriate polymerizable groups such as thiophene [61], pyrrole [62], aromatic amine [63], diphenylamine [64], carbazole [65], and triphenylamine [66] have long been known to undergo oxidative electropolymerization. In addition to the reductive electropolymerization discussed in the previous section, we are also interested in making thin films of redox-active ruthenium complexes using triphenylamine as the polymerizable groups. Some representative examples are discussed in this section.
Triphenylamine usually undergoes dimerization upon oxidation to afford tetraphenylbenzidine. Upon further oxidation, tetraphenylbenzidine is either transformed into a delocalized radical cation or a close-shell dication. Both of them are unreactive for further polymerization (Scheme 2a). However, if a metal complex containing two distal decoupled triphenylamine units is used as the monomer, the oxidative electropolymerization could occur smoothly (Scheme 2b). Upon electrochemical oxidation, the two triphenylamine units are transformed into localized radical cation intermediates. After carbon-carbon bond formation on the para-position of the terminal benzene rings, followed by a reductive de-doping process, a dimeric structure is produced. Upon further oxidation, the chain propagation takes from the two terminal triphenylamine groups of the dimer to give polymers. This strategy was adopted for the preparation of thin films of metal complexes and organic materials by Leung, Liou and others [67, 68].

|
Download:
|
| Scheme. 2. Mechanism of oxidative dimerization and electropolymerization of triarylamine compounds. | |
By using a similar strategy, we prepared two cyclometalated diruthenium complexes [Ru2(tpb)(Nptpy)2]2+ (72+) [Ru2(tp- pyr)(Nptpy)2]2+ (82+) and (Fig. 7) [69], where Nptpy is 4'-(p-N, N- diphenylamino)phenyl-2, 2':6', 2''-terpyridine. Both complexes can be successfully deposited on electrode surfaces by oxidative electropolymerization (Fig. 7b and 7d). The resulting polymers are composed of two types of alternating constituent units: bridged cyclometalated diruthenium units and bis-triarylamine segments. These films exhibited four well-defined anodic redox couples as a result of the stepwise oxidations of these two units. In addition to the two Ru(III/II) processes, two new redox waves appeared at +0.90 and +1.04V. These two waves are assigned to the stepwise oxidations of the two amine units from the newly generated tetraphenylbenzidine structure of the polymer. The Ru(III/II) potentials basically remain unchanged with respect to those of corresponding monomers.

|
Download:
|
| Figure 7. (a, c) Schematic representation for the oxidative electropolymerization of 72+ and 82+. (b, d) CV recorded during the electropolymerization at a glass ITO electrode of 72+ and 82+, respectively. See details in ref [69]. | |
Because of the presence of four consecutive redox processes in the anodic scan, the poly -72+ and poly -82+ film display reversible four-step NIR electrochromism (Fig. 8). In the first two-step oxidations, the increase and decrease of the IVCT transitions of the diruthenium segment were observed. The operating wavelength is 1185 and 2150 nm for poly -72+ and poly -82+, respectively. Similar spectral changes have been discussed for the poly -12+ and poly -22+ films in Figs. 1 and 2. In the third- and fourth-step switching processes, both polymers show the increase and decrease of the NIR transitions at 1500 nm. This band is associated with the IVCT transition of the one-electron-oxidized tetraphenylbenzidine unit [70].

|
Download:
|
| Figure 8. Absorption spectral changes of poly -72+ (a-d) and poly -82+ film (e-h) on ITO electrode upon stepwise oxidation. The potential is referenced vs. Ag/AgCl. See details in ref [69]. | |
Recently, resistive memory devices based on thin films of redox-active materials have received much attention because they hold great promise for high-density data storage with a miniaturized device size [71]. A resistive memory operates as an electrical switch between high and low conductivity states and remembers its present resistance when the electric power supply is turned off.
In addition to organic and polymeric materials [72], the incorporation of transition metal complexes into resistive memory devices has been the focus of several studies [73]. It should be noted that the thin films for most known resistive memory devices are prepared by either spin-coating or vapor deposition. The applications of electropolymerized films in resistive memory devices remained largely unexplored.
By using triphenylamine as the polymerizable group, we recently prepared the thin film of poly -94+ containing a diruthenium-diamine conjugated structural unit (Fig. 9a) [74]. In this polymer, two Ru ions are connected by a 2, 3, 5, 6-tetrakis(2-pyridyl)pyrazine (tppz) bridging ligand, which becomes electron-deficient upon coordination with ruthenium. As a result, this polymer is readily reduced at - 0.34 V vs. Ag/AgCl and has a low LUMO level of -5.6 eV. The diamine unit is readily oxidized at +0.90 V and the polymer has a HOMO level of -4.4 eV. Using poly -94+ as the active-layer, the sandwiched ITO/ polymer/Al electrical devices show excellent resistive memory switching with low operational voltage, large ON/OFF current ratio (100~1000), good stability (500 cycles tested), and long retention time.
Fig. 9c shows the typical I-V characteristics of the above device. In the first voltage sweep from 0 to +5 V, an abrupt increase in the current was observed at a threshold voltage of +3.4V. This indicates that the device was switched from a low-conductivity state (OFF state) to a high-conductivity state (ON state), corresponding to the "write" process. The high-conductivity state was retained during the second sweep from 0 to +5 V, implying the data were memorized. The OFF state can be recovered by simply applying a reverse voltage in the third sweep, where an abrupt drop in current occurred at a threshold voltage of -4.3 V. This serves as the "erase" process for the memory device. The fourth sweep from 0to-5V showed the I-V characteristics right after the erase process, indicating that the device remained in the stable OFF state. These I-V characteristics defined the electrical bistability of the device. The mechanism of the field-induced conductivity of the poly -94+ film is probably due to the formation of a charge transfer state from the diamine unit to the tppz segment as supported by DFT calculations and electrochemical analysis.

|
Download:
|
| Figure 9. (a, d) poly -94+ and poly -102+. (b, e) CV of the poly -94+ and poly -102+ film on ITO surface. (c, f) Typical I-V characteristics of the ITO/polymer/Al device with poly -94+ or poly -102+ as the active polymer layer. See details in ref [74]. | |
For the purpose of comparison, a monoruthenium complex 102+ with two appended triphenylamine units was also prepared, from which poly -102+ was prepared by oxidative electropolymerization (Fig. 9d). In stark contrast, the ITO/poly -102+/Al device shows much poorer memory performance with respect to the poly -94+ device. A hysteretic I-V characteristic was observed for the device with poly -102+ film (Fig. 9f). However, no abrupt decrease or increase in current occurred, and the best ON/OFF ratio achieved was less than 15. Poly-102+ and poly -94+ have similar HOMO levels. Based on the electrochemical data, the LUMO of poly -102+ is estimated to be -3.5 eV vs. vacuum, which is 0.9 eV more destabilized with respect to that of poly -94+. Such a high LUMO level is unfavorable for the formation of a potential charge transfer state of 102+. This highlights the critical roles of the molecular design and electronic properties of materials in developing excellent molecular devices.
4. ConclusionElectropolymerization is a convenient and practical method for making thin films of redox-active metal complexes. The resulting films are stable, adhesive, and suitable for further electrochemical characterization and solid device fabrications. They are useful for multi-state NIR electrochromism, ion sensing, and information storage. The polymerization can be initiated by either a reductive or oxidative method. The reductive electropolymerization ofvinyl- substituted metal complexes basically retains the electrochemical and spectroscopic properties of the monomer. However, the oxidative electropolymerization of triphenylamine-substituted complexes introduces a new tetraphenylbenzidine functional unit, which may strongly affect the electronic and spectroscopic properties of the polymer.
The reductive and oxidative electropolymerization is not incompatible with each other. For instance, a one-pot layer-bylayer deposition of thin films of a vinyl-functionalized ruthenium complex and a carbazole-functionalized fluorene derivative has been recently reported via the alternation of reductive and oxidative electropolymerization [75]. Such a method would be very convenient for the preparation of thin films of mixed components and it seems that there is no big interference between the reductive and oxidative polymerization. However, more experiments are needed to prove the capability of this method. In addition, the electropolymerization method is not limited to the film formation of mono- and di-metallic complexes. The oxidative electropolymerization of a supramolecular hexakistriphenylamine platinum metallacycles was recently reported to afford structurally defined porous membranes with molecular-sieving functions [76]. With these efforts, the applications of electropolymerized films of redox-active materials are believed to be significantly expanded in the future.
Acknowledgment We thank the National Natural Science Foundation of China (Nos. 21271176, 21472196, 21521062, and 21501183) and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB 12010400) for funding support.| [1] | R.J. Mortimer. Electrochromic materials. Chem. Soc. Rev. 26 (1997) 147–156. DOI:10.1039/cs9972600147 |
| [2] | P.M. Beaujuge, J.R. Reynolds. Color control in p-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 110 (2010) 268–320. DOI:10.1021/cr900129a |
| [3] | X.D. Wu, Y.S. Wu, C.Y. Zhang, et al. Polyurethanes prepared from isocyanates containing triphenylamine derivatives: synthesis and optical, electrochemical, electrochromic and memory properties. RSC Adv. 5 (2015) 58843–58853. DOI:10.1039/C5RA09361A |
| [4] | L. Beverina, G.A. Pagani, M. Sassi. Multichromophoric electrochromic polymers: colour tuning of conjugated polymers through the side chain functionalization approach. Chem. Commun. 50 (2014) 5413–5430. DOI:10.1039/c4cc00163j |
| [5] | E.L. Runnerstrom, A. Llordés, S.D. Lounis, D.J. Milliron. Nanostructured electrochromic smart windows: traditional materials and NIR-selective plasmonic nanocrystals. Chem. Commun. 50 (2014) 10555–10572. DOI:10.1039/C4CC03109A |
| [6] | J.W. Liu, J. Zheng, J.L. Wang, et al. Ultrathin W18O49 nanowire assemblies for electrochromic devices. Nano Lett. 13 (2013) 3589–3593. DOI:10.1021/nl401304n |
| [7] | B. Yao, F.K. Chen, H. Jiang, J. Zhang, X.H. Wan. Isomer effect on the near-infrared electrochromism of anthraquinone imides. Electrochim. Acta 166 (2015) 73–81. DOI:10.1016/j.electacta.2015.03.057 |
| [8] | M. Higuchi. Stimuli-responsive metallo-supramolecular polymer films: design, synthesis and device fabrication. J. Mater. Chem. C 2 (2014) 9331–9341. |
| [9] | R.J. Mortimer, D.R. Rosseinsky, P.M.S. Monk, Electrochromic Materials and Devices, Wiley-VCH, Weinheim, 2015, pp. 185-210. |
| [10] | S. Wang, X.Z. Li, S.D. Xun, X.H. Wan, Z.Y. Wang. Near-Infrared electrochromic and electroluminescent polymers containing pendant ruthenium complex groups. Macromolecules 39 (2006) 7502–7507. DOI:10.1021/ma061751+ |
| [11] | G. Sonmez. Polymeric electrochromics. Chem. Commun. (2005) 5251–5259. |
| [12] | U.S. Schubert, C. Eschbaumer. Macromolecules containing bipyridine and terpyridine metal complexes: towards metallosupramolecular polymers. Angew. Chem. Int. Ed. 41 (2002) 2892–2926. DOI:10.1002/1521-3773(20020816)41:16<2892::AID-ANIE2892>3.0.CO;2-6 |
| [13] | I. Eryazici, C.N. Moorefield, G.R. Newkome, square-planar Pd(II). Pt(II), and Au(III) terpyridine complexes: their syntheses, physical properties, supramolecular constructs, and biomedical activities. Chem. Rev. 108 (2008) 1834–1895. DOI:10.1021/cr0781059 |
| [14] | E.A. Medlycott, G.S. Hanan. Designing tridentate ligands for ruthenium(II) complexes with prolonged room temperature luminescence lifetimes. Chem. Soc. Rev. 34 (2005) 133–142. DOI:10.1039/b316486c |
| [15] | J.A.G. Williams. The coordination chemistry of dipyridylbenzene: N-deficient terpyridine or panacea for brightly luminescent metal complexes. Chem. Soc. Rev. 38 (2009) 1783–1801. DOI:10.1039/b804434c |
| [16] | M.J. Sun, H.J. Nie, J.N. Yao, Y.W. Zhong. Bis-triarylamine with a cyclometalated diosmium bridge: a multi-state redox-active system. Chin. Chem. Lett. 26 (2015) 649–652. DOI:10.1016/j.cclet.2015.03.032 |
| [17] | M. Abe, H. Futagawa, T. Ono, et al. An electropolymerized crystalline film incorporating axially-bound metalloporphycenes: remarkable reversibility, reproducibility, and coloration efficiency of ruthenium(II/III)-based electrochromism. Inorg. Chem. 54 (2015) 11061–11063. DOI:10.1021/acs.inorgchem.5b02129 |
| [18] | M. Chhatwal, A. Kumar, S.K. Awasthi, M. Zharnikov, R.D. Gupta. An electroactive metallo-polypyrene film as a molecular scaffold for multi-state volatile memory devices. J. Phys. Chem. C 120 (2016) 2335–2342. DOI:10.1021/acs.jpcc.5b12597 |
| [19] | A. Maier, A.R. Rabindranath, B. Tieke. Fast-switching electrochromic films of zinc polyiminofluorene-terpyridine prepared upon coordinative supramolecular assembly. Adv. Mater. 21 (2009) 959–963. DOI:10.1002/adma.v21:9 |
| [20] | S.J. Vickers, M.D. Ward. Facile preparation of a visible- and near-infrared-active electrochromic film by direct deposition of a ruthenium dioxolene complex on an ITO/glass surface. Electrochem. Commun. 7 (2005) 389–393. DOI:10.1016/j.elecom.2005.02.013 |
| [21] | C.S. Grange, A.J.H.M. Meijer, M.D. Ward. Trinuclear ruthenium dioxolene complexes based on the bridging ligand hexahydroxytriphenylene: electrochemistry, spectroscopy, and near-infrared electrochromic behaviour associated with a reversible seven-membered redox chain. Dalton Trans. 39 (2010) 200–211. DOI:10.1039/B918086A |
| [22] | B.B. Cui, Y.W. Zhong, J.N. Yao. Three-state near-infrared electrochromism at the molecular scale. J. Am. Chem. Soc. 137 (2015) 4058–4061. DOI:10.1021/jacs.5b00586 |
| [23] | I.K. Ding, J. Melas-Kyriazi, N.L. Cevey-Ha, et al. Deposition of hole-transport materials in solid-state dye-sensitized solar cells by doctor-blading. Org. Electron. 11 (2010) 1217–1222. DOI:10.1016/j.orgel.2010.04.019 |
| [24] | P. Denisevich, H.D. Abruña, C.R. Leidner, et al. Electropolymerization of vinylpyridine and vinylbipyridine complexes of iron and ruthenium: homopolymers, copolymers, reactive polymers. Inorg. Chem. 21 (1982) 2153–2161. DOI:10.1021/ic00136a006 |
| [25] | Y.W. Zhong, C.J. Yao, H.J. Nie. Electropolymerized films of vinyl-substituted polypyridine complexes: synthesis, characterization, and applications. Coord. Chem. Rev. 257 (2013) 1357–1372. DOI:10.1016/j.ccr.2013.01.001 |
| [26] | C. Friebe, M.D. Hager, A. Winter, U.S. Schubert. Metal-containing polymers via electropolymerization. Adv. Mater. 24 (2012) 332–345. DOI:10.1002/adma.201103420 |
| [27] | H.D. Abrun ã, P. Denisevich, M. Uman ã, T.J. Meyer, R.W. Murray. Rectifying interfaces using two-layer films of electrochemically polymerized vinylpyridine and vinylbipyridine complexes of ruthenium and iron on electrodes. J. Am. Chem. Soc. 103 (1981) 1–5. DOI:10.1021/ja00391a001 |
| [28] | J.M. Calvert, R.H. Schmehl, B.P. Sullivan, et al. Synthetic and mechanistic investigations of the reductive electrochemical polymerization of vinyl-containing complexes of iron(II), ruthenium(II), and osmium(II). Inorg. Chem. 22 (1983) 2151–2162. DOI:10.1021/ic00157a013 |
| [29] | O. Fussa-Rydel, H.T. Zhang, J.T. Hupp, C.R. Leidner. Electrochemical assembly of metallopolymeric films via reduction of coordinated 5-chlorophenanthroline. Inorg. Chem. 28 (1989) 1533–1537. DOI:10.1021/ic00307a022 |
| [30] | S.C. Huang, C.Y. Lin. Reductive electropolymerization of N-methyl-3-pyridylethynyl-porphyrins. Chem. Commun. 51 (2015) 519–521. DOI:10.1039/C4CC08157A |
| [31] | M.O. Wolf. Transition-metal-polythiophene hybrid materials. Adv. Mater. 13 (2001) 545–553. DOI:10.1002/(ISSN)1521-4095 |
| [32] | A. Deronzier, J.C. Moutet. Polypyrrole films containing metal complexes: syntheses and applications. Coord. Chem. Rev. 147 (1996) 339–371. DOI:10.1016/0010-8545(95)01130-7 |
| [33] | C. Gu, Z.B. Zhang, S.H. Sun, et al. In situ electrochemical deposition and doping of C60 films applied to high-performance inverted organic photovoltaics. Adv. Mater. 24 (2012) 5727–5731. DOI:10.1002/adma.v24.42 |
| [34] | C.B.Fan, C.Q. Ye, X.M. Wang, et al. Synthesis and electrochromic properties of new terpyridine-triphenylamine hybrid polymers. Macromolecules 48 (2015) 6465–6473. DOI:10.1021/acs.macromol.5b00493 |
| [35] | H.D. Abruña. Coordination chemistry in two dimensions: chemically modified electrodes. Coord. Chem. Rev. 86 (1988) 135–189. DOI:10.1016/0010-8545(88)85013-6 |
| [36] | S. Gould, T.R. O'Toole, T.J. Meyer. Photochemical ligand loss as a basis for imaging and microstructure formation in a thin polymeric film. J. Am. Chem. Soc. 112 (1990) 9490–9496. DOI:10.1021/ja00182a007 |
| [37] | R.M. Leasure, W. Ou, J.A. Moss, R.W. Linton, T.J. Meyer. Spatial electrochromism in metallopolymeric films of ruthenium polypyridyl complexes. Chem. Mater. 8 (1996) 264–273. DOI:10.1021/cm950371s |
| [38] | T. Kajita, R.M. Leasure, M. Devenney, D. Friesen, T.J. Meyer. Electropolymerized films of macromeric assemblies. Inorg. Chem. 37 (1998) 4782–4794. DOI:10.1021/ic971279w |
| [39] | K.T. Potts, D.A. Usifer, A. Guadalupe, H.D. Abruna. 4-vinyl-,6-vinyl-, and 4'-vinyl-2,2':6',2"-terpyridinyl ligands: their synthesis and the electrochemistry of their transition-metal coordination complexes. J. Am. Chem. Soc. 109 (1987) 3961–3967. DOI:10.1021/ja00247a021 |
| [40] | G.R. Newkome, G.E. Kiefer, N. Matsumura, W.E. Puckett. Chemistry of heterocyclic compounds series. 110. Synthesis of vinyl derivatives of phenanthroline and bipyridine. J. Org. Chem. 50 (1985) 3807–3810. DOI:10.1021/jo00220a025 |
| [41] | M.E. Wright, S.R. Pulley. An improved synthesis of 4-vinyl-2,6-di-tert-butylpyridine and its suspension copolymerization with styrene and divinylbenzene. J. Org. Chem. 52 (1987) 1623–1624. DOI:10.1021/jo00384a051 |
| [42] | H.J. Nie, J.Y. Shao, J. Wu, J.N. Yao, Y.W. Zhong. Synthesis and reductive electropolymerization of metal complexes with 5,5'-divinyl-2,2'-bipyridine. Organometallics 31 (2012) 6952–6959. DOI:10.1021/om300730f |
| [43] | Y.W. Zhong, Z.L. Gong, J.Y. Shao, J.N. Yao. Electronic coupling in cyclometalated ruthenium complexes. Coord. Chem. Rev. 312 (2016) 22–40. DOI:10.1016/j.ccr.2016.01.002 |
| [44] | C.J. Yao, Y.W. Zhong, J.N. Yao. Charge delocalization in a cyclometalated bisruthenium complex bridged by a noninnocent 1,2,4,5-tetra(2-pyridyl) benzene ligand. J. Am. Chem. Soc. 133 (2011) 15697–15706. DOI:10.1021/ja205879y |
| [45] | C.J. Yao, H.J. Nie, W.W. Yang, J.N. Yao, Y.W. Zhong. Combined experimental and computational study of pyren-2,7-diyl-bridged diruthenium complexes with various terminal ligands. Inorg. Chem. 54 (2015) 4688–4698. DOI:10.1021/ic503117k |
| [46] | C.J. Yao, Y.W. Zhong, H.J. Nie, H.D. Abruña, J.N. Yao. Near-IR electrochromism in electropolymerized films of a biscyclometalated ruthenium complex bridged by 1,2,4,5-tetra(2-pyridyl)benzene. J. Am. Chem. Soc. 133 (2011) 20720–20723. DOI:10.1021/ja209620p |
| [47] | C.J. Yao, J.N. Yao, Y.W. Zhong. Metallopolymeric films based on a biscyclometalated ruthenium complex bridged by 1,3,6,8-tetra(2-pyridyl)pyrene: applications in near-infrared electrochromic windows. Inorg. Chem. 51 (2012) 6259–6263. DOI:10.1021/ic3004646 |
| [48] | H.J. Nie, Y.W. Zhong. Near-infrared electrochromism in electropolymerized metallopolymeric films of a phen-1,4-diyl-bridged diruthenium complex. Inorg. Chem. 53 (2014) 11316–11322. DOI:10.1021/ic5019967 |
| [49] | C.J. Yao, R.H. Zheng, Q. Shi, Y.W. Zhong, J.J. Yao. 1,4-Benzene-bridged covalent hybrid of triarylamine and cyclometalated ruthenium: a new type of organicinorganic mixed-valent system. Chem. Commun. 4 (2012) 5680–5682. |
| [50] | B.B. Cui, C.J. Yao, J.N. Yao, Y.W. Zhong. Electropolymerized films as a molecular platform for volatile memory devices with two near-infrared outputs and long retention time. Chem. Sci. 5 (2014) 932–941. DOI:10.1039/C3SC52815D |
| [51] | G. de Ruiter, E. Tartakovsky, N. Oded, M.E. van der Boom. Sequential logic operations with surface-confined polypyridyl complexes displaying molecular random access memory features. Angew. Chem. Int. Ed. 49 (2010) 169–172. DOI:10.1002/anie.200905358 |
| [52] | B.B. Cui, J.H. Tang, J.N. Yao, Y.W. Zhong. A molecular platform for multistate nearinfrared electrochromism and flip-flop, flip-flap-flop, and ternary memory. Angew. Chem. Int. Ed. 54 (2015) 9192–9197. DOI:10.1002/anie.201504584 |
| [53] | H.N. Kim, Z.Q. Guo, W.H. Zhu, J. Yoon, H. Tian. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 40 (2011) 79–93. DOI:10.1039/C0CS00058B |
| [54] | Y.Y. Yao, L. Zhang, Z.F. Wang, J.K. Xu, Y.P. Wen. Electrochemical determination of quercetin by self-assembled platinum nanoparticles/poly(hydroxymethylated-3,4-ethylenedioxylthiophene) nanocomposite modified glassy carbon electrode. Chin. Chem. Lett. 25 (2014) 505–510. DOI:10.1016/j.cclet.2014.01.028 |
| [55] | Y.W. Gao, H. Bai, G.Q. Shi. Electrosynthesis of oligo(methoxyl pyrene) for turn-on fluorescence detection of volatile aromatic compounds. J. Mater. Chem. 20 (2010) 2993–2998. DOI:10.1039/b924992c |
| [56] | Z.L. Gong, B.B. Cui, W.W. Yang, J.N. Yao, Y.W. Zhong. Reversible multichannel detection of Cu2+ using an electropolymerized film. Electrochim. Acta 130 (2014) 748–753. DOI:10.1016/j.electacta.2014.03.096 |
| [57] | Z.L. Gong, Y.W. Zhong. Stepwise coordination followed by oxidation mechanism for the multichannel detection of Cu2+ in an aqueous environment. Organometallics 32 (2013) 7495–7502. DOI:10.1021/om400999h |
| [58] | Y.X. Gao, J. Qi, J. Zhang, et al. Fabrication of both the photoactive layer and the electrode by electrochemical assembly: towards a fully solution-processable device. Chem. Commun. 50 (2014) 10448–10451. DOI:10.1039/C4CC04788E |
| [59] | C. Gu, Y.C. Chen, Z.B. Zhang, et al. Electrochemical route to fabricate film-like conjugated microporous polymers and application for organic electronics. Adv. Mater. 25 (2013) 3443–3448. DOI:10.1002/adma.v25.25 |
| [60] | C. Gu, N. Huang, Y. Wu, H. Xu, D.L. Jiang. Design of highly photofunctional porous polymer films with controlled thickness and prominent microporosity. Angew. Chem. Int. Ed. 54 (2015) 11540–11544. DOI:10.1002/anie.201504786 |
| [61] | C. Friebe, B. Schulze, H. Görls, M. Jäger, U.S. Schubert. Designing cyclometalated ruthenium(II) complexes for anodic electropolymerization. Chem. Eur. J. 20 (2014) 2357–2366. DOI:10.1002/chem.201301439 |
| [62] | L.J. Wang, K. Zhang, F.Y. Cheng, J. Chen. Polypyrrole-cobalt-carbon nanocomposites as efficient counter electrode materials for dye-sensitized solar cells. Sci. China Chem. 57 (2014) 1559–1563. DOI:10.1007/s11426-014-5121-z |
| [63] | A. Venkatanarayanan, A.M. Spehar-Délèze, L. Dennany, et al. Ruthenium aminophenanthroline metallopolymer films electropolymerized from an ionic liquid: deposition and electrochemical and photonic properties. Langmuir 24 (2008) 11233–11238. DOI:10.1021/la8011316 |
| [64] | D.F. Qiu, Q. Zhao, X.Y. Bao, et al. Electropolymerization and characterization of an alternatively conjugated donor-acceptor metallopolymer: Poly-[Ru(4'-(4-(Diphenylamino)phenyl)-2,2':6',2"-Terpyridine)2]2+. Inorg. Chem. Commun. 14 (2011) 296–299. DOI:10.1016/j.inoche.2010.11.019 |
| [65] | Y.Y. Zhu, C. Gu, S. Tang, et al. A new kind of peripheral carbazole substituted ruthenium(II) complexes for electrochemical deposition organic light-emitting diodes. J. Mater. Chem. 19 (2009) 3941–3949. DOI:10.1039/b900481e |
| [66] | D.F. Qiu, X.Y. Bao, Q. Zhao, et al. Near-IR electrochromic film prepared by oxidative electropolymerization of the cyclometalated Pt(II) chloride with a triphenylamine group. Inorg. Chem. 54 (2015) 8264–8270. DOI:10.1021/acs.inorgchem.5b00782 |
| [67] | M.K. Leung, M.Y. Chou, Y.O. Su, et al. Diphenylamino group as an effective handle to conjugated donor acceptor polymers through electropolymerization. Org. Lett. 5 (2003) 839–842. DOI:10.1021/ol027474i |
| [68] | H.J. Yen, H.Y. Lin, G.S. Liou. Novel starburst triarylamine-containing electroactive aramids with highly stable electrochromism in near-infrared and visible light regions. Chem. Mater. 23 (2011) 1874–1882. DOI:10.1021/cm103552k |
| [69] | C.J. Yao, Y.W. Zhong, J.N. Yao. Five-stage near-infrared electrochromism in electropolymerized films composed of alternating cyclometalated bisruthenium and Bis-triarylamine segments. Inorg. Chem. 52 (2013) 10000–10008. DOI:10.1021/ic401288g |
| [70] | K. Sreenath, C.V. Suneesh, V.K.R. Kumar, K.R. Gopidas. Cu(II)-mediated generation of triarylamine radical cations and their dimerization. An easy route to tetraarylbenzidines. J. Org. Chem. 73 (2008) 3245–3251. DOI:10.1021/jo800349n |
| [71] | W.P. Lin, S.J. Liu, T. Gong, Q. Zhao, W. Huang. Polymer-based resistive memory materials and devices. Adv. Mater. 26 (2014) 570–606. DOI:10.1002/adma.v26.4 |
| [72] | P.Y. Gu, F. Zhou, J.K. Gao, et al. Synthesis, characterization, and nonvolatile ternary memory behavior of a larger heteroacene with nine linearly fused rings and two different heteroatoms. J. Am. Chem. Soc. 135 (2013) 14086–14089. DOI:10.1021/ja408208c |
| [73] | S.J. Liu, P. Wang, Q. Zhao, et al. Single polymer-based ternary electronic memory material and device. Adv. Mater. 24 (2012) 2901–2905. DOI:10.1002/adma.v24.21 |
| [74] | B.B. Cui, Z.P. Mao, Y.X. Chen, et al. Tuning of resistive memory switching in electropolymerized metallopolymeric films. Chem. Sci. 6 (2015) 1308–1315. DOI:10.1039/C4SC03345K |
| [75] | M. Li, J. Zhang, H.J. Nie, et al. In situ switching layer-by-layer assembly: one-pot rapid layer assembly via alternation of reductive and oxidative electropolymerization. Chem. Commun. 49 (2013) 6879–6881. DOI:10.1039/c3cc43629b |
| [76] | X.D. Xu, C.J. Yao, L.J. Chen, et al. Facile construction of structurally defined porous membranes from supramolecular hexakistriphenylamine metallacycles through electropolymerization. Chem. Eur. J. 22 (2016) 5211–5218. DOI:10.1002/chem.201504480 |
 2016, Vol. 27
2016, Vol. 27 


