Azulene is a special aromatic hydrocarbon constructed of fused ring architecture of pentagon and heptagon. Its resonance structure is shown in Fig. 1, which contains electrically negative pentagon and electrically positive heptagon, leading to an intramolecular dipole moment about 1.04 D [1]. In contrast, its isoelectronic isomer naphthalene has zero dipole moment. Azulene was firstly discovered from oil extraction [2]. Because of its low concentration in nature source and difficulty in synthesis, the development of azulene-based functional molecules has lagged far behind its isomer naphthalene.

|
Download:
|
| Figure 1. Azulene and its polarized resonance structure. | |
Despite the difficulty to access azulene derivatives, their unique electronic structures have intrigued lots of interest in the fields of optical and electronic investigations. Azulene is fascinating not only for its polar nature, but also for its distinct energy transition feature. Azulene presents small HOMO-LUMO band gap. This is related to its non-alternant nature, which induces the HOMO and LUMO having no mirror image relation, thereby reducing the mutual repulsion between electrons. Consequently, its HOMO- LUMO gap is depressed compared to common alternant aromatic molecules [3]. Azulene gives strong absorption associated with the S0→S2 optical transition [4], while its S0→S1 transition gives weak absorption in the visible range, which accounts for its blue color [5, 6]. Another peculiar feature of azulene is that its fluorescence mainly originated from the S2 state, whereas S1→S0 transition is negligible [7, 8]. This transition pattern violates Kasha's rule [9]. The reason for its abnormal fluorescence nature is that the energy gap between S1 and S2 state is relatively large (△E(S2-S1) over 10000 cm-1) [10], which leads to the decrease of the transition rate from S2 to S1 state, thus emission from S2 to S0 becomes the dominant pathway. Because of its unique properties, azulene has gained increasing attention in the last few years. This review will firstly introduce the typical functionalized azulene derivatives and the representative synthesis methods. Then we will focus on the physical properties of azulene-containing molecules and their applications in various optoelectronic devices.
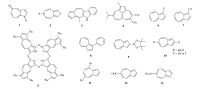
2. The synthesis of azulene derivativesBeside pristine azulene, a variety of molecules containing fused pentagon and heptagon have been synthesized, which can be considered as azulene derivatives. For instance, there are azulene derivatives with carbonyl groups (1) [11], compound with heterocycles (2) [12] and azulene fused with more ring structures (3, 4) (Fig. 2)[13, 14]. Expanding the conjugation structure can shift the absorption of azulene toward longer wavelength, which encouraged researchers explore azulene in optical applications. For example, the porphyrinanalogue azulenocyanine (5) [15] is an azulene-fused structure that exhibits strong near-IR absorption.
|
Download:
|
| Figure 2. Typical azulene analogues and derivatives. | |
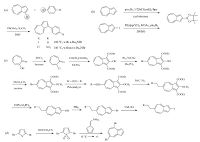
Many simple azulene derivatives have been synthesized by attaching various functionalized groups (6-13) [4, 16-25]. Scheme 1 summarizes the typical synthesis methods to obtain different azulene derivatives. Azulene can easily interact with electrophilic reactants at its electron-rich 1- and 3-positions resulting in structures like compounds 6 [16] and 7 [17]. To attain biaryl compounds, Suzuki [26] and Stille coupling [25] reactions are the most popular synthesis methods. Activation of C-H bond of azulene via Pd catalyst in the presence of aryl halide was rarely described [27]. Compound 8 [18] was the first arylated azulene- based structure synthesized by direct palladium catalysis coupling reaction (Scheme 1a).
|
Download:
|
| Scheme. 1. Synthetic route to azulene derivatives substituted at (a) 1-position; (b) 2-position; (c) 2, 6-postions; (d) 5, 8-positions. | |
Although 1- and 3-positions are the most active sites in azulene, other positions can also be functionalized via different strategies. For example, compound 9 [19] could be obtained by Suzuki coupling reaction or by direct C-H activation method with iridium catalysis (Scheme 1b). In order to obtain the 2, 6-disubstituted azulene 10 [4, 20-22], the most commonly used method is shown in Scheme 1c. The preparation commenced with tropolone, and the bromination at 6-position and protection by methoxylation of the hydroxyl group at 2-position are crucial for the Sonogashira- Hagihara reaction that is generally applied to anchor alkyl groups. 2-Bromo and 2-iodo derivatives were produced via PBr3 treatment and halogen exchange reaction, respectively.
Instead of starting from azulene, compound 11 [23] could be synthesized via a two-step reaction to construct azulene skeleton. As shown in Scheme 1d, this reaction was accessible through cycloaddition of thiophene-S-S-dioxides and fulvenes. Besides the convenience to get the substituted product, another advantage of this reaction is that it allowed us to get various azulene derivatives with different substitution at the heptagon ring by changing the building block of the 2, 5-dibromothiophene.
3. Functional materials containing azulene structures 3.1. Nonlinear optical materialsMaterials with second-order nonlinear optical (NLO) properties are attractive for applications in telecommunications [28], information processing [29], and laser beam modulation like second harmonic generation (SHG) [30]. The well-established molecule design strategy for second-order NLO is to construct push-pull structures with low-lying charge-transfer excited state, which is associated with efficient charge separation, leading to enhanced NLO efficiency [31, 32]. Additionally, lack of a symmetric center and noncentrosymmetric orientation in the bulk are also essential for high activity of SHG [33].
Azulene itself is a polar structure, which makes it an ideal fragment in NLO materials. Fig. 3 listed some typical molecules used in NLO related researches [34-39]. Molecule 14 [34] is comprised of an azulene moiety, an azo (-N=N-) linker and a nitrobenzene ring. In this molecule, azulene acts as a donor in the architecture. It should be noted that electron transfer was involved in the whole π-conjugated structure thanks to the electron- donating effect of azulene and electron-accepting nitro group. Also importantly, this molecule crystallized in noncentrosymmetric fashion. Strikingly, it exhibited second-order NLO characteristics with hyperpolarizability (β) slightly higher than that of the widely used 2-[N-ethyl-4-(4-nitrophenylazo) phenyl-amino] ethanol (DR1), and the SHG efficiency was 420 times higher than that of urea. Iftime [35] reported that by attaching methoxy group in the molecule 15, improved NLO efficiency was observed in solid state compared with the unsubstituted compound. This may be ascribed to higher hyperpolarizability of molecule 15 induced by the methoxy group.

|
Download:
|
| Figure 3. Azulene-based molecules for nonlinear optics. | |
Migalska-Zalas [36] offered theoretical support on how the alkyl chains affect NLO properties. They found that by substituting the alkyl groups in the seven-membered ring of molecule 17, the HOMO-LUMO energy gap, and the ground state dipole moment decreased, resulting in an increase of NLO coefficient. They also observed asymmetric topology of HOMO and LUMO, which accounted for the large change of dipole moments of compounds 16-19 during the transition from ground states to excited states. The electronic delocalization contributed dominantly to their NLO properties.
Asato [37] investigated the dependence of NLO performances on the accepting strength, conjugation length and alkyl chains. Comparison between compounds 20 and 23 indicated that using a stronger acceptor enhanced the nonlinear optical properties (βμ = 1323 × 10-48 esu for 20, βμ = 911 × 10-48 esu for 23).From the comparison of compounds 22 and 23, it was concluded that increasing the conjugation length led to gain in NLO properties by a factor of 3. The experimental data confirmed that alkyl substitution (22 vs. 21) on the azulene moiety gave higher NLO coefficient.
Stilbazolium salts [38] have been intensively investigated as SHG-active materials and showed impressive quadratic NLO responses. Asselberghs [39] reported that azulen-1-yl substituent in molecule 24 and 25 was a stronger electron donor than the widely studied (dimethylamino)phenyl substituent. Nevertheless, its strong donating ability hampered the azulene-based chromo- phores to gain high second-order NLO activity. It was mainly due to the fact that the charge-separated form was involved in their ground states at a high level, which caused reduced △μ, defined as the change of dipole moment between the ground state and the excited state. As △μ was the main factor that influences quadratic molecular hyperpolarizabilities (β), its reduction led to decrease of β value, hence poor NLO efficiencies.
It should be noted that azulene can act as electron-accepting moiety as well. Molecule 26 [40] combined azulene with ferrocene, in which the azulene unit acted as an electron acceptor, whereas the ferrocene fragment acted as an electron donor. This was evidenced from electrochemistry measurement as its oxidation wave was assignable to ferrocene for first anodic wave and the reduction wave was assignable to azulene part for wave below -2.0 V. The tested hyperpolarizability tensor was observed in the range of the reference compound 4-(N, N-dimethylamino)cinnamic aldehyde.
3.2. Organic semiconductors for field-effect transistorsOrganic Field-Effect Transistors (OFETs) are important classes of electronic devices having important applications in mechanically flexible displays [41], radio frequency identification tags [42], chemical sensors [43] and complementary logic circuits [44]. Traditionally, benzene [45, 46], thiophene [46, 47], naphthalene [48, 49] and pyridine [50, 51] units have been widely used as building blocks for organic semiconductors for OFETs. But azulene, which is characteristic of dipolar structure and low band gap, was rarely studied as the building block in OFET materials. Katagiri and co-workers [52] have reported azulene-based oligomers showing field-effect transport behavior. The two molecules, 27 and 28 shown in Fig. 4, both took planer configurations and herringbone packing mode, revealing p-channel transport properties, with features of a hole mobility up to 5.0 × 10-2 cm-2V-1s-1 for molecule 28. Based on similar molecular design strategy, they reported another oligomer containing solely azulene motif as the repeating unit (compound 29) [53]. The molecule had a narrow band gap with HOMO level being -5.56 eV and LUMO level being -3.93 eV. It exhibited only n-type response with the highest carrier mobility to be 0.29 cm2V-1s-1. The absence of hole transport activity can be rationalized by its non-uniform HOMO distribution. DFT calculation indicated that the HOMO mainly localized in one end of the molecule, whereas the LUMO delocalized over the whole molecular plane. The biased HOMO distribution led to limited degree of orbital overlap, resulting in silent response of p-type transistor.

|
Download:
|
| Figure 4. Azulene-based molecules for OFETs and the transistor behaviors of 30 having different connection mode of azulene moiety (Adapted with permission from ref [54]. Copyright 2015 American Chemical Society). | |
Zhang's group [54] constructed three D-A conjugated polymers (DPPA1, DPPA2, DPPA3 shown in Fig. 4) incorporating dithienyl- diketopyrrolopyrrole (DPP) as the accepting unit and azulene as the donating unit. They successfully modulated their semiconducting behaviors by altering the linkage positions of azulene in the DPP backbones. For DPPA3, lower LUMO level and more uniform distributed LUMO were observed compared with its analogues DPPA1 and DPPA2. Therefore, p-type transport properties were obtained for the films of DPPA1 and DPPA2, in which electron-rich pentagon ring of azulene was linked with DPP. While DPPA3 with heptagon ring connected with DPP moiety acted as ambipolar semiconductor (Fig. 5).

|
Download:
|
| Figure 5. Azulene-based molecules for solar cells (33-37) and singlet fission (38), one study important for solar cell performance improvement (Reprinted with permission from ref [66]. Copyright 2014 American Chemical Society). | |
Squaraines (SQs) are attractive due to its distinct absorption and fluorescence in red-IR region [55], which made it favorable building blocks for optoelectronics. Leeuw, Djukic et al.has implemented both SQs and guaiazulene into OFETs. Compounds 31 [56] and 32 [57] both have low band gaps (1.2 eV) and perform as ambipolar semiconductors with balanced hole and electron mobilities in the order of 10-4 cm-2V-1s-1. Distinctively, by simultaneously activate hole and electron transport from opposite end of the channel, compound 31 can emit light in the nearinfrared region as a result of hole and electron recombination. They also estimated the position of recombination at varied Vg (gate potential) and Vd (drain potential) through theoretical calculation. By taking advantages of the strong absorption in the near-infrared (NIR) region, compound 32 was used in NIR light sensor, and the drain current was sensitive to light and increased under illumination.
3.3. Solar cellsThe unique dipolar structure and optical properties of azulene make it an attractive candidate to construct photovoltaic materials with self-assembly capability. In the report of Zhang [54], the thin film of DPPA1 and PC71BM blend gave power conversion efficiency (PCE) value of 2.04%.
Imahori [58] explored two series of D-A polymers encompassing azulene as electron donor and DPP or 2, 1, 3-benzothiadiazole (BT) as electron acceptors for solar cells. Due to the strong electron- withdrawing feature of DPP, molecule 33 had relatively low LUMO energy level comparable with PC71BM, which imparted the main reason for its lower PCE (0.08%) compared with that of PAzBT1 (0.39%). Alkyl groups are also one of the important factors affecting PCE. PAzBT2 (PCE = 0.03%) and PAzBT3 (PCE = 0.02%) based devices had inferior power conversion efficiencies compared with that based on PAzBT1. It was mainly attributed to the twisted backbone structure induced by alkylation, which hampered efficient light harvesting and charge transport, thus gave poor PCE values.
Apart from being used as the active layer in solar cells, Emrick and co-workers [4] implemented zwitterionic azulene-substituted sulfobetaine methacrylate copolymer as the cathode modification interlayer in bulk heterojunction (BHJ) solar cells. They observed uniform coverage after spin-coating of the azulene-containing modifier. In addition, a higher open-circuit voltage (Voc) arising from work function (WF) reduction of the cathode was additionally responsible for the enhancement of PCE value (7.9% with copolymer 35 vs. 2.4% without any modifier).
Azulene and its derivative have been reported to be able to absorb light and then transfer the photo-induced electrons to the conduction band of TiO2 from S2 state [59] or S1 state [60]. These findings opened up possibilities for azulene in dye-sensitized solar cells (DSSCs). Importantly, the excited state of azulene can be tuned by attaching varied substituents [6, 61]. Zhang [62] constructed four azulene-based molecules, 36a-36d, with the alteration of conjugation length and alkyl substitution, and explored their applications in DSSCs. The elongated conjugation (36b vs. 36a, 36d vs. 36c) and alkyl substitutions (36c vs. 36a, 36d vs. 36b) red shifted the absorption band, and the alkyl substitutions restricted dye aggregation. This restriction played an important role in blocking other deactivation pathways of the exited state. Thus, higher photoelectric conversion efficiencies were found in 36c and 36d based TiO2 electrodes. As for the intrinsic mechanism of this photo-induced electron transfer process, the contribution of S2 state was larger than S1 state mainly due to the fast decay of S1 state and larger driving force from S2 state as well as long lifetime of S2 state.
Recently, Murata's group [63] exploited azulene cored twodimensional system 37 as the hole-transporting material (HTM) in perovskite solar cells. A high cell performance was observed (PCE = 16.5%) which was superior to that of Spiro-OMeTAD prepared under the same condition. They elucidated that high performances derived from HTM design require good hole mobility and well control of HOMO and LUMO levels, which should be appropriately higher than the valence band and conduction band of perovskite, respectively. Additionally, higher hole-collection ability determined by time-resolved microwave conductivity (TRMC) measurement over that of Spiro-OMeTAD was another significant factor for the superior performance. Ultimately, the two-dimensional molecular skeleton rather than twisted configuration like Spiro-OMeTAD provided preferable condition to enhance horizontal face-on orientation on perovskite, which facilitated charge collection.
Singlet fission (SF) has the potential to improve the PCE of solar cells [64]. SF is normally defined as a process in which a photoexcited singlet exciton splits into two triplet excitons in chromophore materials. There are two basic rules that SF organic chromophores must meet: E(S1)>2E(T1) and E(T2) > 2E(T1). In these two formula, E represents the energy of electronic states, S and T stand for singlet state and triplet state, and the subscripts denote first and second excited state for 1 and 2, respectively [65] . Azulene has two prominent advantages over its isomer naphthalene. One is a smaller HOMO-LUMO band gap (3.33 eV vs. 4.79 eV) [66] which is beneficial for visible-region light absorption. The other is the seemingly greater diradical character which has been investigated to have correlation with the ability to satisfy SF criteria described above [67]. These merits render azulene promising starting structures for SF research. Zeng and co-workers [66] have developed a perturbation strategy by introducing boron (B) and nitrogen (N) atoms into azulene structure (38). Their works are based on computational calculation. It was argued that the B and N atoms served as captodative stabilization centers on the diradical Lewis structure. The hetero-substituted azulene was calculated to have lower HOMO-LUMO gap (2.36 eV) than its parent azulene. And importantly, the S1 and T1 energies located close to those of pentacene, a favorable SF chromophore.
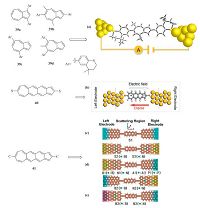
3.4. Molecular devicesMolecules with π-conjugated systems have potential applications in single-molecule devices, which integrate an individual molecule into electronic devices. The progress of single-molecule conductance has boosted the development of field-effect transistors [68], diodes [69], and electroluminescence materials [70]. A typical single-molecule device includes three components: two electrodes, suitable anchoring groups and a conjugated organic molecule. Generally, molecular structure [71], electrode type [72] and molecule-electrode bonding [73] are key factors affecting the measured molecular conductance. Molecules in the junction with p-delocalized feature and low HOMO-LUMO gaps are desirable for high performances. Benzene [74], thiophene [73], furan [75], ethynylene [76] have been extensively incorporated as building blocks for active molecular devices. Azulene, possessing planer conformation with 10π electrons and a low HOMO-LUMO energy gap, is a potential target molecule for investigating singlemolecule conductance. Campos et al.[77] reported on azulene- based molecules coupled with thiochroman as anchoring groups connecting with Au electrodes (Fig. 6a). The four molecules 39a, 39b, 39c, and 39d with different substitution pattern all exhibited conductance activity. The transmission plot from calculation showed that unlike naphthalene, 39a and 39d did not have a transmission value of zero at E = 0, indicating single-molecule conduction response. The four molecules with different connectivity had different conducting path. 39b and 39c had conducting paths across the binding sites, whereas 39a and 39d were assumed to have conducting paths with π-system interaction with one side of the Au electrodes [78].
|
Download:
|
| Figure 6. Azulene-like molecules for rectifiers and model diagram for evaluation of rectification performance. In the model diagram for 41,blue,pristine zigzag edged graphene nanoribbon (zGNR); yellow,single atom doped armchair edged graphene nanoribbon (aGNR); fuchsia,pair atoms doped aGNR. (c) Molecularjunctions constructed between symmetric electrodes,denoted as S1,S2,S3. (d) Molecularjunctions constructed between a pristine electrode and a single molecule-doped aGNRelectrode,denoted as B1,N1,A1,and P1 representing boron,aluminum,nitrogen,and phosphorus doping atoms,respectively,and B2,N2 representing exchanged electrodes as to B1,N1,respectively. (e) Molecular junctions constructed between a pristine electrode and a double-doped aGNR electrode,denoted as B3 and N3 representing boron and nitrogen doping atoms. Reprinted with permission from ref [77]. Copyright 2014 American Chemical Society (a). [Original citation] - Reproduced by permission of the PCCP Owner Societies (b). Reprinted with permission from ref [72]. Copyright 2014 American Chemical Society (c-e). | |
Azulene's dipole moment could be altered by modification through inserting functional groups or changing its aromatic structure [79]. This feature provides azulene preferred material for rectifier applications. Zhang and co-workers [80] firstly employed azulene-like fused structures as molecular rectifiers. They demonstrated that by increasing the length of aromatic linker between the pentagon and heptagon rings, the dipole moment increased, which gave rise to high rectification ratio. Meanwhile, the high conductance independent from molecular length was attributed to the highly conjugated rigid structure. While dipole moment is not the only factor influencing the rectification effect, HOMO-LUMO orbital distribution and asymmetric shift of frontier orbital levels under different bias are as well key to the better performance of 40 over those with larger dipole moment.
Since the backbone of molecule 40 has been verified to have significant rectification ratio, Wang' group [72] studied the same backbone anchored with carbon atomic chains between graphene nanoribbon electrodes. By doping the graphene nanoribbon electrodes with B, N, Al and P atoms, they observed that the position of the electrodes' impurity-subband in the energy gap, the overlapping of relevant subbands and the location of the charge transfer channel of the molecular diode 41 had determining influences on their rectification performances. The comprehensive effects resulted in asymmetric doping of B shown in Fig. 6d exhibited pronounced rectification behavior (with ratio up to 104) better than that of systems with other dopants. Moreover, to study the effect of the concentration of dopants on rectification behavior, they investigated the configuration shown in Fig. 6e with double doping atoms per two carbon unit cells. They found that it gave rise to improved rectification performance for double B-doping configuration in comparison with its single B-doping analogue. Nevertheless, the double N-doping motif did not bring a better performance compared with the single N-doping motif. The different responses of them can be attributed to that for the B- doping system, subbands 2π* and 3π* of the left electrode overlapped partly with π* of the right electrode, and the charge transfer channel h of azulene-like molecule was inside the overlapping region. While for the double N-doping configuration, there only existed 2π* and π* overlapping, resulting in less obvious transmission spectra through the molecular orbital h.
4. Summary and perspectivesAzulene possesses various advantages over its isomer naphthalene for applications in optoelectronic fields. Firstly, azulene has a narrow HOMO-LUMO energy gap. Many azulene derivatives benefit from this characteristic such as azulenocyanine (5) featuring near-IR absorption and terthiophene (29) appearing strengthened long-wavelength absorption stemming from azu- lene. Secondly, azulene has a larger molecular area [81] compared with naphthalene, which could offer azulene-based π-extended molecules enhanced intermolecular interactions through π-π coupling. Thirdly, azulene is a non-alternant structure with a high dipole moment orientating from the electron-deficient heptagon ring to the electron-sufficient pentagon ring. This structure feature makes it possible for constructing versatile dipolar molecules as nonlinear optical materials and molecular rectifiers, etc.
Azulene-based materials have met much success in NLO and showed much promise in OFETs and solar cells. Despite the fact that azulene has been considered as an ideal constructive unit in NLO field, so far their optoelectronic properties are still lower than the state-of-art NLO materials [82]. This is partly due to the difficulties in the synthesis of azulene derivatives; hence, their full potential has not been fully explored. Of particular note is that there are still limited materials to be tested in various optoelectronic applications. Further structural optimization of azulene- containing materials shall bring about much improved performances. Although several researches have suggested that azulene exhibited interesting properties in molecular electronics, there has yet been no experimental test of azulene rectifier. Experimental exploration on the azulene-based rectifiers is a highly desired research in near future. However, the synthesis of azulene derivatives is still the main obstacle preventing its applications. The development of cost-effective methods for synthesizing azulene derivatives will remain an important topic in recent years for organic chemists. It is expected that more azulene aromatics necessary will be synthesized with their properties characterized in the coming years, which will provide more understanding of their physical chemistry characteristics, and shed light on the structure-property relationships.
Acknowledgments This work is supported by National Basic Research Program of China (973 Program, No. 2012CB933102), National Natural Science Foundation of China (Nos. 51525303, 21233001, 21190034), the Fundamental Research Funds for the Central Universities and 111 Project.| [1] | A.G. Anderson, B.M. Steckler. Azulene. VIII. A study of the visible absorption spectra and dipole moments of some 1- and 1,3-substituted azulenes. J. Am. Chem. Soc. 81 (1959) 4941–4946. DOI:10.1021/ja01527a046 |
| [2] | A.E. Sherndal. On the blue hydrocarbon occurring in some essential oils. J. Am. Chem. Soc. 37 (1915) 167–171. DOI:10.1021/ja02270a016 |
| [3] | D.M. Lemal, G.D. Goldman. Synthesis of azulene, a blue hydrocarbon. J. Chem. Educ. 65 (1988) 923–925. DOI:10.1021/ed065p923 |
| [4] | E. Puodziukynaite, H.W. Wang, J. Lawrence, et al. Azulene methacrylate polymers: synthesis, electronic properties, and solar cell fabrication. J. Am. Chem. Soc. 136 (2014) 11043–11049. DOI:10.1021/ja504670k |
| [5] | H. Korichi, F. Zouchoune, S.M. Zendaoui, B. Zouchoune, J.Y. Saillard. The coordination chemistry of azulene: a comprehensive DFT investigation. Organometallics 29 (2010) 1693–1706. DOI:10.1021/om901089z |
| [6] | S.V. Shevyakov, H.R. Li, R. Muthyala, et al. Orbital control of the color and excited state properties of formylated and fluorinated derivatives of azulene. J. Phys. Chem. A 107 (2003) 3295–3299. DOI:10.1021/jp021605f |
| [7] | M. Myahkostupov, C.V. Pagba, L. Gundlach, P. Piotrowiak. Vibrational state dependence of interfacial electron transfer: hot electron injection from the S1 state of azulene into TiO2 nanoparticles. J. Phys. Chem. C 117 (2013) 20485–20493. DOI:10.1021/jp406662n |
| [8] | J.P. Heritage, A. Penzkofer. Relaxation dynamics of the first excited electronic singlet state of azulene in solution. Chem. Phys. Lett. 44 (1976) 76–81. DOI:10.1016/0009-2614(76)80413-7 |
| [9] | M. Kasha. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 9 (1950) 14–19. DOI:10.1039/df9500900014 |
| [10] | T. Itoh. Fluorescence and phosphorescence from higher excited states of organic molecules. Chem. Rev. 112 (2012) 4541–4568. DOI:10.1021/cr200166m |
| [11] | L.T. Scott, C.M. Adams. Quinones of azulene. 4. Synthesis and characterization of the parent 1, 5- and 1,7-quinones. J. Am. Chem. Soc. 106 (1984) 4857–4861. DOI:10.1021/ja00329a037 |
| [12] | Y. Sugihara, T. Yagi, I. Murata, A. Imamura. 1-Phenylthieno[3, 4-d]borepin: a new 10.pi. electron system isoelectronic with azulene. J. Am. Chem. Soc. 114 (1992) 1479–1481. DOI:10.1021/ja00030a052 |
| [13] | B.C. Hong, Y.F. Jiang, E.S. Kumar. Microwave-assisted 6+4-cycloaddition of fulvenes and alpha-pyrones to azulene-indoles: facile syntheses of novel antineoplastic agents. Bioorg. Med. Chem. Lett. 11 (2001) 1981–1984. DOI:10.1016/S0960-894X(01)00349-3 |
| [14] | E.H. Ghazvini Zadeh, A.W. Woodward, D. Richardson, M.V. Bondar, K.D. Belfield. Stimuli-responsive cyclopenta[ef]heptalenes: synthesis and optical properties. Eur. J. Org. Chem. (2015) 2271–2276. |
| [15] | A. Muranaka, M. Yonehara, M. Uchiyama. Azulenocyanine: a new family of phthalocyanines with intense near-IR absorption. J. Am. Chem. Soc. 132 (2010) 7844–7845. DOI:10.1021/ja101818g |
| [16] | R.S. Muthyala, R.S.H. Liu. Synthesis of fluorinated azulenes. J. Fluorine Chem. 89 (1998) 173–175. DOI:10.1016/S0022-1139(98)00139-0 |
| [17] | H.Q. Do, O. Daugulis. Copper-catalyzed cyanation of heterocycle carbon hydrohydrogen bonds. Org. Lett. 12 (2010) 2517–2519. DOI:10.1021/ol100772u |
| [18] | G. Dyker, S. Borowski, J. Heiermann, et al. First intermolecular palladium catalyzed arylation of an unfunctionalized aromatic hydrocarbon. J. Organomet. Chem. 606 (2000) 108–111. DOI:10.1016/S0022-328X(00)00224-2 |
| [19] | K. Kurotobi, M. Miyauchi, K. Takakura, T. Murafuji, Y. Sugihara. Direct introduction of a boryl substituent into the 2-position of azulene: application of the Miyaura and Smith methods to azulene. Eur. J. Org. Chem. (2003) 3663–3665. |
| [20] | S. Ito, M. Ueda, R. Sekiguchi, J. Kawakami. Efficient synthesis and redox behavior of a series of 6-alkyl-2-phenylazulenes. Tetrahedron 69 (2013) 4259–4269. DOI:10.1016/j.tet.2013.03.084 |
| [21] | K. Nakagawa, T. Yokoyama, K. Toyota, et al. Synthesis and liquid crystalline behavior of azulene-based liquid crystals with 6-hexadecyl substituents on each azulene ring. Tetrahedron 66 (2010) 8304–8312. DOI:10.1016/j.tet.2010.08.012 |
| [22] | M. Koch, O. Blacque, K. Venkatesan. Syntheses and tunable emission properties of 2-alkynyl azulenes. Org. Lett. 14 (2012) 1580–1583. DOI:10.1021/ol300327b |
| [23] | E. Amir, R.J. Amir, L.M. Campos, C.J. Hawker. Stimuli-responsive azulene-based conjugated oligomers with polyaniline-like properties. J. Am. Chem. Soc. 133 (2011) 10046–10049. DOI:10.1021/ja203267g |
| [24] | S. Ito, T. Kubo, N. Morita, et al. Preparation of azulenyllithium and magnesium reagents utilizing halogen-metal exchange reaction of several iodoazulenes with organolithium or magnesium ate complex. Tetrahedron Lett. 45 (2004) 2891–2894. DOI:10.1016/j.tetlet.2004.02.059 |
| [25] | S. Ito, T. Okujima, N. Morita. Preparation and Stille cross-coupling reaction of the first organotin reagents of azulenes. Easy access to poly(azulen-6-yl) benzene derivatives, J. Chem. Soc.. Perkin Trans. 1 (2002) 1896–1905. |
| [26] | K. Tsurui, M. Murai, S.Y. Ku, C.J. Hawker, M.J. Robb. Modulating the properties of azulene-containing polymers through controlled incorporation of regioisomers. Adv. Funct. Mater. 24 (2014) 7338–7347. DOI:10.1002/adfm.v24.46 |
| [27] | T. Shoji, A. Maruyama, T. Araki, S. Ito, T. Okujima. Synthesis of 2-and 6-thienylazulenes by palladium-catalyzed direct arylation of 2-and 6-haloazulenes with thiophene derivatives. Org. Biomol. Chem. 13 (2015) 10191–10197. DOI:10.1039/C5OB01317H |
| [28] | S. Kumar, J. Shao, X. Liang. Impulse response of nonlinear Schrodinger equation and its implications for pre-dispersed fiber-optic communication systems. Opt. Express 22 (2014) 32282–32292. DOI:10.1364/OE.22.032282 |
| [29] | D. Cotter. Nonlinear optics for high-speed digital information processing. Science 286 (1999) 1523–1528. DOI:10.1126/science.286.5444.1523 |
| [30] | C. Wang, T. Zhang, W. Lin. Rational synthesis of noncentrosymmetric metalorganic frameworks for second-order nonlinear optics. Chem. Rev. 112 (2012) 1084–1104. DOI:10.1021/cr200252n |
| [31] | S.R. Marder, C.B. Gorman, B.G. Tiemann, L.T. Cheng. Stronger acceptors can diminish nonlinear optical response in simple donor-acceptor polyenes. J. Am. Chem. Soc. 115 (1993) 3006–3007. DOI:10.1021/ja00060a071 |
| [32] | J.M. Raimundo, P. Blanchard, N. Gallego-Planas, et al. Design and synthesis of push-pull chromophores for second-order nonlinear optics derived from rigidified thiophene-based pi-conjugating spacers. J. Org. Chem. 67 (2002) 205–218. DOI:10.1021/jo010713f |
| [33] | Z. Yang, M. Jazbinsek, B. Ruiz, et al. Molecular engineering of stilbazolium derivatives for second-order nonlinear optics. Chem. Mater. 19 (2007) 3512–3518. DOI:10.1021/cm070764e |
| [34] | P.G. Lacroix, I. Malfant, G. Iftime, et al. Azo-azulene derivatives as second-order nonlinear optical chromophores. Chem. Eur. J. 6 (2000) 2599–2608. DOI:10.1002/(ISSN)1521-3765 |
| [35] | G. Iftime, P.G. Lacroix, K. Nakatani, A.C. Razus. Push-pull azulene-based chromophores with nonlinear optical properties. Tetrahedron Lett. 39 (1998) 6853–6856. DOI:10.1016/S0040-4039(98)01495-6 |
| [36] | A. Migalska-Zalas, Y. El Kouari, S. Touhtouh. Methodologies for computing UV-VIS spectra and nonlinear properties of azo-azulene derivatives. Opt. Mater. 34 (2012) 1639–1643. DOI:10.1016/j.optmat.2012.03.021 |
| [37] | A.E. Asato, R.S.H. Liu, V.P. Rao, Y.M. Cai. Azulene-containing donor-acceptor compounds as second-order nonlinear chromophores. Tetrahedron Lett. 37 (1996) 419–422. DOI:10.1016/0040-4039(95)02202-3 |
| [38] | B.J. Coe, J.A. Harris, I. Asselberghs, et al. Quadratic nonlinear optical properties of N-aryl stilbazolium dyes. Adv. Funct. Mater. 12 (2002) 110–116. DOI:10.1002/(ISSN)1616-3028 |
| [39] | L. Cristian, I. Sasaki, P.G. Lacroix, et al. Donating strength of azulene in various azulen-1-yl-substituted cationic dyes: application in nonlinear optics. Chem. Mater. 16 (2004) 3543–3551. DOI:10.1021/cm0492989 |
| [40] | R. Herrmann, B. Pedersen, G. Wagner, J.H. Youn. Molecules with potential applications for non-linear optics: the combination of ferrocene and azulene. J. Organomet. Chem. 571 (1998) 261–266. DOI:10.1016/S0022-328X(98)00872-9 |
| [41] | H. Sirringhaus. Integrated optoelectronic devices based on conjugated polymers. Science 280 (1998) 1741–1744. DOI:10.1126/science.280.5370.1741 |
| [42] | S. Steudel, K. Myny, V. Arkhipov, et al. 50 MHz rectifier based on an organic diode. Nat. Mater. 4 (2005) 597–600. DOI:10.1038/nmat1434 |
| [43] | J.H. Park, J.E. Royer, E. Chagarov, et al. Atomic imaging of the irreversible sensing mechanism of NO2 adsorption on copper phthalocyanine. J. Am. Chem. Soc. 135 (2013) 14600–14609. DOI:10.1021/ja403752r |
| [44] | Y. Zhao, Y. Guo, Y. Liu. 25th anniversary article: recent advances in n-type and ambipolar organic field-effect transistors,. Adv. Mater. 25 (2013) 5372–5391. DOI:10.1002/adma.201302315 |
| [45] | H. Xu, Y.C. Zhou, X.Y. Zhou, et al. Molecular packing-induced transition between ambipolar and unipolar behavior in dithiophene-4, 9-dione-containing organic semiconductors. Adv. Funct. Mater. 24 (2014) 2907–2915. DOI:10.1002/adfm.v24.19 |
| [46] | C. Kanimozhi, M. Naik, N. Yaacobi-Gross, et al. Controlling conformations of diketopyrrolopyrrole-based conjugated polymers: role of torsional angle. J. Phys. Chem. C 118 (2014) 11536–11544. DOI:10.1021/jp501526h |
| [47] | B. He, A.B. Pun, D. Zherebetskyy, et al. New form of an old natural dye: bayannulated indigo (BAI) as an excellent electron accepting unit for high performance organic semiconductors. J. Am. Chem. Soc. 136 (2014) 15093–15101. DOI:10.1021/ja508807m |
| [48] | J. Kim, M.H. Yun, G.H. Kim, et al. Synthesis of PCDTBT-based fluorinated polymers for high open-circuit voltage in organic photovoltaics: towards an understanding of relationships between polymer energy levels engineering and ideal morphology control. ACS Appl. Mater. Interfaces 6 (2014) 7523–7534. DOI:10.1021/am500891z |
| [49] | M.M. Durban, P.D. Kazarinoff, Y. Segawa, C.K. Luscombe. Synthesis and characterization of solution-processable ladderized n-type naphthalene bisimide copolymers for OFET applications. Macromolecules 44 (2011) 4721–4728. DOI:10.1021/ma2004822 |
| [50] | B. Sun, W. Hong, Z. Yan, H. Aziz, Y. Li. Record high electron mobility of 6.3 cm2V-1s-1 achieved for polymer semiconductors using a new building block. Adv. Mater. 26 (2014) 2636–2642. DOI:10.1002/adma.v26.17 |
| [51] | Y.Y. Liu, C.L. Song, W.J. Zeng, et al. High and balanced hole and electron mobilities from ambipolar thin-film transistors based on nitrogen-containing oligoacences. J. Am. Chem. Soc. 132 (2010) 16349–16351. DOI:10.1021/ja107046s |
| [52] | Y. Yamaguchi, Y. Maruya, H. Katagiri, K.I. Nakayama, Y. Ohba. Synthesis, properties, and OFET characteristics of 5,5'-di(2-azulenyl)-2,2'-bithiophene (DAzBT) and 2,5-di(2-azulenyl)-thieno[3,2-b]thiophene (DAzTT). Org. Lett. 14 (2012) 2316–2319. DOI:10.1021/ol3007327 |
| [53] | Y. Yamaguchi, K. Ogawa, K. Nakayama, Y. Ohba, H. Katagiri. Terazulene: a highperformance n-type organic field-effect transistor based on molecular orbital distribution control. J. Am. Chem. Soc. 135 (2013) 19095–19098. DOI:10.1021/ja410696j |
| [54] | J. Yao, Z. Cai, Z. Liu, et al. Tuning the semiconducting behaviors of new alternating dithienyldiketopyrrolopyrrole-azulene conjugated polymers by varying the linking positions of azulene. Macromolecules 48 (2015) 2039–2047. DOI:10.1021/acs.macromol.5b00158 |
| [55] | J.Q. Jiang, C.L. Sun, Z.F. Shi, H.L. Zhang. Squaraines as light-capturing materials in photovoltaic cells. RSC Adv. 4 (2014) 32987–32996. DOI:10.1039/C4RA03972F |
| [56] | E.C.P. Smits, S. Setayesh, T.D. Anthopoulos, et al. Near-infrared light-emitting ambipolar organic field-effect transistors. Adv. Mater. 19 (2007) 734–738. DOI:10.1002/(ISSN)1521-4095 |
| [57] | P.H. Woebkenberg, J.G. Labram, J.M. Swiecicki, et al. Ambipolar organic transistors and near-infrared phototransistors based on a solution-processable squarilium dye. J. Mater. Chem. 20 (2010) 3673–3680. DOI:10.1039/b919970e |
| [58] | T. Umeyama, Y. Watanabe, T. Miyata, H. Imahori. Electron-rich five-membered ring of azulene as a donor unit in donor-acceptor alternating copolymers for polymer solar cell applications. Chem. Lett. 44 (2015) 47–49. DOI:10.1246/cl.140904 |
| [59] | C. Pagba, G. Zordan, E. Galoppini, et al. Hybrid photoactive assemblies: electron injection from host-guest complexes into semiconductor nanoparticles. J. Am. Chem. Soc. 126 (2004) 9888–9889. DOI:10.1021/ja0475252 |
| [60] | M. Myahkostupov, C.V. Pagba, L. Gundlach, P. Piotrowiak. Vibrational state dependence of interfacial electron transfer: hot electron injection from the S1 state of azulene into TiO2 nanoparticles. J. Phys. Chem. C 117 (2013) 20485–20493. DOI:10.1021/jp406662n |
| [61] | R.S.H. Liu, R.S. Muthyala, X.S. Wang, et al. Correlation of substituent effects and energy levels of the two lowest excited states of the azulenic chromophore. Org. Lett. 2 (2000) 269–271. DOI:10.1021/ol990324w |
| [62] | X.H. Zhang, C. Li, W.B. Wang, et al. Photophysical, electrochemical, and photoelectrochemical properties of new azulene-based dye molecules. J. Mater. Chem. 17 (2007) 642–649. DOI:10.1039/B613703B |
| [63] | H. Nishimura, N. Ishida, A. Shimazaki, et al. Hole-transporting materials with a two-dimensionally expanded pi-system around an azulene core for efficient perovskite solar cells. J. Am. Chem. Soc. 137 (2015) 15656–15659. DOI:10.1021/jacs.5b11008 |
| [64] | M.C. Hanna, A.J. Nozik. Solar conversion efficiency of photovoltaic and photoelectrolysis cells with carrier multiplication absorbers. J. Appl. Phys. 100 (2006) 074510. DOI:10.1063/1.2356795 |
| [65] | M.B. Smith, J. Michl. Singlet fission. Chem. Rev. 110 (2010) 6891–6936. DOI:10.1021/cr1002613 |
| [66] | T. Zeng, N. Ananth, R. Hoffmann. Seeking small molecules for singlet fission: a heteroatom substitution strategy. J. Am. Chem. Soc. 136 (2014) 12638–12647. DOI:10.1021/ja505275m |
| [67] | T. Minami, S. Ito, M. Nakano. Theoretical study of singlet fission in oligorylenes. J. Phys. Chem. Lett. 3 (2012) 2719–2723. DOI:10.1021/jz3011749 |
| [68] | H. Song, Y. Kim, Y.H. Jang, et al. Observation of molecular orbital gating. Nature 462 (2009) 1039–1043. DOI:10.1038/nature08639 |
| [69] | I. Diez-Perez, J. Hihath, Y. Lee, et al. Rectification and stability of a single molecular diode with controlled orientation. Nat. Chem. 1 (2009) 635–641. DOI:10.1038/nchem.392 |
| [70] | C.W. Marquardt, S. Grunder, A. Blaszczyk, et al. Electroluminescence from a single nanotube-molecule-nanotube junction. Nat. Nanotechnol. 5 (2010) 863–867. DOI:10.1038/nnano.2010.230 |
| [71] | M. Taniguchi, M. Tsutsui, R. Mogi, et al. Dependence of single-molecule conductance on molecule junction symmetry. J. Am. Chem. Soc. 133 (2011) 11426–11429. DOI:10.1021/ja2033926 |
| [72] | Y. Song, Z. Xie, Y. Ma, Z.L. Li, C.K. Wang. Giant rectification ratios of azulene-like dipole molecular junctions induced by chemical doping in armchair-edged graphene nanoribbon electrodes. J. Phys. Chem. C 118 (2014) 18713–18720. DOI:10.1021/jp504448n |
| [73] | K. Yokota, M. Taniguchi, M. Tsutsui, T. Kawai. Molecule electrode bonding design for high single-molecule conductance. J. Am. Chem. Soc. 132 (2010) 17364–17365. DOI:10.1021/ja108032q |
| [74] | E. Leary, M.T. Gonzalez, C. van der Pol, et al. Unambiguous one-molecule conductance measurements under ambient conditions. Nano Lett. 11 (2011) 2236–2241. DOI:10.1021/nl200294s |
| [75] | W. Chen, H. Li, J.R. Widawsky, et al. Aromaticity decreases single-molecule junction conductance. J. Am. Chem. Soc. 136 (2014) 918–920. DOI:10.1021/ja411143s |
| [76] | C. Wang, A.S. Batsanov, M.R. Bryce, et al. Oligoyne single molecule wires. J. Am. Chem. Soc. 131 (2009) 15647–15654. DOI:10.1021/ja9061129 |
| [77] | J. Xia, B. Capozzi, S. Wei, et al. Breakdown of interference rules in azulene, a nonalternant hydrocarbon. Nano Lett. 14 (2014) 2941–2945. DOI:10.1021/nl5010702 |
| [78] | R. Stadler. Comment on "Breakdown of interference rules in azulene, a nonalternant hydrocarbon". Nano Lett. 15 (2015) 7175–7176. DOI:10.1021/acs.nanolett.5b03468 |
| [79] | R.S.H. Liu, R.S. Muthyala, X.S. Wang, et al. Correlation of substituent effects and energy levels of the two lowest excited states of the azulenic chromophore. Org. Lett. 2 (2000) 269–271. DOI:10.1021/ol990324w |
| [80] | K.G. Zhou, Y.H. Zhang, L.J. Wang, et al. Can azulene-like molecules function as substitution-free molecular rectifiers. Phys. Chem. Chem. Phys. 13 (2011) 15882–15890. DOI:10.1039/c0cp02693j |
| [81] | T. Hartman, K. Collins, R. Wehlitz. Isomer effects in the double-to-single photoionization ratio of aromatic hydrocarbons. Phys. Rev. A 88 (2013) 024701. DOI:10.1103/PhysRevA.88.024701 |
| [82] | Y. Shi, D. Frattarelli, N. Watanabe, et al. Ultra-high-response, multiply twisted electro-optic chromophores: influence of p-system elongation and interplanar torsion on hyperpolarizability. J. Am. Chem. Soc. 137 (2015) 12521–12538. DOI:10.1021/jacs.5b04636 |
 2016, Vol. 27
2016, Vol. 27 


