b School of Life Sciences and Engineering, Southwest Jiaotong University, Chengdu 610031, China
Titanium (Ti) and its alloys are widely used in the orthopedic and dental fields because of their remarkable biological and mechanical properties. However, after implantation in vivo, these Ti materials cannot establish bone bonding and only develop mechanical fixation with the bone [1]. How to improve the osteointegration between an implant and surrounding natural bone tissue, in turn to extend the lifetime of the implant, still remains unresolved. The biological behaviors of cells are highly regulated by the local chemical and topographical microenvironment [2, 3]. Previous studies confirmed that a nano-porous rough structure can enhance the specific surface area and surface energy of a material, which played a role in promoting the proliferation and differentiation of osteoblasts and regulating the secretion of growth factors and cytokines [4-6]. However, the biological activity resulted from this modification is limited, requiring further modifications to the material.
Layer-by-layer self-assembly technology is based on the electrostatic attractions between multiple self-assembly film systems. This technique has important applications in electronic biosensors, cell engineering and nanotechnology. This is a simple and effective method for constructing a bioactive surface that has the following advantages: no special requirement for chemical structures or active functional groups on material surface; the construction of the layered structure does not require additional conditions, such as energy supply; various biocompatible watersoluble polymers (especially various bioactive biological macromolecules, such as proteins and nucleic acids) can be used for assembly [7].
Poly-L-lysine (PLL) shows good biocompatibility and contains functional groups such as amino and carboxyl groups. Jessel et al. [8, 9] deposited two types of plasmids (plasmids containing the green fluorescent protein gene sequence and a transcription factor gene sequence) at various locations in multilayer films of PLL and polyglutamic acid to regulate the expression timing of the two plasmids in cells. DNA is an important genetic substance and is also an anionic polyelectrolyte. DNA molecules contain a large number of phosphate groups; the high affinity between phosphate and calcium ions was shown to favor calcium deposition during the osteogenic process [10]. Using layer-by-layer self-assembly technology, DNA, as a polyanion, can successfully form a layered structure on a material’s surface. Blacklock et al. [11] revealed that a multilayer film of polypeptide TAT/DNA containing disulfide bonds was degraded within 24 h in a reductive environment.
Our preliminary studies demonstrated that PLL and DNA could achieve layer-by-layer self-assembly on Ti surface and effectively improve the biological properties of the Ti surface [12, 13]. Cell-nanotopography interactions are believed to represent a promising management to precisely control seed cell function and differentiation in bone tissue engineering, because bone itself has a structural hierarchy at the first level in the nanometer range [5, 14, 15]. If PLL and DNA can achieve selfassembly on a nano-porous rough surface, the bioactivity of Ti and its alloys should be further improved. However, further study is required to confirm whether PLL and DNA can form multilayer films through layer-by-layer self-assembly on a nano-porous rough surface and whether these films could enhance biological activity. This study fabricated PLL/DNA multilayer films through self-assembly on the surface of TiO2 nanotubes, and evaluated the physical chemical properties and biological properties of the material.
2. Experimental 2.1. MaterialsCommercial pure titanium (CP-Ti, 99.7%) was cut into ∅ 10 mm × 1.5 mm. Poly-L-lysine (PLL, (MW: 30-70 kDa)), N-[2- hydroxyethyl] piperazine-N'-[2-ethanesulfonic acid] (HEPEs, free acid, high purity grade) and Gold view Ⅰ were purchased from Sigma (USA). Deoxyribonucleic acid (DNA, fish sperm, sodium salt) was obtained from AppliChem (Germany).
2.2. Fabrication of TiO2 nanotubesTitanium disks were polished with silicon carbide sandpaper of no. 240, 400, 800, 1000 and 1500 grits in series, and then washed in an ultrasonic cleaner with distilled water, acetone, ethanol and distilled water sequentially and finally dried in air at room temperature.
The samples were pre-processed using a previously reported method [16]. Briefly, the titaniumdisks were anodic oxidized in a 2mol/LH3PO4 + 0.15 mol/L HF electrolyte solution at 20 V for 1 h, followed by heating at 450 ℃ to produce anatase (TiO2) nanotubes. The obtained samples were labeled as T. T was then processed in a 5 mol/L NaOH solution at 80 ℃ for 6 h, followed by ultrasonic cleaning and drying. This dried sample was labeled as TNT.
2.3. Preparation of polyelectrolyte multilayer filmsThe assembling process was similar to the previous study [13]. The isoelectric point of TiO2 is 4.2-5.5 [17] and the isoelectric points of PLL and DNA are 9.74 and 4-4.5, respectively [13]. So PLL was first assembled on the TNT surface. TNT was immersed in 1 mg/mL PLL/HEPEs buffer (20 mmol/L HEPEs, 75 mmol/L NaCl, pH 7.4) for 15 min, cleaned and dried to acquire sample TNT/P. Subsequently, TNT/P was incubated in 1 mg/mL DNA/HEPEs buffer (20 mmol/L HEPEs, 75 mmol/L NaCl, pH 7.4) for another 15 min and then cleaned and dried to obtain sample TNT/P/D. Finally, TNT/ P/D was incubated in the PLL/HEPEs buffer again and cleaned and dried, resulting in sample TNT/P/D/P.
2.4. Surface characterizationAll samples were dried in a vacuum desiccator under room temperature for at least 12 h before the measurements. The morphologies of T and TNT were observed by scanning electron microscopy (SEM, FEI Quanta 200, The Netherlands). The chemical compositions of the self-assembled surfaces determined by X-ray photoelectron spectroscopy (XPS, XSAM800, Kratos Ltd, Britain), and the data were processed using Kratos VISION 2000.
2.5. Biomimetic mineralizationBecause of rapid deposition, the self-assembled samples were slant immersed in centrifuge tubes with a double concentration of simulated body fluid (2SBF) at 37 ℃ [18, 19]. The 2SBF was changed every 2 days to ensure the freshness and constant ionic concentration of the solution. The samples were retrieved from the solution at day 2 and day 7 and then cleaned and dried in air. The crystal structure of samples were measured by X-ray diffraction (XRD, X0pert pro-MPD, PANalytical, The Netherlands) using a Cu-Kα radiation in the regular range 2θ = 20°-50° with a step rate of 0.01°/s. The morphology of samples was observed by SEM at an accelerating voltage of 20 kV.
2.6. Osteoblast cultureOsteoblast cells were isolated from newborn (2-4 days) rats, as previously described [20]. Cells were cultured in a-minima essential medium (Gibco), supplemented with 10% fetal bovine serum (FBS, Gibco). Then, the cultured third-generation of osteoblasts was digested with Trypsin-EDTA to acquire a cell suspension. The density of the cells in the suspension was adjusted to 2.5 × 104 cells/mL. The samples were sterilized and placed into a 24-well plate with 1 mL of the cell suspension in each well. The plates were then placed in a 37 ℃, 5% CO2 cell incubator. The culture medium was renewed every two days. All culture experiments have been done in triplicates: each experimental point is presented as the mean of three measures on three titanium disks.
2.7. Cell viability assayAlamar blue, a fluorescent indicator dye, was used for assay of cell viability. In brief, at day 3 and day 7, the samples were rinsed with PBS and 300 μL of dye solution (10% v/v FBS/10% Alamar blue/ 80% M199) was added to each well. After incubating at 37 ℃ for 4 h, 200 μL of solubilization/stop solution was added into a 96- well plate. The relative cell number was determined by measuring light absorbance (OD) at a wavelength of 570 nm with an automatic micro-plate (ELISA) reader (Molecular Devices, Sunnyvale, CA). The data was normalized to the results of the blank samples. The absorbance of the blank well without material extracts was regarded as 100%, and the percentage of absorbance for each well was calculated.
2.8. Alkaline phosphatase activity assayAlkaline phosphatase (ALP) was employed to evaluate the early differentiation ability of the cells. After 7 days, cells were washed 3 times in PBS and incubated in Triton X-100 (1% w/v). Then the 24- well plate was placed into a refrigerator to be frozen at -80 ℃ for 2 h. After running three freeze-thaw cycles to homogenize the solutions, 50 μL of the solutions was added into a 96-well plate for the ALP activity test. ALP substrate solution (200 μL; 4-NPP, ELPN- 500, Bio-Assay Systems) was added to each well, and the solutions were mixed at room temperature. The absorbance of each solution was measured at a wavelength of 405 nm with an ELISA Reader.
2.9. Cell morphologyAfter culture for 3 and 7 days, the cells on the samples were washed with PBS. Subsequently, cells were fixed with 2.5% glutaraldehyde in 0.8 mol/L PBS for 12 h at 4 ℃ and then thoroughly washed with PBS. The fixed and washed samples were dipped in Rhodamine123 (Sigma) solution containing 1% PBS for 30 min at room temperature, washed with abundant water for 3 times, dried, and then measured using a fluorescence microscope (DMIL, Leica, Germany).
2.10. Statistical analysisAll data were expressed as means ± SD for n = 3. Further, the one way ANOVA was used to determine the level of significance. P < 0.05 was set as significant and P < 0.01 was set as highly significant.
3. Results and discussionPLL was used as cationic building blocks. DNA was chosen as the anionic building block, considering the unique structure of DNA molecules and their potentially positive effects on implantology instead of the encoded genetic information.
Fig. 1 shows SEM images of the samples without and with heat and alkali treatments. After anodic oxidation, there are highly ordered TNT arrays with diameters of 100 nm formed on the Ti surface. Afterwards, with the heat and alkali treatments, the nanotubes on the TNT surface retained its original orderly shapes and the walls of the nanotubes were slightly thicker.

|
Download:
|
| Figure 1. SEM image of the samples: (a) T (without heat and alkali treatment) and (b) TNT (with heat and alkali treatment). | |
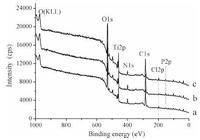
The full XPS spectra of samples TNT/P, TNT/P/D and TNT/P/D/P are shown in Fig. 2. Characteristic peaks of C 1s, O 1s and N 1s were detected for all samples, suggesting that all of the samples contained PLL. P 2p peaks were detected on TNT/P/D and TNT/P/D/ P, but not on TNT/P, which suggests that DNA was assembled onto the samples of TNT/P/D and TNT/P/D/P successfully. Ti 2p peaks of all samples with assembled PLL or/and DNA, suggest that the films were so thin that the titanium signals could be detected by XPS and also could not completely cover the substrates.
|
Download:
|
| Figure 2. XPS overview spectra of the self-assembled samples: (a) TNT/P, (b) TNT/P/D and (c) TNT/P/D/P. | |
Fig. 3 shows the high-resolution XPS spectra and the fitting results for O 1s, N 1s and P 2p peaks of each sample. O 1s spectra in Fig. 3a shows two characteristics, including oxygen in Ti-O (530.4 eV), -COOH (531.4 eV). The other one at 532.4 eV is O 1s in PO3- 4 , which was only detected in Fig. 3b and c. The N 1s peaks include two characteristic peaks: amino (NH2) peak at 399.3 eV; protonated amino peak (NH3+ ) at 400.2 eV. The P 2p in P-O- (133.1 eV) was detected only for samples TNT/P/D and TNT/P/D/P (Fig. 3b and c). These results confirmed the successful assembly of PLL and DNA on the TNT surfaces through an electrostatic driving force. In addition, the presence of Cl on samples should come from NaCl in HEPEs buffer, in which PLL and DNA dissolved. Shi et al. [21] demonstrated that, in the presence of NaCl, the DNA molecules were coiled on the PLL layer. This configuration should allow a greater number of DNA molecules to pack onto the PLL layer.

|
Download:
|
| Figure 3. The high resolution maps of N 1s, O 1s and P 2p and fitting results of the self-assembled samples: (a) TNT/P, (b) TNT/P/D and (c) TNT/P/D/P. | |
Surface functional groups play important roles including nucleation and deposition of calcium phosphate onto titanium surface. Different functional groups can have different nucleation ability for calcium phosphate. Fig. 4 shows the SEM results of the samples after mineralization for one day. After the assembly, deposits were observed on the sample surfaces, and more deposits were found on the surface of TNT/P/D/P (Fig. 4c). No deposits were observed on the TNT surface after immersion on 2SBF for 1 day (photo not shown).

|
Download:
|
| Figure 4. SEM images of samples soaked in 2SBF for 1 day with (a) TNT/P, (b) TNT/P/D and (c) TNT/P/D/P. | |
Fig. 5 shows the XRD spectra of the samples after incubation in the 2SBF solution for 2 days. Before mineralization treatment, there were peaks of Ti and anatase. Following mineralization for two days, diffraction peaks of HA were detected on both the nonassembled and assembled sample surfaces. The coatings formed in 2SBF had a low HA crystallinity level and the HA grains were fine. In addition, the coatings also were very thin. As a result, it is difficult to detect the diffraction peaks of HA at high scattering angle. In our previous work Ca/P ratio and IR spectra have been used to confirm that it was HA [13, 22]. Besides, the results of XRD detection of samples after 7 days were consistent with the results in Fig. 5 and it can also be reflected in Fig. 6. This result suggested that the deposits in Fig. 4 were HA crystals.

|
Download:
|
| Figure 5. XRD patterns of TNT and samples after biomimetic mineralization treatment for 2 days: (a) TNT (before mineralization), (b) TNT, (c) TNT/P, (d) TNT/P/D and (e) TNT/P/D/P. | |

|
Download:
|
| Figure 6. SEM images of samples soaked in 2SBF for 2 days and 7 days with (a) TNT, (b) TNT/P, (c) TNT/P/D and (d) TNT/P/D/P. | |
Fig. 6 is the SEM images of the mineralized samples at day 2 and day 7. After 2 days, the mineralized layers were evenly distributed, and their surface showed a few microcracks. Because the anatase peak and Ti peak were still detected, as shown in Fig. 5, the thickness of the HA layer could be very thin. After mineralization for 7 days, the microcracks on the sample surface were even more significant, which may be the result of high internal stress caused by fast depositing to thick coating. Based on our previous study [13], the TiO2 nanotubes surface with PLL/DNA multilayers can more quickly induce HA formation than the polished titanium surface with the some multilayers. One possible reason for this finding was that after alkali treatment, fibrous sodium titanate [23] was produced on top of the TiO2 nanotubes. After self-assembled, PLL and DNA cannot cover the whole substrate that exists in the naked TiO2 nanotubes. Then fibrous sodium titanate could rapidly induce the nucleation and growth of Ca-P in SBF. In addition, the assembled sample surface contained multiple functional groups, including -COOH, -PO4H2, -NH2 and -CONH2, which all favored the formation of hydroxyapatite [24]. Ca2+ ions in SBF were electrostatically attracted onto the multilayers by negatively charged -COOH, -PO4H2 groups and subsequently combined PO43- ions. In summary, the mineralization results showed a more positive effect on hydroxyapatite deposition with the sample TNT/ P/D/P.
The Alamar Blue test reflects cell vitality, and the OD value is proportional to the number of living cells within a certain range. Fig. 7 shows the cell vitality on four different samples at day 3 and day 7. The number of cells on each sample increased over time, which suggested that the surface chemistry of the samples favored the proliferation of the cells. The multilayered samples exhibited larger cell numbers than TNT, and the highest number on TNT/P/D/ P. Cell numbers on samples followed the order: TNT/P/D/P > TNT/ P > TNT/P/D > TNT. In addition, the cell proliferation on TiO2 nanotubes with PLL-DNA multilayers was obviously superior to that on polished titanium with the same multilayers [12].

|
Download:
|
| Figure 7. The Alamar blue assay of osteoblast cells cultured on samples after 3 and 7 days incubation compared with control TNT, *P < 0.05, **P < 0.01. | |
Fig. 8 shows fluorescence microscopy images of cell cultures on four different samples for day 3 and day 7. The numbers of living cells on all of the samples increased over time. The fluorescent signal of the cells on the TiO2 nanotubes with the multilayers was stronger than that without the multilayers, suggesting that there were more cells on the assembled nanotubes than the nonassembled nanotubes. Specifically, the number of living cells on the TNT/P/D/P surface with three assembled layers and PLL on the outmost layer, was significantly greater compared with the other samples. The result was consistent with Alamar Blue assay.

|
Download:
|
| Figure 8. Osteoblast alkaline-phosphatase-specific activity after 7 days incubation on samples and control TNT, *P < 0.05. | |
Cell adhesion, spreading, and subsequent proliferation are closely related to the surface properties of the substrate, such as composition, roughness, wettability, and morphology. It is well known that these surface properties have a direct effect on osteoblast attachment, proliferation, and differentiation [17, 25]. We can find that when cells were cultured on the TiO2 nanotubes with PLL or DNA, the effects of material composition were significant. The carboxyl, hydroxyl, amino, imino and amide groups can promote cell adhesion and proliferation [26]. In particular, PLL on the outmost layer, with positive charges, can promote the attachment of negatively charged osteoblasts. In addition, TNT/P/D/P can provide more carboxyl and amino groups. Therefore, sample TNT/P/D/P, with three layers containing the outermost layer of PLL, showed significantly more cells on its surface compared with the other samples. After 7 days, the cells were radially and fully extended and covered the entire sample surface. The pseudopodia between cells evenly spread and cells connected into a network. Compared with our previous work that osteoblasts grew on polished Ti with the self-assembled layers [12], the cells in this study exhibited better adhesion and proliferation on TiO2 nanotubes with the self-assembled layers. The above results showed that the self-assembled PLL and DNA layers on TiO2 nanotubes were beneficial for cell adhesion and spreading, thus further enhancing the proliferation ability of the cells. Moreover, the cell number on the sample surface was greater when the outmost layer consisted of PLL compared with when outmost layer consisted of DNA.
ALP, a mineralization-related protein for osteogenesis of osteoblasts [27], can indicate the early osteogenic differentiation tendency of cells. The ALP activity (OD value) of cells cultured with various samples for 7 days are shown in Fig. 9. The ALP activity was the lowest for the samples without self-assembled layers. In another word, self-assembly modification could effectively enhance the cell activity of TiO2 nanotubes on titanium surfaces. Moreover, sample TNT/P/D/P, with three assembled layers and the outmost layer of PLL, showed the highest ALP activity. It suggested that the self-assembled layer could not only benefit the proliferation of osteoblasts but also promote the early differentiation of osteoblasts.

|
Download:
|
| Figure 9. Fluorescent light microscopic images of osteoblast cells on samples after 3 and 7 days incubation: (a) TNT, (b) TNT/P, (c) TNT/P/D and (d) TNT/P/D/P. | |
4. Conclusions
In this work, PLL and DNA were shown to be able to selfassemble onto TiO2 nanotubes through an electrostatic interaction. In vitro investigation showed that the PLL and DNA assembly layers on the TiO2 nanotubes surfaces benefited the deposition of hydroxyapatite. In particular, the sample with assembled three layers containing the outmost layer of PLL showed a more positive effect on hydroxyapatite deposition. And the self-assembled PLL and DNA layers on TiO2 nanotubes can promote cell adhesion, extension, proliferation and differentiation. Using PLL and DNA to modify Ti nanotubes through the layer-by-layer self-assembly technology was an effective method for promoting bone tissue regeneration and enhancing the biocompatibility of the material.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 31570955) and Applied Basic Research Programs of Sichuan Province, China (No. 2015JY0036).
| [1] | Y.L. Zhang, L.J. Chen, C.D. Liu, et al. , Self-assembly chitosan/gelatin composite coating on icariin-modified TiO2 nanotubes for the regulation of osteoblast bioactivity. Mater. Des. 92 (2016) 471–479. |
| [2] | S. Lavenus, D.J. Poxson, N. Ogievetsky, et al. , Stem cell behavior on tailored porous oxide surface coatings. Biomaterials 55 (2015) 96–109. DOI:10.1016/j.biomaterials.2015.03.033 |
| [3] | Q.C. Wang, M. Libera. Microgel-modified surfaces enhance short-term osteoblast response. Colloid Surf B 118 (2014) 202–209. DOI:10.1016/j.colsurfb.2014.04.002 |
| [4] | L. Xia, B. Feng, P.Z. Wang, et al. , In vitro and in vivo studies of surface-structured implants for bone formation. Int. J. Nanomed. 7 (2012) 4873–4881. |
| [5] | K.S. Brammer, C.J. Frandsen, S. Jin. TiO2 nanotubes for bone regeneration. Trends. Biotechnol. 30 (2012) 315–322. DOI:10.1016/j.tibtech.2012.02.005 |
| [6] | K.C. Popat, L. Leoni, C.A. Grimes, et al. , Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 28 (2007) 3188–3197. DOI:10.1016/j.biomaterials.2007.03.020 |
| [7] | T. Groth, A. Lendlein. Layer-by-layer deposition of polyelectrolytes a versatile tool for the in vivo repair of blood vessels. Angew. Chem. Int. Ed. 43 (2004) 926–928. DOI:10.1002/(ISSN)1521-3773 |
| [8] | N. Jessel, M. Oulad-Abdeighani, F. Meyer, et al. , Multiple and time-scheduled in situ DNA delivery mediated by beta-cyclodextrin embedded in a polyelectrolyte multilayer. Proc. Natl. Acad. Sci. 103 (2006) 8618–8621. DOI:10.1073/pnas.0508246103 |
| [9] | Z. Guo, G.Q. Huang, J. Li, et al. , Graphene oxide-Ag/poly-L-lysine modified glassy carbon electrode as an electrochemical sensor for the determination of dopamine in the presence of ascorbic acid. J. Electroanal. Chem. 759 (2015) 113–121. DOI:10.1016/j.jelechem.2015.11.001 |
| [10] | S. Kamei, N. Tomita, S. Tamai, et al. , Histologic and mechanical evaluation for bone bonding of polymer surfaces grafted with a phosphate-containing polymer. J. Biomed. Mater. Res. 37 (1997) 384–393. DOI:10.1002/(ISSN)1097-4636 |
| [11] | J. Blacklock, H. Handa, D.S. Manickam, et al. , Disassembly of layer-by-layer films of plasmid DNA and reducible TAT polypeptide. Biomaterials 28 (2007) 117–124. DOI:10.1016/j.biomaterials.2006.08.035 |
| [12] | W.L. Gao, B. Feng, X. Lu, et al. , Characterization and cell behavior of titanium surfaces with PLL/DNA modification via a layer-by-layer technique. J. Biomed. Mater. Res. A 100 (2012) 2176–2185. |
| [13] | W.L. Gao, B. Feng, Y.X. Ni, et al. , Protein adsorption and biomimetic mineralization behaviors of PLL-DNA multilayered films assembled onto titanium. Appl. Surf. Sci. 257 (2010) 538–546. DOI:10.1016/j.apsusc.2010.07.029 |
| [14] | C.J. Bettinger, R. Langer, J.T. Borenstein. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. 48 (2009) 5406–5415. DOI:10.1002/anie.v48:30 |
| [15] | S. Clair, F. Variola, M. Kondratenko, et al. , Self-assembled monolayer of alkanephosphoric acid on nanotextured Ti. J. Chem. Phys. 128 (2008) 144705. DOI:10.1063/1.2876421 |
| [16] | J. Shi, B. Feng, X. Lu, et al. , Adsorption and release behavior of BSA and FN on nanostructural Ti surface. J. Inorg. Mater. 26 (2011) 1299–1303. DOI:10.3724/SP.J.1077.2011.01299 |
| [17] | B. Feng, J. Weng, B.C. Yang, et al. , Surface characterization of titanium and adsorption of bovine serum albumin. Mater. Charact. 49 (2003) 129–137. |
| [18] | T. Sun, W.C. Lee, M. Wang. A comparative study of apatite coating and apatite/collagen composite coating fabricated on NiTi shape memory alloy through electrochemical deposition. Mater. Lett. 65 (2011) 2575–2577. DOI:10.1016/j.matlet.2011.05.107 |
| [19] | Y. Wang, H.J. Yu, C.Z. Chen, et al. , Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 85 (2015) 640–652. |
| [20] | A. Nanci, S. Zalzal, Y. Gotoh, et al. , Ultrastructural characterization and immunolocalization of osteopontin in rat calvarial osteoblast primary cultures. Microsc. Res. Tech. 33 (1996) 214–231. DOI:10.1002/(ISSN)1097-0029 |
| [21] | X.Y. Shi, R. Sanedrin, F.M. Zhou. Structural characterization of multilayered DNA and polylysine composite films: influence of ionic strength of DNA solutions on the extent of DNA incorporation. J. Phys. Chem. B 106 (2002) 1173–1180. |
| [22] | B. Feng, J.Y. Chen, S.K. Qi, et al. , Carbonate apatite coating on titanium induced rapidly by precalcification. Biomaterials 23 (2002) 173–179. DOI:10.1016/S0142-9612(01)00093-X |
| [23] | S.H. Oh, R.R. Finones, C. Daraio, et al. , Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 26 (2005) 4938–4943. DOI:10.1016/j.biomaterials.2005.01.048 |
| [24] | Q. Liu, J. Ding, F.K. Mante, et al. , The role of surface functional groups in calcium phosphate nucleation on titanium foil: a self-assembled monolayer technique. Biomaterials 23 (2002) 3110–3111. |
| [25] | K. Anselme, M. Bigerelle, B. Noel, et al. , Effect of grooves titanium substrate on human osteoblastic cell growth. J. Biomed. Mater. Res. 60 (2002) 529–540. DOI:10.1002/jbm.v60:4 |
| [26] | J. Yang, J.Z. Bei, S.G. Wang. Enhanced cell affinity of poly (D. L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 23 (2002) 2607–2614. DOI:10.1016/S0142-9612(01)00400-8 |
| [27] | H.Y. Lin, J.H. Chen. Osteoblast differentiation and phenotype expressions on chitosan-coated Ti-6Al-4V. Carbohydr. Polym. 97 (2013) 618–626. DOI:10.1016/j.carbpol.2013.05.048 |
 2016, Vol. 27
2016, Vol. 27 


