Direct methanol fuel cells (DMFC) have been widely studied as a promising power source for portable electronic devices due to the ease of carry and high specific energy density [1-5]. The noble metal Pt is still the most efficient anode catalyst because of its high catalytic activity, but the high cost and low tolerance to poisoning limit its use in fuel cells. So the improvement of Pt-based catalyst has great practical significance [6].
Alloying a non-noble metal with pure platinum has become a popular method to increase the electrochemical activity and corrosion resistance [7-10], wherein tin as a non-noble alloyed metal component received extensive attention [11, 12]. Studies suggested that Sn, as an alloy component is beneficial for the decomposition of H2O on the surface of tin, thus, enhanced CO oxidation into CO2. However, its bonding with Pt weakened the adsorption of oxide species on Pt by altering electronic properties of Pt [13-15]. Current methods of synthesis of Pt-Sn alloy mostly are complex [16-18], for example, high temperature organic phase co-reduction method [19], in which precursor reduced at 180-280 ℃ in 1-octadecene with oleylamine as reducing agent and oleylamine/oleic acid as surfactants. The Pt/oxide-C materials show increasing electrochemical activity, for instance, presence of Sn-oxide on conventional Pt/C exhibits remarkable promotional effects by reducing CO poisoning, where CO oxidation on Pt/SnO2- C samples take place at lower electrode potentials compared to Pt/ C due to changes in their electronic properties of Pt caused by the interaction of Pt and the oxide [20, 21].
In this paper, we successfully prepared PtSn2-SnO2/C nanocatalyst by co-reduction of Pt and Sn precursor using NaBH4 as the reducing agent at 15 8C, integrating advantages of alloy and oxide. Electrochemical tests proved its enhanced resistance to CO poisoning and the methanol oxidation activity was up to 3 times than that of commercial Pt/C.
2. Experimental 2.1. ChemicalsAnalytical grade hexachloroplatinic (Ⅳ) acid hexahydrate (H2PtCl6·6H2O, 99.9%, 0.0193 mol L-1 aqueous solution) and anhydrous ethanol were obtained from Tianjin Chemical Reagents, China. Stannous chloride (SnCl2·2H2O), sodium borohydride (NaBH4) and the Pt/C (20 wt%. Pt nanoparticles on Vulcan XC-72 carbon support) catalyst was purchased from Alfa Aesar (Ward Hill, MA, USA). Nafion (5% in a mixture of lower aliphatic alcohols and water) was purchased from Sigma Aldrich. All the chemicals were used as received without further purification.
2.2. Preparation of catalystCarbon black (0.05 g), stannous chloride (0.0344 g), chloroplatinic acid (1.32 mL), together with ethanol (5 mL), were stirred at 15 ℃ for 2 h, followed by addition of 0.04 g sodium borohydride aqueous solution and stirred for another 2 h. Then centrifuged, dried at 80 ℃ to obtain PtSn2-SnO2/C catalyst. Reduce the amount of stannous chloride, and cut off the air, we can obtain PtSn2/C. As a comparison, samples prepared without Sn or Pt precursors were denoted as Pt/C and Sn/C, respectively, with all the other parameters the same as above.
2.3. Physical characterizationXRD was performed using a Brucker D8 Focus diffractometer with Cu Ka radiation (λ = 0.154 nm, 40 kV, 40 mA). The transmission electron microscopy (TEM) investigations were performed using a 200 kV TEM Philips Tecnai G2F20 instrument. X-ray dispersion spectroscopy (EDX) can be used to determine the composition of the sample. The elemental composition of the surfaces was measured with XPS (ESCALAB 250Xi spectrometer, XR6 X-ray source).
2.4. Electrochemical measurementThe test was performed at r.t. on the CHI 660D electrochemical work station measurement. The electrodes were glassy carbon electrodes (support for the working electrodes with a diameter of 3 mm) with Ag/AgCl as the reference electrode and Pt wire as the counter electrode. All potentials used in tests are versus Ag/AgCl. Five microliter catalyst in water suspension (2 mg of catalyst with 1 mL water) was deposited on a glassy carbon electrode (3 mm in diameter, mirror polished) and dried at room temperature. Five microliter Nafion was deposited over the catalyst and dried at r.t. Then, these three electrodes are placed in the well-prepared electrolytic cell mixture (2 mol L-1 methanol 3 mL, 0.1 mol L-1 HClO4 3 mL). The Electrochemical Impedance Spectra (EIS) was also carried on the CHI660D electrochemical workstation measurement at open circuit potential with a 5 mV sine perturbation. The measuring frequency range was 105-10-2 Hz.
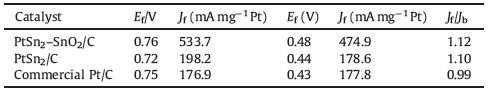
3. Results and discussion 3.1. Physical characterizationFig. 1 shows XRD patterns of PtSn2-SnO2/C and Pt/C in comparison with reference data of PtSn2 and SnO2. In the case of PtSn2-SnO2/C, the characteristic diffraction peaks at 2θ = 24.05°, 39.65°, 46.875° and 57.31°, which correspond to the (1 1 1), (2 2 0), (3 1 1) and (4 0 0) planes of PtSn2 structure (JCPDS PDF #65-2991), and the diffraction peaks at 2θ = 33.89°, 51.78° correspond to (1 0 1) and (2 1 1) planes of SnO2 standard (JCPDS PDF #71-652). These results indicate the formation of PtSn2-SnO2/C, being consistent with the results of TEM test which will be showed in detail in the following part.
|
Download:
|
| Figure 1. XRD patterns of PtSn2-SnO2/C, PtSn2 standard and SnO2 standard | |
Structure analysis of the samples prepared above is examined by TEM measurements as shown Fig. 2. According to the statistical survey results, the average diameter of particles is about 2-6 nm (Fig. 2c). As confirmed by the EDS in Fig. 2d, the constitution of PtSn2-SnO2/C nanoparticles is measured to be 16.34 at% Pt and 83.66 at% Sn. The composition is also supported by EDS elemental mapping of Pt, Sn and O (Fig. 2e and f). As can be seen, nanoparticles are composed by Pt, Sn and O, meanwhile, some SnO2 homogeneously distribute at the surface of carbon black. The XPS was employed to further analyze the compositions and atom binding states of PtSn2-SnO2/C, it revealed that Pt and partial Sn are in zero valence state while the other part of Sn is tetravalent (Fig. 2h and i).

|
Download:
|
| Figure 2. TEM micrograph (a, b and c) and EDX (d) of PtSn2-SnO2/Ccatalyst. (e, f) EDS maps of Pt, Sn and O. (g-h) XPS survey spectra of PtSn2-SnO2/C. | |
3.2. Electrochemical measurement
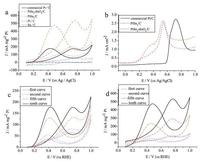
Fig. 3a shows the comparison of CV curves for PtSn2-SnO2/C, PtSn2/C, Pt/C Sn/C and commercial Pt/C catalysts in the 0.1 mol L-1 HClO4 and 1.0 mol L-1 CH3OH at a scan rate of 20 mV s-1. Here, the electro-catalytic current density is normalized in references to the loading amount of Pt. The oxidation of methanol molecules on the electrode surface occurs in the first anodic peak, which is usually used to evaluate the catalytic performance of the catalysts. During the forward scan, some methanol is incompletely oxidized resulting in an increase in the catalyst surface coverage by adsorbed CO intermediates, which can be oxidized when the kickback continues, forming another oxidation peak. Therefore, the current intensity ratio of these two oxidation peaks often act as an indicator to estimate the poison tolerance of catalysts. The forward peak potential (Ef), forward scan current intensity (Jf), reverse scan peak potential (Eb), reverse scan peak current intensity (Jb) and Jf/Jb of catalysts are analyzed and their values are shown in Table 1. It is obvious that the current strength of PtSn2-SnO2/C (533.7) is 3.02 times high than that of commercial Pt/C (176.9). When Sn and Pt are alloyed, maybe Sn promotes H2O decomposition to enhance CO oxidation into CO2, which would increase methanol oxidation effectively [19]. The Jf/Jb of PtSn2-SnO2/C (1.12) is high than that of PtSn2/C (1.10), and commercial Pt/C (0.99), which proves the enhanced ability of resistance to oxygen species poison.
|
Download:
|
| Figure 3. CV curves reflecting methanol electro-oxidation of catalysts and commercial Pt/C in 0.1 mol L-1 HClO4 and 1.0 mol L-1 CH3OH with a potential sweeping rate at 20 mV s-1. (b) CO stripping experiments curves of PtSn2-SnO2/C, PtSn2/C and commercial Pt/C at 0.1 mol L-1 HClO4. (c) CV curves of commercial Pt/C. (d) CV curves of PtSn2-SnO2/C. | |
|
|
Table 1 Forward peak potential (Ef), forward scan current intensity (Jf), reverse scan peak potential (Eb), reverse scan peak current intensity (Jb) and Jf/Jb of catalysts in 0.1 mol L-1 HClO4 and 1.0 mol L-1 CH3OH. |
The CO stripping experiments further prove the catalyst activity (Fig. 3b). The electrochemical cell (0.1 mol L-1 HClO4) is purged for 30 min with N2 before experiment to avoid the impact of oxygen, and then introduces into CO (10% CO, 90% He) for 15 min. As can be seen from the figure, commercial Pt/C catalyst has a sharp stripping CO oxidation peak at 0.72 V. While the peak position of PtSn2/C and PtSn2-SnO2/C catalyst has a negative shift to 0.551 V and 0.534 V, respectively. The negative direction indicates that the catalyst is capable of oxidizing CO at a lower voltage, implying SnO2 has a positive effect on the stripping removal of CO absorbed on the Pt surface, which will lower CO poisoning effectively. [22]
To further investigate the stability of the catalyst, chronoamperometry (CA) experiments were performed (Fig. 4a). As can be seen, the current density PtSn2-SnO2/C catalyst declined more slowly than that of commercial Pt/C, after 5 h it still remains a large current density.

|
Download:
|
| Figure 4. (a) CA curves of PtSn2-SnO2/Cand commercial Pt/C in 0.1 mol L-1 HClO4 and 1 mol L-1 CH3OH, the potential is 0.7 V. (b) Cyclic voltammograms of commercial Pt/C and PtSn2-SnO2/C catalysts in 0.1 mol L-1 HClO4. (c) The EIS of commercial Pt/C and the PtSn2-SnO2/C. | |
Fig. 4b displays the hydrogen adsorption and desorption test of catalysts in 0.1 mol L-1 HClO4. The electrochemical cell is purged for 30 min with N2 before experiment. The cyclic voltammetry curves have four distinct potential zones. -0.2-0.1 V, 0.1-0.3 V, 0.3-0.6 V and 0.6-1.2 V are respect for hydrogen adsorption dissociation interval, oxygen adsorption and oxide reduction zone, double plateau region and oxygen precipitation range, respectively. The process of catalyst’s adsorption of H+ in the acid solution shows hydrogen adsorption and desorption of PtSn2-SnO2/C catalyst are weakened. It can also prove that when Sn is added, the adsorption of the oxygen-containing species such as CO was weakened.
The electrochemical impedance spectra (EIS) is shown in Fig. 4c. As can be seen, the depressed semicircle in each curve is related to the double-layer capacitance between the interface of the electrode and the electrolyte [23]. The smaller arc diameter of PtSn2-SnO2/C indicates the better ability of charge transfer and ion diffusion rate than that of commercial Pt/C.
4. ConclusionPtSn2-SnO2 nanocatalyst is prepared by a relatively simple method. XRD and TEM of the nanocatalyst show the formation of metal alloy. The alloying of PtSn2 and existence of SnO2 improves the electrical properties of methanol oxidation and enhanced the resistance ability to CO oxidation, which are proved by stripping voltammetry curves from electrochemical measurements. This synthetic method is convenient, strong, stable and environment friendly.
AcknowledgmentThis work was supported by NSFC (Nos. 21373116 and 21421001), the Tianjin Natural Science Research Fund (No. 13JCYBJC18300), RFDP (No. 20120031110005) and the MOE Innovation Team (No. IRT13022) of China.
| [1] | J.A. Turner. A realizable renewable energy future. Science 285 (1999) 687–689. DOI:10.1126/science.285.5428.687 |
| [2] | A.S. Aricò, P. Bruce, B. Scrosati, J.M. Tarascon, W. Van Schalkwijk. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4 (2005) 366–377. DOI:10.1038/nmat1368 |
| [3] | R. Borup, J. Meyers, B. Pivovar, et al. , Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 107 (2007) 3904–3951. DOI:10.1021/cr050182l |
| [4] | Z.H. Wen, J. Liu, J.H. Li. Core/shell Pt/C nanoparticles embedded in mesoporous carbon as a methanol-tolerant cathode catalyst in direct methanol fuel cells. Adv. Mater. 20 (2008) 743–747. DOI:10.1002/(ISSN)1521-4095 |
| [5] | Y.H. Kwon, S.C. Kim, S.Y. Lee. Nanoscale phase separation of sulfonated poly (arylene ether sulfone)/poly(ether sulfone) Semi-IPNs for DMFC membrane applications. Macromolecules 42 (2009) 5244–5250. DOI:10.1021/ma900781c |
| [6] | C.C. Huang, Q. Wang, D.B. Xiang, H.B. Shao. Surface interrogation mode of scanning electrochemical microscopy for oxidation study of adsorbed CO generated from serine on platinum. Chin. Chem. Lett. 22 (2011) 1481–1484. DOI:10.1016/j.cclet.2011.07.017 |
| [7] | J. Kua, W.A. Goddard Ⅲ. Oxidation of methanol on, 2nd and 3rd row group VⅢ transition metals (Pt, Ir, Os, Pd, Rh, and Ru): application to direct methanol fuel cells. J. Am. Chem. Soc. 121 (1999) 10928–10941. DOI:10.1021/ja9844074 |
| [8] | D. Kaplan, M. Alon, L. Burstein, Y. Rosenberg, E. Peled. Study of core-shell platinum-based catalyst for methanol and ethylene glycol oxidation. J. Power Sources 196 (2011) 1078–1083. DOI:10.1016/j.jpowsour.2010.08.022 |
| [9] | V.R. Stamenkovic, B.S. Mun, M. Arenz, et al. , Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6 (2007) 241–247. DOI:10.1038/nmat1840 |
| [10] | N.M. Marković, P.N. Ross Jr.. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 45 (2002) 117–229. DOI:10.1016/S0167-5729(01)00022-X |
| [11] | F.E. Ló pez-Suárez, A. Bueno-Ló pez, K.I.B. Eguiluz, G.R. Salazar-Banda. Pt-Sn/C catalysts prepared by sodiumborohydride reduction for alcohol oxidation in fuel cells: effect of the precursor addition order. J. Power Sources 268 (2014) 225–232. DOI:10.1016/j.jpowsour.2014.06.042 |
| [12] | H.B. Zhu, D.H. Anjum, Q.X. Wang, et al. , Sn surface-enriched Pt-Sn bimetallic nanoparticles as a selective and stable catalyst for propane dehydrogenation. J. Catal. 320 (2014) 52–62. DOI:10.1016/j.jcat.2014.09.013 |
| [13] | M.A. Taher, Z. Daliri, H. Fazelirad. Simultaneous extraction and preconcentration of copper, silver and palladium with modified alumina and their determination by electrothermal atomic absorption spectrometry. Chin. Chem. Lett. 25 (2014) 649–654. DOI:10.1016/j.cclet.2013.12.025 |
| [14] | B.E. Hayden, M.E. Rendall, O. South. Electro-oxidation of carbon monoxide on well-ordered Pt(1, 1 1)/Sn surface alloys. J. Am. Chem. Soc. 125 (2003) 7738–7742. DOI:10.1021/ja0214781 |
| [15] | H.A. Gasteiger, N.M. Marković, P.N. Ross Jr.. Structural effects in electrocatalysis: electrooxidation of carbon monoxide on Pt3 Sn single-crystal alloy surfaces. Catal. Lett. 36 (1996) 1–8. DOI:10.1007/BF00807197 |
| [16] | C.T. Hsieh, Y.S. Chang, A.K. Roy, P.Y. Yuan, K.M. Yin. Fast synthesis of binary Pt-Sn nanocatalysts onto graphene sheets for promoted catalytic activity. Electrochim. Acta 149 (2014) 278–284. DOI:10.1016/j.electacta.2014.10.108 |
| [17] | A.R. Rautio, P. Mäki-Arvela, A. Aho, K. Eränen, K. Kordas. Chemoselective hydrogenation of citral by Pt and Pt-Sn catalysts supported on TiO2 nanoparticles and nanowires. Catal. Today 241 (2015) 170–178. DOI:10.1016/j.cattod.2013.12.052 |
| [18] | L.D. Deng, T. Shishido, K. Teramura, T. Tanaka. Effect of reduction method on the activity of Pt-Sn/SiO2 for dehydrogenation of propane. Catal. Today 232 (2014) 33–39. DOI:10.1016/j.cattod.2013.10.064 |
| [19] | Y. Liu, D.G. Li, V.R. Stamenkovic, et al. , Synthesis of Pt3 Sn alloy nanoparticles and their catalysis for electro-oxidation of CO and methanol. ACS Catal. 1 (2011) 1719–1723. DOI:10.1021/cs200430r |
| [20] | B.R. Camacho, C. Morais, M.A. Valenzuela, N. Alonso-Vante. Enhancing oxygen reduction reaction activity and stability of platinum via oxide-carbon composites. Catal. Today 202 (2013) 36–43. DOI:10.1016/j.cattod.2012.03.033 |
| [21] | B. Ruiz-Camacho, M.A. Valenzuela, R.G. Gonzá lez-Huerta, et al. , Electrochemical and XAS investigation of oxygen reduction reaction on Pt-TiO2-C catalysts. Int. J. Hydrogen. Energy 38 (2013) 12648–12656. DOI:10.1016/j.ijhydene.2013.01.002 |
| [22] | B. Ruiz-Camacho, H.H.R. Santoyo, J.M. Medina-Flores, O. Á lvarez-Martínez. Platinum deposited on TiO2-C and SnO2-C composites for methanol oxidation and oxygen reduction. Electrochim. Acta 120 (2014) 344–349. DOI:10.1016/j.electacta.2013.12.055 |
| [23] | D.Soundararajan, J.H. Park, K.H. Kim, J.M.Ko. Pt-Ni alloy nanoparticles supportedon CNF as catalyst for direct ethanol fuel cells. Curr. Appl. Phys. 12 (2012) 854–859. DOI:10.1016/j.cap.2011.11.020 |
 2016, Vol. 27
2016, Vol. 27