Aerosols exhibit a broad range of impacts on the atmosphere, directly by interfering with the solar radiative transfer and indirectly by influencing cloud formation [1]. In atmosphere, the aerosols phase will change with the relative humidity (RH) and temperature [2]. The different phase of atmospheric particles affects their physical, chemical, and optical properties [3, 4]. For example, when the dry salt constituents of a particle uptake water to form an aqueous particle, the diameter increases by several folds [5]. These larger particles scatter visible light much more efficiently. So the hygroscopic response of atmospheric particles is responsible for reduced visibility associated with smog. Beyond their physical and optical effects, atmospheric aerosols also provide the milieu for many important chemical transformations. For example, aqueous phase oxidation is believed responsible for the acidification of rain in most regions of the world [6, 7]. However, a phase transition to a crystalline particle removes this oxidation pathway. In common, inorganic compounds exhibit the strong hygroscopic properties and undergo phase changes with cycles of RH [8-10]. Prominent examples include most nitrates and sulfates [11-13].
The precipitation of crystalline in an aqueous droplet at low RH is also often called efflorescence, and the corresponding RH is efflorescence RH (ERH). Crystallization is a kinetically controlled process due to the free energy barrier associated with nucleation of a crystalline solid in an aqueous solution. As a result, the ERH is often lower than deliquescence RH (DRH), which refers to when particles take up water to form solution droplets. In the absence of heterogeneous nuclei, crystallization occurs by homogeneous nucleation; otherwise, crystallization can occur by heterogeneous nucleation.Organic aerosol particles, constitute up to 20%-90%mass fraction of ultrafine particles in some tropospheric environments [14], have complex effects on the hygroscopic growth of inorganic salts resulted fromspecific organic-inorganic interactions [15, 16]. In our group, the hygroscopic properties of glycerol/NaNO3 and mixed phthalic acid/ammonium sulfate particles have been observed by Raman technique, which showed that glycerol suppressed the NaNO3 crystallization and the physical state of phthalic acid/ ammonium sulfate particles experienced well-mixed liquid state, liquid-liquid phase-separated state, and crystalline state [17, 18].
Sulfate, originating from a variety of sources including volcanic ash, sea wave spray, and oxidation of sulfur dioxide (SO2) and other sulfur-containing species, plays an important role in tropospheric chemistry and the atmospheric environment [19, 20]. Knowledge of the hygroscopic properties (including crystallization) of sulfates would be beneficial to understanding their role in the atmosphere. The hygroscopic behaviors of Na2SO4 and its mixture, such as, Na2SO4/NaCl, Na2SO4/MgSO4, Na2SO4/(NH4)2SO4, NaNO3/Na2SO4, have been well investigated [21-25]. Hexadecyltrimethylammoniumbromide (CTAB) is a presentative surfactant, which often form assemblies in aqueous solution above critical micelle concentration (CMC). In the following, we use FTIR-ATR technology to investigate the crystallization of Na2SO4 droplets containing CTAB. For comparison purposes we also investigated the crystallization of aqueousNa2SO4 droplets.We gained the ERHofNa2SO4 andNa2SO4/ CTAB aerosols and determined the heterogeneous nucleation rates (number of nucleation events per unit volume of the aqueous droplet per unit time) of crystalline Na2SO4 in aqueous Na2SO4 droplets and Na2SO4/CTAB droplets. We also calculated the Na2SO4 crystal amount in Na2SO4/CTAB mixed system. This combined analysis provides insight into the kinetics of nucleation in aqueous droplets in the atmosphere.
2. ExperimentalThe analytical grade Na2SO4 and chemical grade CTAB were purchased from Beijing Chemical Reagent Company and used without further purification. 3.551 g Na2SO4 and 0.167 g CTAB are dissolved into 50 mL triply distilled water to get the mixed solution. In mixed Na2SO4/CTAB solution, the concentration of Na2SO4 is 0.5 mol L×1 and that of CTAB is 9.2 - 10×3 mol L×1. The aerosol particles were generated from a nebulizer.
The FTIR-ATR setup has been elaborated in the previous studies [26] and FTIR-ATR spectra of the aerosol droplets were collected by a Nicolet Magan-IR 560 FTIR spectrometer. A liquid nitrogencooled MCT detector was used to record the FTIR spectra. In experiment, the nebulized droplets were deposited onto a ZnSe substrate by a vacuum pump, which is put onto a baseline horizontal ATR accessory (Spectra-Tech Inc. USA) in the bottom of the sample chamber. The sizes of aerosol particles span 1 μm to 5 μm in diameter, so the mass median diameter is 3 μm. The RH and temperature were determined by a thermo-hygrograph (Centertek Center 313) with a precision of ±2.5% RH and ±0.5 ℃, which was fixed at the exit of the cell. Each IR spectrum was gained in the range of 650-4000 cm-1 with a resolution of 4 cm-1 by accumulating 32 scans. All measurements were taken at ~298 K.
3. Results and discussionFig. 1 gives the FTIR spectra of Na2SO4, CTAB and mixed Na2SO4/ CTAB aerosols with the RH decreasing linearly. For Na2SO4 aerosols (Fig. 1a), the O-H stretching band (3700-3200 cm-1) and O-H bending mode (~1640 cm-1) of water decrease with the RH. The v3-SO42- and v1-SO42- bands are 1094 cm-1 and 981 cm-1 at 94.3% RH, respectively, which blue-shifted gradually. At 5.2% RH, They positioned at 1132 cm-1 and 996 cm-1, together with the complete disappearance of water band, meaning the complete crystallization of Na2SO4. It is noted that the above feature bands all changed suddenly at 75.1% RH, indicating the efflorescence relative humidity (ERH) of 75.1%, which is consistent well with previous report [26]. For CTAB (Fig. 1b), there are two obvious bands at 2917 cm-1 and 2850 cm-1, which are assigned to the asymmetric and symmetric methylene stretching vibrations. On dehydration, they are both keeping constant, suggesting unchanged hydrocarbon chains. For the water envelope at 3427 cm-1, the intensity decreased continuously with the RH without ERH (seen in inset of Fig. 1b). When CTAB is mixed with Na2SO4 (Fig. 1c), the Na2SO4 bands change with the same trend to pure Na2SO4 aerosols. The feature band is at 1132 cm-1 for Na2SO4 crystal and at 1094 cm-1 for Na2SO4 solution. As the RH decreases, the 1094 cm-1 band decreases, till the weak band at 1132 cm-1 occurs at 74.2% RH. So it can be concluded that the ERH of Na2SO4 in mixed Na2SO4/CTAB aerosols is 74.2%. The band in the range of 3600-3000 cm-1 decreased with the RH and was still visible at 5.7% RH, implying incomplete crystallization in mixed Na2SO4/ CTAB aerosols.

|
Download:
|
| Figure 1. FTIR spectra of Na2SO4 (a), CTAB (b) and Na2SO4/CTAB (c) aerosols on dehydration. | |
In order to gain the quantitative data of Na2SO4 crystals in dehumidifying process. The differential spectra during the crystallization process, which are got by every spectrum at various RHs subtracting that at 74.2% RH by using OMINIC software, were used. Fig. 2 describes the differential IR spectra for Na2SO4 and mixed Na2SO4/CTAB aerosols in the efflorescence process. It can be found that the crystal peaks are increasing accompanied by the solution peak decrease, and an obvious isosbestic point reflected the transition between solution and crystal.

|
Download:
|
| Figure 2. FTIR differential spectra of Na2SO4 (a) and Na2SO4/CTAB in the efflorescence process. | |
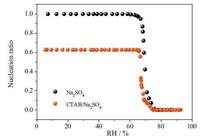
In general, the band height can characterize the concentration of the species. Herein, the band heights corresponding to crystal and solution peak are adopted to calculate the amounts of Na2SO4 crystals and Na2SO4 solutions. At first, the maximal height values of positive crystal peak (Hc, m) and negative solution peak (Ha, m) for pure Na2SO4 aerosols were acquired by Omnic software. And then, the ratio Hc, m/Ha, m = 1.08 was gained which is used as the standard of the 100% Na2SO4 crystallization. Second, the Hc, m/Ha, m value for Na2SO4/CTAB mixed aerosols was also obtained, which is 0.677. Third, 0.677 divided by 1.08 is 0.627, meaning 62.7% Na2SO4 crystals formed and still Na2SO4 solutions remaining in Na2SO4/CTAB mixed aerosols even at the minimal RH. Calculating the ratio Hc/Ha at arbitrary RH, where Hc is the peak height of crystal band and Ha represents that of solution band. And then Hc/Ha divided by 1.08 can get the nucleation ratio at corresponding RH. The nucleation ratio of Na2SO4 crystal dependent upon RH is presented in Fig. 3. It can be seen that the nucleation happened very rapid and the ERH range is about 10%.
|
Download:
|
| Figure 3. The nucleation ratio of Na2SO4 in pure Na2SO4 and Na2SO4/CTAB aerosols. | |
Next, the nucleation rate is calculated by combining FTIR data with classical nucleation theory [27]. In present experiment, the aerosol droplets are deposited onto ZnSe substrate and the heterogeneous nucleation is preferred. The heterogeneous nucleation rate, Jhet, defined as the number of nucleation events per unit surface area of aqueous solution per unit time, can be calculated from following nucleation rate equation:
| $$\begin{align} & {{J}_{\text{het}}}\left( \text{RH} \right)=-\frac{r}{A\cdot N\left( \text{RH} \right)}\cdot \frac{\text{d}N\left( \text{RH} \right)}{\text{dRH}}=-\frac{\text{dRH/d}t}{A\cdot N\left( \text{RH} \right)}\cdot \frac{\text{d}N\left( \text{RH} \right)}{\text{dRH}} \\ & =-\frac{1}{A\cdot N\left( \text{RH} \right)}\cdot \frac{\text{d}N\left( \text{RH} \right)}{\text{d}t} \\ \end{align}$$ | (1) |
where r is the rate of RH change, A is the average surface area of the ZnSe substrate interacting per liquid droplet, dN (RH) is the number of droplets observed to crystallize between RH and (RH × dRH), and N(RH) is the number of liquid droplets (not including droplets that have crystallized). For mixed Na2SO4/CTAB aerosols, the crystallization of Na2SO4 is incomplete. So the crystallized particles are used to calculate the nucleation rate. In FTIR spectra of aqueous Na2SO4 and mixed Na2SO4/CTAB, the maximal integrated area of crystal peak at 1132 cm-1 is AT, which corresponds to the total crystallized number of the liquid droplets N. When the peak area of crystal is Ac, the number of droplets at a certain RH can be described as followed:
| $$N\left( \text{RH} \right)=\frac{{{A}_{T}}-{{A}_{c}}}{{{A}_{T}}}N$$ | (2) |
So the following equation can be known:
| $$\begin{align} & {{J}_{\text{het}}}\left( \text{RH} \right)=-\frac{1}{A}\frac{\text{d}\left( {{A}_{c}}/{{A}_{T}} \right)/\left( \left( {{A}_{T}}-{{A}_{c}} \right)/{{A}_{T}} \right)}{\text{d}t} \\ & =-\frac{1}{A}\frac{\text{d}\left( {{A}_{c}}/{{A}_{T}} \right)/\left( \left( {{A}_{c}}/{{A}_{T}} \right)-1 \right)}{\text{d}t} \\ \end{align}$$ | (3) |
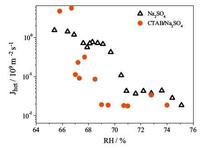
In our work, the mass median diameter of the droplets is 3 mm and the contact angel between Na2SO4 droplets and the surface of ZnSe substrate is approximately 91.1°. So the average contact surface area, A = πr2, can be calculated. From the differential spectra shown in Fig. 2, the peak areas AT and Ac at different RHs can be measured by OMINIC software. The dt can be read from experiment record. Therefore, the Jhet at a certain RH will be calculated based on the above parameters and Eq. (3). The heterogeneous nucleation rate of Na2SO4 crystal verse RH in pure Na2SO4 and mixed Na2SO4/CTAB aerosols can be seen from Fig. 4. The rate of Na2SO4 in Na2SO4 aerosols is higher than Na2SO4/CTAB mixture, suggesting that CTAB suppressed the Na2SO4 nucleation.
|
Download:
|
| Figure 4. The heterogeneous nucleation rate of Na2SO4 in pure Na2SO4 and Na2SO4/CTAB aerosols. | |
It is well known that CTAB is easy to assemble into reversed micelle above the critical micelle concentration (CMC) of 9.2 - 10-4 mol L-1. In present experiment, the origin concentration of CTAB in Na2SO4/CTAB mixture is 9.2 - 10-3 mol L-1 in aerosol droplets, which is higher than CMC and means reversed micelles formation. So part Na2SO4 droplets maybe are contained into the hydrophilic core and some is free. As the RH decreases, the free Na2SO4 droplets in mixed aerosols can dehydrate and effloresce into crystalline below ERH. While, the other Na2SO4 droplets wrapped up by CTAB assemblies cannot crystallize owing to no water evaporating into gas, reflected by incomplete crystallization (62.7%). In addition, the counter ion Br- for CTAB reversed micelle can interact with Na+ ions, which decreases the crystallization rate of free Na2SO4 droplets and ERH is delayed.
4. ConclusionIn this study, we proposed a FTIR-ATR method to calculate the nucleation rate in Na2SO4 and Na2SO4/CTAB aerosols quantitatively. Because theCTABreversedmicelles canforma thin filmonsurface of part Na2SO4 droplets, only uncovered 62.7% Na2SO4 droplets can effloresce.Moreover, the interaction between Br- and Na+ make the crystallization rate of free Na2SO4 decrease and ERH is delayed.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (Nos. 21373026, 21473009.)
| [1] | R. Makkonen, A. Asmi, V.M. Kerminen, et al. , Air pollution control and decreasing new particle formation lead to strong climate warming. Atmos. Chem. Phys. 12 (2012) 1515–1524. DOI:10.5194/acp-12-1515-2012 |
| [2] | C.K. Chan, Z.Y. Ha, M.Y. Choi. Study of water activities of aerosols of mixtures of sodium and magnesium salts. Atmos. Environ. 34 (2000) 4795–4803. DOI:10.1016/S1352-2310(00)00252-1 |
| [3] | B. Zuberi, A.K. Bertram, T. Koop, L.T. Molina, M.J. Molina. Heterogeneous freezing of aqueous particles induced by crystallized (NH4)2SO4, Ice, and Letovicite. J. Phys. Chem. A 105 (2001) 6458–6464. DOI:10.1021/jp010094e |
| [4] | S.T. Martin. Phase transitions of aqueous atmospheric particles. Chem. Rev. 100 (2000) 3403–3454. DOI:10.1021/cr990034t |
| [5] | B. Jing, S.R. Tong, Q.F. Liu, et al. , Hygroscopic behavior of multicomponent organic aerosols and their internal mixtures with ammonium sulfate. Atmos. Chem. Phys. Discuss. 15 (2015) 23357–23405. DOI:10.5194/acpd-15-23357-2015 |
| [6] | M. Kajino, M. Aikawa. A model validation study of the washout/rainout contribution of sulfate and nitrate in wet deposition compared with precipitation chemistry data in Japan. Atmos. Environ. 117 (2015) 124–134. DOI:10.1016/j.atmosenv.2015.06.042 |
| [7] | L. Sun, Y. Wang, T.X. Yue, et al. , Evaluation of the behavior of clouds in a region of severe acid rain pollution in southern China: species, complexes, and variations. Environ. Sci. Pollut. Res. 22 (2015) 14280–14290. DOI:10.1007/s11356-015-4674-5 |
| [8] | P.D. Lu, T. He, Y.H. Zhang. Relative humidity anneal effect on hygroscopicity of aerosol particles studied by rapid-scan FTIR-ATR spectroscopy. Geophys. Res. Lett. 35 (2008) L20812. DOI:10.1029/2008GL035302 |
| [9] | Q.N. Zhang, Y. Zhang, C. Cai, et al. , In situ observation on the dynamic process of evaporation and crystallization of sodium nitrate droplets on a ZnSe substrate by FTIR-ATR. J. Phys. Chem. A 118 (2014) 2728–2737. DOI:10.1021/jp412073c |
| [10] | J.P. Darr, S.Q. Davis, Y. Kohno, K. McKenna, P. Morales. Morphological effects on the hygroscopic properties of sodium chloride-sodium sulfate aerosols. J. Aerosol Sci. 77 (2014) 158–167. DOI:10.1016/j.jaerosci.2014.08.002 |
| [11] | M. Gysel, E. Weingartner, U. Baltensperger. Hygroscopicity of aerosol particles at low temperatures., 2. Theoretical and experimental hygroscopic properties of laboratory generated aerosols. Environ. Sci. Technol. 36 (2002) 63–68. DOI:10.1021/es010055g |
| [12] | C. Rodriguez-Navarro, E. Doehne, E. Sebastian. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cem. Concr. Res. 30 (2000) 1527–1534. DOI:10.1016/S0008-8846(00)00381-1 |
| [13] | E.R. Gibson, P.K. Hudson, V.H. Grassian. Physicochemical properties of nitrate aerosols: implications for the atmosphere. J. Phys. Chem. A 110 (2006) 11785–11799. DOI:10.1021/jp063821k |
| [14] | M. Kanakidou, J.H. Seinfeld, S.N. Pandis, et al. , Organic aerosol and global climate modelling: a review. Atmos. Chem. Phys. 5 (2005) 1053–1123. DOI:10.5194/acp-5-1053-2005 |
| [15] | A.J. Prenni, P.J. DeMott, S.M. Kreidenweis. Water uptake of internally mixed particles containing ammonium sulfate and dicarboxylic acids. Atmos. Environ. 37 (2003) 4243–4251. DOI:10.1016/S1352-2310(03)00559-4 |
| [16] | C. Marcolli, B.P. Luo, T. Peter. Mixing of the organic aerosol fractions: liquids as the thermodynamically stable phases. J. Phys. Chem. A 108 (2004) 2216–2224. DOI:10.1021/jp036080l |
| [17] | J.Y. Yu, Y. Zhang, G. Zeng, et al. , Suppression of NaNO3 crystal nucleation by glycerol: micro-raman observation on the efflorescence process of mixed glycerol/NaNO3/water droplets. J. Phys. Chem. B 116 (2012) 1642–1650. DOI:10.1021/jp210824e |
| [18] | Q. Zhou, S.F. Pang, Y. Wang, J.B. Ma, Y.H. Zhang. Confocal raman studies of the evolution of the physical state of mixed phthalic acid/ammonium sulfate aerosol droplets and the effect of substrates. J. Phys. Chem. B 118 (2014) 6198–6205. DOI:10.1021/jp5004598 |
| [19] | T. Koop, H.P. Ng, L.T. Molina, M.J. Molina. A new optical technique to study aerosol phase transitions: the nucleation of ice from H2SO4 aerosols. J. Phys. Chem. A 102 (1998) 8924–8931. DOI:10.1021/jp9828078 |
| [20] | V.G. Ciobanu, C. Marcolli, U.K. Krieger, A. Zuend, T. Peter. Efflorescence of ammonium sulfate and coated ammonium sulfate particles: evidence for surface nucleation. J. Phys. Chem. A 114 (2010) 9486–9495. DOI:10.1021/jp103541w |
| [21] | J.L. Dong, H.S. Xiao, L.J. Zhao, Y.H. Zhang. Spatially resolved Raman investigation on phase separations of mixed Na2SO4/MgSO4 droplets. J. Raman Spectrosc. 40 (2009) 338–343. DOI:10.1002/jrs.v40:3 |
| [22] | H.J. Tong, J.P. Reid, J.L. Dong, Y.H. Zhang. Observation of the crystallization and supersaturation of mixed component NaNO3-Na2SO4 droplets by FTIR-ATR and Raman spectroscopy. J. Phys. Chem. A 114 (2010) 12237–12243. DOI:10.1021/jp1080548 |
| [23] | Q. Qu, L. Li, W. Bai, C.W. Yan. Initial atmospheric corrosion of zinc in presence of Na2SO4 and (NH4)2SO4. Trans. Nonferrous Met. Soc. China 16 (2006) 887–891. DOI:10.1016/S1003-6326(06)60345-2 |
| [24] | P.V. Jentzsch, B. Kampe, P. Rö sch, J. Poop. Raman spectroscopic study of crystallization from solutions containing MgSO4 and Na2SO4: Raman spectra of double salts. J. Phys. Chem. A 115 (2011) 5540–5546. DOI:10.1021/jp200142n |
| [25] | R.M. Garland, M.E. Wise, M.R. Beaver, et al. , Impact of palmitic acid coating on the water uptake and loss of ammonium sulfate particles. Atmos. Chem. Phys. 5 (2005) 1951–1961. DOI:10.5194/acp-5-1951-2005 |
| [26] | X.N. Feng, H.N. Chen, Y.M. Luan, et al. , In-situ FTIR-ATR spectroscopic observation on the dynamic efflorescence/deliquescence processes of Na2SO4 and mixed Na2SO4/glycerol droplets. Chem. Phys. 430 (2014) 78–83. DOI:10.1016/j.chemphys.2013.12.009 |
| [27] | G. Vali. Freezing rate due to heterogeneous nucleation. J. Atmos. Sci. 51 (1994) 1843–1856. DOI:10.1175/1520-0469(1994)051<1843:FRDTHN>2.0.CO;2 |
 2016, Vol. 27
2016, Vol. 27 

 , Zhang Yun-Hong
, Zhang Yun-Hong