b Department of Chemistry, Dr. K.N. Modi University, Newai 304 021, Rajasthan, India ;
c Microbiology Division, Defence Research Development Establishment, Gwalior 474 002, India
Curcumin is a bright yellow aromatic powder obtained from the rhizome of turmeric (Curcuma longa L.), a plant of the ginger (Zingiberaceae) family. Chemically, it is 1, 7-bis (4-hydroxy-3- methoxy phenyl)-1, 6-heptadiene-3, 5-dione; it consists of two back bones, each with a ketone and a -CH2 group separating the backbones and a terminal meta-methoxy-para hydroxyl phenyl ring on each side. Itwas originally used forflavoringand coloring inAsian cooking recipes, but recently it has been widely used as a dietary additive in a variety of foods includingcurries, mustards, ice-creams, gelatins, meats, soups, pickles and in both alcoholic and nonalcoholic beverages [1]. Over a long period, in a number of in vivo and in vitro studies, curcumin has been found to possess awide range of pharmacological activities, such as antibacterial [2], antifungal [3], antiviral [4], anti-HIV-1 integrase [5], anti-Alzheimer’s [6], anti- Parkinson’s [7], anti-arthritic [8], antioxidant [9], anti-angiogenic [10], hypoglycemic [11], anti-inflammatory [12], anti-malarial [13], anti-diabetic [14], anti-protozoan [15], wound treatment [16], anticancer [17], anti-depressant [18], free-radical scavenging activity [19], anti-venom [20] and antitumor properties [21]. In addition to this, curcumin has also been used in dentistry [22] for a long time. It has been suggested that the antioxidant activity of the curcumin molecule depends upon the presence of a phenolic group [23]. On the other hand, other studies concluded that the hydrogens of active methylene group are important for antioxidant activity [24]. Litwinienko and Ingold [25] demonstrated that active methylene group and phenolic groups are responsible as well.
However, the truly unique feature of this molecule is its lack of toxicity. Large quantities of curcumin can be consumed without toxicity, suggesting this molecule may serve as a valuable scaffold for therapeutic development. These distinctive properties make curcumin a valuable lead compound for drug development and it remains the focus of several clinical trials [26]. Recently, the Biginelli reaction has been performed under a wide variety of conditions, and several improvements on the experimental procedures have been developed. It has been traditionally catalyzed using Bronsted, Lewis and protonic acids [27]. In the present communication, a chitosamine hydrochloride catalyzed one-pot multi-component condensation of curcumin, aromatic benzaldehyde, and urea/thiourea/guanidine under solvent-free conditions using microwave irradiation is disclosed. To the best of our knowledge, such curcumin 3, 4-dihydropyrimidinones as antioxidative and anti-inflammatory agents previously have not been reported.
2. ExperimentalMelting points of the synthesized compounds were recorded in open capillary tube and were uncorrected. All the chemicals used in the experiment were purchased from Himedia, Rankem, and Alfa Essar chemical companies and used as received. LG, MS 1927 microwave starter kit was used for microwave irradiation. Microwaves were generated at 300W in open to air conditions. 13C NMR and 1H NMR spectra were recorded on a BRUKER AVANCE Ⅱ using DMSO-d6, and CDCl3 as solvent. All chemical shifts were given in ppm relative to tetramethyl silane. UV spectra were recorded on a Chemito spectrascan 2600 model in acetonitrile. IR spectra were recorded on a Schimadzu Prestize 21 model using KBr pellet at room temperature. Mass spectra were recorded on a JEOLAccuTOF JMST100LC Mass spectrometer.
2.1. Synthesis of curcumin derivativesA 100 mL round bottom flask was charged with curcumin (2.0μmol), aromatic aldehyde (2.0μmol), urea/thiourea/guanidine (2.0μmol), and (0.02μmol) chitosamine hydrochloride as catalyst, and the flask was irradiated in the microwave oven under solvent free conditions for about 10-16min. The reaction was monitored by TLC using acetone/hexane (4:6) ratio as eluent. After completion of the reaction, the contents were dissolved in ethanol and stirred for about 10 min and recrystallized from appropriate solvents.
5-(4-Hydroxy-3-methoxyphenylethylene carbonyl)-6-(4-hydroxy, 3-methoxyphenylethylene)-4-phenyl-3, 4-dihydropyrimidin- 2 (1H)-one (4a): Recrystallization from DMF/MeOH (1:5) mixture, dark red powder, soluble in ethanol and methanol, stable at room temperature, non hygroscopic in nature, λmax(CH3CN)/nm 394; IR (KBr, cm-1): v 3509 (O-Hstr), 3215 (C-Hstr), 2931 (C-Hstr), 1565 (C55Ostr), 1518 (O-Hstr), 1432 (O-Hstr), 1271 (O-Hstr), 1034 (O-Hstr), 969 (C-Hdef); 1H NMR (400 MHz, CDCl3): δ 3.93 (s, 6H, 3', 3" , OCH3), 5.79 (s, 2H, 4', 4" , OH), 7.58 (d, 2H, J = 15.76 Hz, H-1, 7), 6.47 (d, 2H, J = 15.76 Hz, H-50, 500), 6.92 (d, 2H, J = 8.61 Hz, H-20, 200), 7.19 (m, 5H, C6H5), 5.39 (d, 1H, J = 10.44 Hz, CH), 7.41 (s, 1H, NH), 8.04 (1H, NH); 13C NMR (400 MHz, DMSO-d6): δ 48.10, 56.02, 115.46, 112.31, 115.46, 124.06, 126.17, 128.68, 143.22, 144.64, 149.03, 151.46, 152.70, 192.11; ESI-MS: m/z (M+H)+ 498.
2.2. Evaluation of antioxidant activity 2.2.1. CUPRAC assayCUPRAC (Cupric reducing antioxidant capacity) assay was performed according to the literature [28] with slight modification. The CUPRAC method is comprised of mixing the antioxidant solution directly with a copper (Ⅱ) chloride solution, a neocuproine (2, 9-dimethyl-1, 10-phenanthroline) alcoholic solution, and an ammonium acetate aqueous buffer at pH 7. The subsequent CUPRAC assay was performed by adding 1 mL CuCl2, 1 mL Nc solution, and 1 mL NH4Ac solution to the 2 mL test solution, followed by 1 mL water. The absorbance of the final solution was measured at 450 nm against a reagent blank after 30 min standing at room temperature.
2.2.2. FRAP assayFRAP (Ferric Reducing Antioxidant Power) assay was performed as reported in the literature [29] with slight modification. In this method 1 mL sample was added to 2 mL freshly prepared FRAP reagent which was prepared by mixing 300 μmol/L acetate buffer of pH 3.6, 10 mL HCl containing TPTZ (2, 4, 6-tripyridyl-5-triazine) and 20 μmol/L ferric chloride solution in the ratio of 10:1:1 (v/v/v). The mixture was incubated at 37 ℃ for 10 min. The absorbance was measured at 593 mm by Chemito Spectrascan 2600 Spectrophotometer.
2.2.3. DRSA assayThe DRSA (DPPH radical scavenging activity) of the synthesized CDHPMs was carried out as described by Bozin et al. [30] with minor modifications. In this study 90 μmol/L DPPH solution was prepared and 950 μL was pipetted out and added to 50 μL of the samples, (20 mg/mL concentration) and the final volume was adjusted to 5 mL with methanol. The mixtures were shaken vigorously and then incubated at room temperature for 30 min. The color of the mixture changes by scavenging of the free radicals, which was measured at 517 nm by Chemito Spectrascan 2600 Spectrophotometer. The scavenging capacity of the samples was measured by comparison of sample color with the control. The percentage of inhibition can be calculated using the formula:
DPPH radical scavenging activity (%)
where Ac = absorbance of control; As = absorbance of sample.
2.3. Evaluation of anti-inflammatory activitySynthesized compounds were screened for their anti-inflammatory activity using carrageenan-induced method as reported in the literature [31]. In this method, a total number of 20 mice were weighed and divided in four groups (five mice per cage) and were fasted for 2 h before the experiment. One of the groups acted as the negative control (normal saline solution was injected peritoneally), the second group was administered with positive control (Diclofenac 100 mg/kg, i.p.), the third group was administered with curcumin (200 mg/kg, i.p.), and the fourth group received 200 mg/ kg, body weight of CDHPMs. After 1 h, a freshly prepared 0.1 mL of 1% suspension of carrageenan in saline solution was injected into the sub planter region of the right hind paws of the mice to all the four groups to induce acute inflammation. The paw volumes were measured using a plethysmometer (UGO Basile, 7140 Italy) at 2 h, 3 h, and 4 h after carrageenan injection. Thus, % inhibition was calculated using the following formula:
where Vc = edema volume of control; Vt = edema volume of test.
3. Results and discussion 3.1. ChemistryFor the preparation of all compounds described in this paper, curcumin was used as the starting material (Scheme 1). Curcumin 3, 4-dihydropyrimidinones/thiones/imines were obtained by reacting curcumin with urea/thiourea/guanidine and substituted aromatic aldehydes under microwave irradiation using chitosamine hydrochloride as a non-toxic acid catalyst through an improved procedure. In the initial experiments, in order to evaluate the catalytic efficiency of chitosamine hydrochloride catalyst in the three component reaction of curcumin, benzaldehyde and urea was selected as the model reaction. It showed that only 20% of product could be obtained when a mixture of curcumin, substituted aromatic aldehyde, and urea/thiourea/ guanidine was reacted at 60 ℃ for 3 h in the absence of catalyst, which indicated that the catalyst should be necessary for this transformation. The effect of amount of catalyst on the yield and rate was also investigated. It was found that the use of 0.08 g catalyst was sufficient to promote the reaction. Lower amounts gave a low yield even after long reaction time, and higher amounts did not improve the efficiency of this transformation. Analytical and physicochemical data of synthesized CDHPMs using chitosamine hydrochloride have been given in Table 1.
|
Download:
|
| Figure 1. Synthesis of 3, 4-dihydropyrimidinones/thiones/imines under microwave irradiations. | |
|
|
Table 1 Analytical and physicochemical data of synthesized CDHPMs using chitosamine hydrochloride catalyst.a |
After optimization of the conditions, in order to investigate the scope of this approach, we carried out the three component cyclocondensation reaction of curcumin and urea/thiourea with a series of aldehydes under similar conditions. The reactions were completed after 10-16 min, affording corresponding CDHPMs in good to excellent yields.
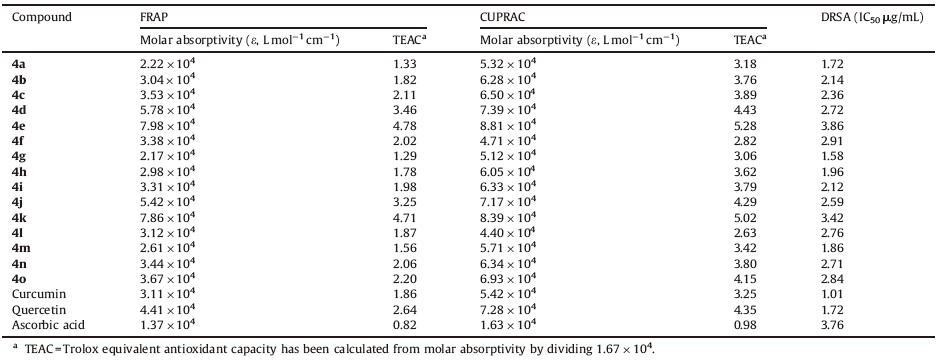
3.2. Antioxidant activityAll the synthesized curcumin 3, 4-dihydropyrimidinones/ thiones/imines (4a-o) were screened for antioxidant activity using FRAP, CUPRAC and DRSA assays. DPPH is a stable free radical compound and has been widely used to test the radical scavenging activity of various chemicals, including natural products and synthesized compounds. The TEAC (trolox equivalent antioxidant capacity) is defined as the millimolar concentration of a trolox solution having the antioxidant capacity equivalent to a 1.0 nmol/L solution of the substance under investigation. The TEAC values were simply calculated by dividing the molar absorptivity (ε) of the species under investigation by that of trolox under corresponding conditions (e.g. the ε value of trolox solution is 1.67 - 104 L moL-1 cm-1). The TEAC values in Table 2 were found from the absorbances of solutions allowed to stand for 30 min at room temperature after reagent addition.
|
|
Table 2 The trolox equivalent antioxidant capacity and DRSA assay correlation diagram of CDHPMs. |
The antioxidant activity data (Table 2) of the FRAP method showed that the compounds 4c-f, 4i-o have more antioxidant activity than curcumin (standard), all the compounds 4a-o showed more antioxidant activity than ascorbic acid (positive control), and the compounds 4d, 4e, 4j and 4k showed activity more than quercetin (positive control). The data obtained in the CUPRAC method showed that all the compounds 4a-o have greater antioxidant capacity than ascorbic acid (positive control), the compounds 4b-e, 4h-k, 4m-o have greater antioxidant capacity than curcumin (standard), and compounds 4d, 4e, and 4k showed greater antioxidant activity than quercetin (positive control). It is very interesting to note that DRSA activity of all the synthesized compounds 4a-o is greater than curcumin (standard), the compounds 4b-f, 4i-o showed greater antioxidant activity than quercetin (positive control), and the compound 4e showed greater antioxidant activity thanascorbic acid. The results of the antioxidant activity data are in accordance with theoretical expectations, because the number and position of the hydroxyl groups as well as the degree of conjugation of the whole molecule are important. The antioxidant potency of flavonoids of similar conjugation level is roughly proportional to the total number of-OH groups and is positively affected by the presence of o-dihydroxy moiety in the benzene ring [28]. In otherwords, antioxidant activitywas greater in guanidine derivatives and minimal in urea derivatives.
3.3. Anti-inflammatory activityThe in vivo anti-inflammatory activity of the curcumin 3, 4-dihydropyrimidinones 4a-o at a dose of 100, 200, and 300 mg/kg using carrageenan-induced paw edema method has been evaluated [29]. The results of Table 3 showed that the compounds 4c-4o exhibited greater anti-inflammatory activity than curcumin, whereas the compounds 4a and 4b showed lesser activity than curcumin after 2 h. Compounds 4c-4f and 4h-4o exhibited greater anti-inflammatory activity than curcumin, and compounds 4a, 4b, and 4g showed lesser activity than curcumin after 3 h. Compounds 4e, 4i-o exhibited greater activity than curcumin, whereas compounds 4a-d, 4f-h exhibited lower activity than curcumin after 4 h. Hence from the data given in Table 3 we can say that compounds 4e, 4i-o exhibited greater anti-inflammatory activity than curcumin after 2, 3 and 4 h. From the data it is clear that the compounds 4e, 4i-o are more effective than curcumin in inhibiting the synthesis of prostaglandin, which is responsible for inflammation.
|
|
Table 3 Anti-inflammatory activity of CDHPMs. |
3.4. Structure activity relationship (SAR)
For all the pharmacological activity viz. antioxidant and antiinflammatory activity, structure activity relationship for the synthesized curcumin 3, 4-dihydropyrimidinones has been suggested. From SAR it was concluded that anti-inflammatory activity was greatest in the compounds derived from salicylaldehyde and vanillin, and the remaining compounds showed moderate antiinflammatory activity. Antioxidant activity was greatest in the compound derived from 2, 4-dihydroxy benzaldehyde and urea. When urea was replaced by thiourea in the same compound, antioxidant activity slightly decreases. From this, we conclude that antioxidant activity decreases when a hydroxyl group departs from the aldehyde group.
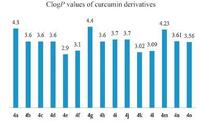
3.5. Rational for molecular designTheoretical physico-chemical parameter Clog P values were calculated using commercially available ChemDraw Ultra 8.0.3 for all synthesized curcumin derivatives (4a-o) and ranged from 2.95 to 4.41. As a general guideline [32], Clog P values must be lower than '5' to by-pass the cell barrier. The Clog P values seem to correlate to some extent with lipophilicity. All of the compounds in the designed series showed Clog P values less than 5 (Fig. 1).

|
Download:
|
| Figure 1. Showing Clog P values of synthesized curcumin derivatives. | |
3.6. Mechanism of antioxidant activity
An odd electron present on the nitrogen atom of DPPH radical is responsible for the absorbance at 515-517 nmand also for a visible deep purple color. When it accepts an electron donated by an antioxidant compound, the DPPH decolorizes, which can be quantitatively measured from the changes in absorbance (Scheme 2). The reversibility of the reaction is evaluated by adding DPPH-H at the end of the reaction. If there is an increase in the percentage of remaining DPPH± at the plateau, the reaction is reversible, otherwise it is a complete reaction (Scheme 3).

|
Download:
|
| Figure 2. Mechanism of addition and elimination of electron in DPPH molecule. | |

|
Download:
|
| Figure 3. Mechanism of DPPH assay of CDHPMs. | |
The DPPH assay measures the ability of the sample to donate hydrogen to the DPPH radical, resulting in bleaching of the DPPH solution. The greater the bleaching action, the higher the antioxidant activity, and this was reflected in a lower IC50 value. FRAP on the other hand measures the ability of the extract to donate an electron to Fe (Ⅲ). The higher the FRAP value, the greater the antioxidant activity. It is interesting to note that for the range of compounds considered here, there is a linear correlation between 1/IC50 and FRAP. This suggests that the lower the IC50 value, the higher the ferric reducing antioxidant power.
4. ConclusionIn conclusion, the resulting curcumin 3, 4-dihydropyrimidinones/ thiones/imines (4a-o) were evaluated for their antioxidant, anti-inflammatory, and analgesic activity and compared with curcumin. Curcumin 3, 4-dihydropyrimidinones/thiones/imines derived from 2-hydroxybenzaldehyde and 4-hydroxy-3-methoxy- benzaldehyde showed more anti-inflammatory activity than curcumin. The compound derived from 2, 4-dihydroxy benzaldehyde and urea showed maximum antioxidant activity, while antioxidant activity decreases in compounds derived from 2- hydroxybenzaldehyde, 3-hydroxybenzaldehyde, and 4-hydroxybenzaldehyde.
AcknowledgmentsThe authors are indebted to the Madhya Pradesh Council of Science and Technology, Bhopal, India; for financial support (No. 4468/CST/R&D/2010) and the Director, Sophisticated Analytical Instrumental Facility, Punjab University, Chandigarh, in carrying out the spectral analysis of the synthesized compounds.
| [1] | J.K. Lin, M.H. Pan, S.Y. Lin-Shiau. Recent studies on the biofunctions and biotransformations of curcumin. Biofactor 13 (2000) 153–158. DOI:10.1002/biof.v13:1/4 |
| [2] | K.S. Parvathy, P.S. Negi, P. Srinivas. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 115 (2009) 265–271. DOI:10.1016/j.foodchem.2008.12.036 |
| [3] | A. Apisariyakul, N. Vanittnakom, D. Buddasukh. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). J. Ethnopharmacol. 49 (1995) 163–169. DOI:10.1016/0378-8741(95)01320-2 |
| [4] | M. Hergenhahn, U. Soto, A. Weninger, et al. , The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells. Mol. Carcinogen. 33 (2002) 137–145. DOI:10.1002/mc.v33:3 |
| [5] | A. Mazumdar, K. Raghavan, J. Weinstein, K.W. Kohn, Y. Pommer. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 49 (1995) 1165–1170. DOI:10.1016/0006-2952(95)98514-A |
| [6] | P. Narlawar, M. Pickhardt, S. Leuchtenberger, et al. , Curcumin-derived pyrazoles and isoxazoles: Swiss army knives or blunt tools for Alzheimer's Disease. Chem. Med. Chem. 3 (2008) 165–172. DOI:10.1002/(ISSN)1860-7187 |
| [7] | M.S. Wang, S. Boddapati, S. Emadi, M.R. Sierks. Curcumin reduces α-synuclein induced cytotoxicity in Parkinson's disease cell model. BMC Neurosci. 11 (2010) 57–66. DOI:10.1186/1471-2202-11-57 |
| [8] | J.L. Funk, J.N. Oyarzo, J.B. Frye, et al. , Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 69 (2006) 351–355. DOI:10.1021/np050327j |
| [9] | M.E.M. Braga, P.F. Leal, J.E. Carvalho, M.A.A. Meireles. Comparison of yield, composition, and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J. Agric. Food Chem. 51 (2003) 6604–6611. DOI:10.1021/jf0345550 |
| [10] | J.L. Arbiser, Curcumin and curcuminoid inhibition of angiogenesis, US Patent 667, 3843 (2004). |
| [11] | M. Kuroda, Y. Mimaki, T. Nishiyama, et al. , Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol. Pharm. Bull. 28 (2005) 937–939. DOI:10.1248/bpb.28.937 |
| [12] | C.L.L. Saw, Y. Huang, A.N. Kong. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 79 (2010) 421–430. DOI:10.1016/j.bcp.2009.08.030 |
| [13] | S. Mishra, K. Karmodiya, N. Surolia, A. Surolia. Synthesis and exploration of novel curcumin analogues, as anti-malarial agents. Bioorg. Med. Chem. 16 (2008) 2894–2902. DOI:10.1016/j.bmc.2007.12.054 |
| [14] | N. Arun, N. Nalini. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rat. Plant Foods Hum. Nutr. 57 (2002) 41–52. DOI:10.1023/A:1013106527829 |
| [15] | C. Changtam, H.P. De-Koning, H. Ibrahim, et al. , Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur. J. Med. Chem. 45 (2010) 941–956. DOI:10.1016/j.ejmech.2009.11.035 |
| [16] | A.B. Hegge, T. Andersen, J.E. Melvik, et al. , Formulation and bacterial phototoxicity of curcumin loaded alginate foams for wound treatment applications: studies on curcumin and curcuminoides XLⅡ. J. Pharm. Sci. 100 (2011) 174–185. DOI:10.1002/jps.22263 |
| [17] | A. Valentini, F. Conforti, A. Crispini, et al. Synthesis, oxidant properties, and antitumoral effects of a heteroleptic palladium(Ⅱ) complex of curcumin on human prostate cancer cells. J. Med. Chem. 52 (2009) 484–491. DOI:10.1021/jm801276a |
| [18] | S.K. Kulkarni, M.K. Bhutani, M. Bishnol. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology 201 (2008) 435–442. DOI:10.1007/s00213-008-1300-y |
| [19] | M.M. Kim, W. Jeong, J. Kang, Y. Chong. Significant enhancement in radicalscavenging activity of curcuminoids conferred by acetoxy substituent at the central methylene carbon. Bioorg. Med. Chem. 19 (2011) 3793–3800. DOI:10.1016/j.bmc.2011.04.055 |
| [20] | A. Gomes, R. Das, S. Sarkhel, et al. , Herbs and herbal constituents active against snake bite. Ind. J. Exp. Biol. 48 (2010) 865–878. |
| [21] | D. Simoni, M. Rizzi, R. Rondanin, et al. , Antitumor effects of curcumin and structurally beta-diketone modified analogs on multidrug resistant cancer cells. Bioorg. Med. Chem. Lett. 18 (2008) 845–849. DOI:10.1016/j.bmcl.2007.11.021 |
| [22] | T.P. Chaturvedi. Uses of turmeric in dentistry: an update. Ind. J. Dental. Res. 20 (2001) 107–109. |
| [23] | L.R.C. Barclay, R. Melinda, M.R. Vinqvist. On the Antioxidant mechanism of curcumin: classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 18 (2000) 2841–2843. |
| [24] | S.V. Jovanovic, C.W. Boone, S. Steenken, M. Trinoga, R.B. Kaskey. How curcumin works preferentially with water soluble antioxidants. J. Am. Chem. Soc. 123 (2001) 3064–3068. DOI:10.1021/ja003823x |
| [25] | G. Litwinienko, K.U. Ingold. Abnormal solvent effects on hydrogen atom abstraction., 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 69 (2004) 5888–5896. DOI:10.1021/jo049254j |
| [26] | A. Goel, A.B. Kunnumakkara, B.B. Aggarwal. Curcumin as “curecumin”: from kitchen to clinic. Biochem. Pharmacol. 75 (2008) 787–809. DOI:10.1016/j.bcp.2007.08.016 |
| [27] | G. Aridoss, Y.T. Jeong. A convenient one-pot Biginelli reaction catalyzed by Y(OAc)3: an improved protocol for the synthesis of, 3, 4-dihydropyrimidin-2(1H)-ones and their sulfur analogues. Bull. Korean Chem. Soc. 31 (2010) 863–868. DOI:10.5012/bkcs.2010.31.04.863 |
| [28] | R. Apak, K. Guclu, M. Ozyurek, S.E. Karademir. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 52 (2004) 7970–7981. DOI:10.1021/jf048741x |
| [29] | I.F. Benzie, J.J. Strain. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239 (1993) 70–76. |
| [30] | B. Bozin, N. Mimica-Dukic, N. Simin, G. Anackov. Characterization of the volatile composition of essential oils of some Lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 54 (2006) 1822–1828. DOI:10.1021/jf051922u |
| [31] | C.A. Winter, E.A. Risley, G.W. Nuss. Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 111 (1962) 545–547. |
| [32] | J. Lal, S.K. Gupta, D. Thavaselvam, D .D. Agarwal, Design, synthesis, synergistic antimicrobial activity and cytotoxicity of 4-aryl substituted 3, 4-dihydropyrimidinones of curcumin. Bioorg. Med. Chem. Lett. 22 (2012) 2872–2876. DOI:10.1016/j.bmcl.2012.02.056 |
 2016, Vol. 27
2016, Vol. 27