b Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China ;
c College of Life Science, Sichuan University, Chengdu 610064, China
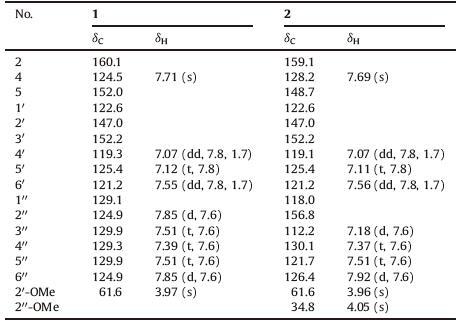
Gymnotheca chinensis Decne, as one of the endemic genera of seed plant in China, is a perennial herb of the family Saururaceae. The whole plants of G. chinensis have long been used as a traditional herbal medicine to treat contusions and strains [1]. In a previous investigation on G. chinensis we had isolated a great amount of new lignans belonging to the dibenzocyclooctene, eupomatilone, eupodienone and norlignan series [2-7]. In our continuing endeavor to discover unique and new structures from this Chinese endemic plant, two novel 2, 5-diphenyl oxazole derivatives, 2-(3'- hydroxy-2'-methoxybenzene-1'-yl)-5-phenyl oxazole (1) and 2- (3'-hydroxy-2'-methoxybenzene-1'-yl)-5-(2"-methoxybenzene- 1"-yl) oxazole (2) (Fig. 1), named gymnotheoxazoles A and B, were isolated from the whole plants of G. chinensis. This kind of alkaloid was found for the first time from this endemic genus. 2, 5-Diphenyl oxazole (DPO) was a class of substances exhibiting radioprotective and radiosensitizing effects [8, 9]. This type of compound is very rare in the natural world. Owing to their attractive structures, there have been total syntheses of some of the members of DPO [10-12]. Herein, we describe the isolation and structure elucidation of gymnotheoxazoles A (1) and B (2), and their cytotoxicity against A549 cell.
|
Download:
|
| Figure 1. The structures of compounds 1 and 2. | |
2. Experimental 2.1. General experimental procedures
UV spectra were recorded on a Perkin-Elmer Lambda 35 UV-VIS spectrophotometer (Perkin-Elmer, Waltham, USA). IR spectra were measured on a Perkin-Elmer one FT-IR spectrometer (KBr) (Perkin- Elmer, Waltham, USA). 1D and 2D-NMR spectra were recorded on a Bruker-AVANCE Ⅲ-400 instrument using TMS as the internal reference (Bruker, Karlsruhe, Germany). HR-ESI-MS spectra were measured on a MicrO TOF-Q Ⅱ mass spectrometer (Bruker, Karlsruhe, Germany). Column chromatography (CC) was performed using 3"-400 mesh silica gel (Qingdao Marine Chemical Inc., Qingdao, China), 75-150 mm MCI CHP-20 gel (Mitsubishi, Kyoto, Japan). Semi-preparative HPLC was performed on LC3000 system (Beijing Chuang Xing Tong Heng Science And Technology Co., Ltd., Beijing, China) equipped with ODS column (5 mm, i.d. 10 - 250 mm, Kromasil) (Akzo Nobel, Amsterdam, Netherlands). 2.2. Plant material
The plant G. chinensis was collected from Jinfoshan in Chongqing city, China, in October 2012, and identified as G. chinensis by Prof. Si-Rong Yi (Chongqing Institute of Pharmaceutical Plant). A voucher specimen (No. 20130125) was deposited at the herbarium of the Chengdu Institute of Biology, Chinese Academy of Sciences.
2.3. Extraction and isolationDried and powdered whole plant of G. chinensis (1.45 kg) were extracted with MeOH at r.t. to give an extract (152 g), which was suspended in H2O (1 L) and extracted with petroleum ether and AcOEt (3×1 L, 3 h each) successively. The petroleum ether extract (8 g) and AcOEt extract (19 g) were combined and then subjected to CC over MCI gel (85 - 100 mm, MeOH/H2O, 90:10). In total, two fractions (A and B) were obtained based on TLC analysis. Fraction A (15 g) was further separated by medium pressure CC (SiO2 (49 - 460 mm), petroleum ether/acetone 100:1→1:1) to give twelve fractions. Fraction 8 was subjected to fractionation by CC (ODS (40-63 µm, 36×310 mm), MeOH/H2O 60:40→100:0) to furnish four main fractions, 8A-8D. Compound 1 (3 mg, tR = 15.1 min) was obtained from fraction ℃ by semi-preparative HPLC (MeCN/H2O 55:45, flow rate 4.5 mL/min). Compound 2 (4 mg, tR = 16.4 min) was isolated from fraction 8D by semipreparative HPLC (MeCN/H2O 56:44, flow rate 4.5 mL/min).
Gymnotheoxazole A (1): White crystal (MeOH); UV (MeOH) λmax (log ε) nm 236 (2.36), 303 (5.21); IR (KBr, cm-1): vmax 3675, 1539, 1433, 1282, 1230, 885; 1H NMR and 13C NMR data, see Table 1; HRESIMS m/z 268.0967 [M+H]+ (calcd. for C16H14NO3+, 268.0968), 290.0779 [M+Na]+ (calcd. for C16H13NO3Na+, 290.0788).
|
|
Table 1 1H (400 MHz) and 13C (100MHz) NMR data of compounds 1 and 2 in acetone-d6 (δ in ppm, J in Hz). |
Gymnotheoxazole B (2): White powder (MeOH); UV (MeOH) lmax (log e) nm 235 (2.58), 316 (5.20); IR (KBr, cm-1): λmax 3400, 1571, 1493, 1462, 1250, 1023, 749; 1H NMR and 13C NMR data, see Table 1; HRESIMS m/z 298.1071 [M+H]+ (calcd. for C17H16NO4+, 298.1074), 320.0898 [M+Na]+ (calcd. for C17H15NO4Na+, 320.0893).
Crystal data for 1: C16H13NO3, M = 267.27, monoclinic, a = 9.717 (4) Å , b = 13.558 (6) Å , c = 9.822 (4) Å , α = 90.008, β = 91.040 (9) 8, γ = 90.008, V = 1293.8 (10) Å3, T = 153 (2) K, space group P21, Z = 4, μ(MoKa) = 0.71073 mm-1, 3930 reflections measured, 2229 independent reflections (Rint = 0.0516). The final R1 values were 0.0454 (I > 2σ(I)). The final wR(F2) values were 0.0993 (I > 2σ(I)). The final R1 values were 0.0734 (all data). The final wR(F2) values were 0.1120 (all data). The goodness of fit on F2 was 0.999.
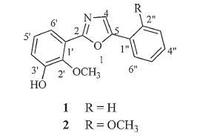
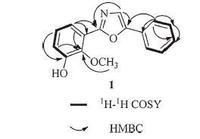
3. Results and discussionCompound 1 was obtained as a white crystal. Its molecular formula, C16H13NO3 which indicated 11 degrees of unsaturation, was established from the quasi-molecular ion peak at m/z 290.0779 [M+Na]+ (calcd. for C16H13NO3Na+: 290.0788) in the HRESIMS. Its IR spectrum exhibited strong absorption bands at 3324 cm-1 due to hydroxyl functional group. The 1H NMR, 13C NMR andHSQCspectra of 1 showed 16 carbon resonances due to a monosubstituted benzene ring [δH 7.85 (d, 2H, J = 7.6 Hz), 7.51 (t, 2H, J = 7.6 Hz), 7.39 (t, 1H, J = 7.6 Hz); δC 129.9, 129.9, 124.9, 124.9, 129.3, 129.1], a 1, 2, 3-trisubstituted phenyl group [δH 7.55 (dd, 1H, J = 7.8, 1.7 Hz), 7.12 (t, 1H, J = 7.8 Hz), 7.07 (dd, 1H, J = 7.8, 1.7 Hz); δC 152.2, 147.0, 125.4, 122.6, 121.2, 119.3], a oxazole moiety [δH 7.71 (1H, s); δC 160.1, 152.0, 124.5], and a methoxyl group [δH 3.97 (3H, s); δC 61.6]. The HMBC correlations (Fig. 2) of OCH3 (δH 3.97) to C-2' (δC 147.0), H-6' to C-2' and H-4' to C-2', indicated that the methoxyl located at C-2'. The HMBC correlations of H-6' to C-1', C-2' and C-2 confirmedthat the 1, 2, 3-trisubstituted phenyl group located at C-2; the HMBC correlations of H-2" to C-1" and C-5 revealed that the mono-substituted benzene ring was attached to C-5. A single crystal of 1 was obtained from MeOH, and X-ray crystallographic analysis with molybdenum radiation was successfully performed (CCDC 996979), which unambiguously determined the complete structure of 1 as deduced (Fig. 3). In summary, the structure of compound 1 was determined to be 2-(3'-hydroxy-2'-methoxybenzene- 1'-yl)-5-phenyl oxazole, named gymnotheoxazole A.
|
Download:
|
| Figure 2. Key 1H–1H COSY and HMBC correlations of compound 1. | |
|
Download:
|
| Figure 3. Crystal structure of compound 1. | |
Compound 2 was obtained as white powder. The molecular formula was determined to be C17H15NO4 by analysis of the HRESIMS ion peak at m/z 320.0898 [M+Na]+ (calcd. for C17H15NO4Na+, 320.0893), indicating 11 degrees of unsaturation. The 1H, 13C NMR and HSQC spectra of 2 showed the presence of a 1, 2-disubstituted benzene ring [δH 7.92 (d, 1H, J = 7.6 Hz), 7.51 (t, 1H, J = 7.6 Hz), 7.37 (t, 1H, J = 7.6 Hz), 7.18 (d, 1H, J = 7.6 Hz); δC 156.8, 130.1, 126.4, 121.7, 118.0, 112.2], a 1, 2, 3-trisubstituted phenyl group [δH 7.56 (dd, 1H, J = 7.8, 1.7 Hz), 7.11 (t, 1H, J = 7.8 Hz), 7.07 (dd, 1H, J = 7.8, 1.7 Hz); δC 152.2, 147.0, 125.4, 122.6, 121.2, 119.1], a oxazole moiety [δH 7.69 (s, 1H); δC 159.1, 148.7, 128.2], two methoxyl groups [δH 4.05, 3.96 (each 3H, s); δC 61.6, 55.9]. Detailed HMBC analysis suggested that the structure of 2 was very similar to those of 1 except for the replacement of the mono-substituted benzene ring by a 1, 2-disubstituted benzene ring at C-5. The HMBC correlations of OCH3-2' to C-2', H-3' to C-2' and C-1', H-60 to C-2' and C-1', confirmed that the methoxyl group located at C-2'. Compound 2 was then assigned as 2-(3'-hydroxy- 2'-methoxybenzene-1'-yl)-5-(2"-methoxybenzene-1"-yl) oxazole, named gymnotheoxazole B.
In the MTT assay, compounds 1 and 2 were evaluated in vitro for the cytotoxic activity against A549 cell line, with the IC50 values above 50 μmol/L.
4. ConclusionTwo novel 2, 5-diphenyl oxazole derivatives were isolated from the endemic medicinal plant of G. chinensis. The results of the cell viability assay indicated that these novel compounds showed no detectable cytotoxicity against the A549 cell.
AcknowledgmentThis work was financially supported by grants from the National Natural Sciences Foundation (Nos. 21572219 and 21302180) of China. We are grateful to Prof. Sirong Yi for the collection and identification of the plant. We also appreciate Prof. Kaibai Yu for the sing-crystal X-ray experiments.
| [1] | Y.Q. Cheng, Flora Republicae Popularis Sinicae, (Zhongguo Zhiwu Zhi), vol. 20 (1), Science Press, Beijing, 1982, p. 9. |
| [2] | D.H. He, L.S. Ding, H.X. Xu, et al. , Gymnothelignans A-O: conformation and absolute configuration analyses of lignans bearing tetrahydrofuran from Gymnotheca chinensis. J. Org. Chem. 77 (2012) 8435–8443. DOI:10.1021/jo301225v |
| [3] | S.J. Xiao, X.X. Lei, B. Xia, et al. , Two novel norlignans from Gymnotheca chinensis. Tetrahedron Lett. 55 (2014) 2869–2871. DOI:10.1016/j.tetlet.2014.03.091 |
| [4] | S.J. Xiao, F. Chen, L.S. Ding, et al. , Two new eupodienone lignans from Gymnotheca chinensis. Chin. Chem. Lett. 25 (2014) 463–464. DOI:10.1016/j.cclet.2013.11.053 |
| [5] | S.J. Xiao, Y.Z. Lao, F. Chen, et al. , Two new lignans from Gymnotheca chinensis. Phytochem. Lett. 8 (2014) 38–39. DOI:10.1016/j.phytol.2014.01.007 |
| [6] | S.J. Xiao, D.M. Fang, B. Xia, et al. Biphenyl lignans with a tetrahydrofuran moiety from Gymnotheca chinensis Decne. Chin. J. Org. Chem 34 (2014) 1677–1681. DOI:10.6023/cjoc201403017 |
| [7] | S.J. Xiao, D.H. He, D.M. Fang, et al. , Further biphenyl lignans with a tetrahydrofuran moiety from Gymnotheca chinensis. Helv. Chim. Acta 97 (2014) 499–550. DOI:10.1002/hlca.v97.4 |
| [8] | L.N. Shishkina, M.A. Smotriaeva, M.V. Kozlov, et al. , Modifying properties of, 2, 5-diphenyloxazole during low-dose X-ray exposure of mice. Radiat. Biol. Radioecol. 45 (2004) 610–615. |
| [9] | J.T. Ahokas, C. Davies, N. Jacobsen, et al. , The metabolism of, 2, 5-diphenyloxazole (PPO) in human lymphocytes and rat liver microsomes. Pharmacol. Toxicol. 61 (1987) 184–190. DOI:10.1111/bcpt.1987.61.issue-3 |
| [10] | O.V. Prezhdo, I.V. Lysova, V.B. Distanov, et al., Synthesis and scintillating efficiencies of 2, 5-diarylthiazoles with intramolecular hydrogen bond, Tetrahedron Lett. 45 (2004) 5291-5294. |
| [11] | C.F. Wan, L.F. Gao, Q. Wang, et al. , Simple and efficient preparation of, 2, 5-disubstituted oxazoles via a metal-free-catalyzed cascade cyclization. Org. Lett. 12 (2010) 3902–3905. DOI:10.1021/ol101596s |
| [12] | S.Z. Yuan, Z. Li, L. Xu, et al. , Convenient one-pot synthesis of, 2, 5-disubstituted oxazoles via a catalytic oxidative dehydrogenation of F3CSO3H·SiO2-DDQ/CuCl2/LiCl. J. Heterocycl. Chem. 50 (2013) 1405–1409. DOI:10.1002/jhet.v50.6 |
 2016, Vol. 27
2016, Vol. 27