b Department of Medicinal Chemistry, Beijing Key Laboratory of Active Substances Discovery and Drugability Evaluation, Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100050, China
Rearrangement is one of the most useful reactions applied in organic synthesis, and it can be used to produce structures that are not easily accessed by conventional transformations. Most of the rearrangements are 1, 2-rearrangement that are exemplified by Hofmann rearrangement [1, 2], Curtius reaction [3, 4], Beckmann reaction [5, 6], Pinacol rearrangement [7, 8], Favorskii rearrangement [9, 10] etc. In contrast, 1, 3-carbon and longer rearrangements are relatively rare. Herein, we disclose an unexpected 1, 3-carbon rearrangement with an unusual dearomatization on the quinoline ring that was observed in one reaction.
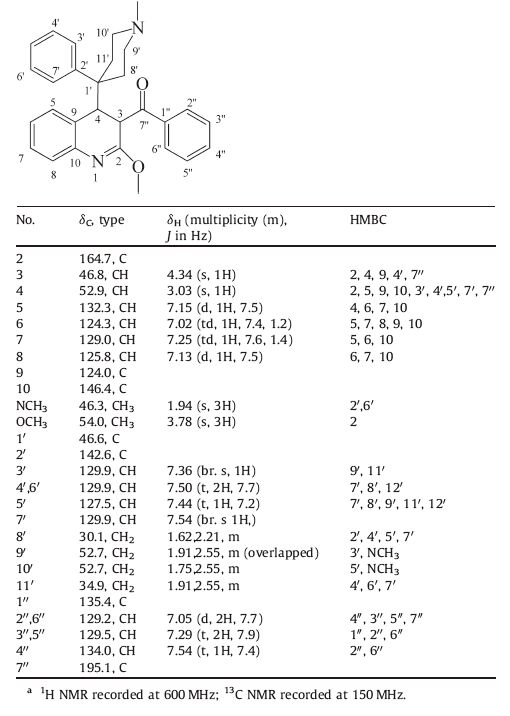
Bedaquiline (TMC-207) , a diarylquinoline (DARQ) derivative [11, 12], was the first one in this class launched for the treatment of tuberculosis [13]. In searching for new anti-tuberculosis drugs [14], we have designed conformationally restricted diarylquinoline analogues to study if a rigid structural motif has impact on antitubercular activity of the molecules. Compound 1 was one of our designed molecules for this study (Fig. 1), and we proposed it could be quickly accessed from 2-methoxyquinolino-3-lithium (2) and N-methyl-4-phenyl-4-benzoylpiperidine (3) (Scheme 1).

|
Download:
|
| Scheme. 1. Nucleophilic addition of 2 to 1-methyl-4-phenyl-4-benzoylpiperidine 3. | |
|
Download:
|
| Figure 1. Structure of TMC-207 and proposed structure 1. | |
2. Experimental
The 1H NMR, 13C NMR, HMBC, HMQC, H-H COSY, DEPT were recorded on a Mercury-300, Mercury-400 or Bruker-AV600 spectrometer in acetone-d6. Chemical shifts are reported as d values using tetramethylsilane (TMS) as the internal standard. HR- ESI-MS data were measured on a Micromas AutoSpec Ultima-TOF spectrometer.
The starting material, 3-bromo-2-methoxyquinoline was prepared using 3-bromo-2-chloroquinoline [15] with sodium methylate in methanol.
2-Methoxy-4-(1-methyl-4-phenylpiperidin-4-yl)-3, 4-dihydroquinolin- 3-yl)(phenyl)methanone (4) : To a solution of n-butyllithium (2.5 mol/L in hexane; 0.30 mL, 0.75 mmol) (1.4 equiv.) in anhydrous tetrahydrofuran (2 mL) at -70 ℃ was added 3-bromo- 2-methoxyquinoline (170 mg, 0.71 mmol, in 2 mL anhydrous tetrahydrofuran) (1.3 equiv.) dropwise via syringe under an atmosphere of N2. After stirring for 1 h, a solution of ketone (0.54 mmol) (1.0 equiv.) in anhydrous tetrahydrofuran (2 mL) was added, the stirring continued at -70 ℃ for 30 min followed by another 30 min of stirring at the room temperature. The reaction was quenched by 10 mL of water, extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by flash column chromatography to afford the corresponding product.
Synthesis of compound 6, 8, 10 and the physical and spectroscopic characterization data of all target compounds were given in Supporting information.
3. Results and discussionWhen the reaction (Scheme 1) was carried out, only product 4 was isolated in a yield of 78.6%. The data of 13C NMR indicated the existence of a carbonyl group (δC 195.1) , which is supposed to be reduced in the reaction (Scheme 1). The ESI-HRMS of 4 ([M + H]+: 439.2380 calcd. for C29H31O2N2: 439.2379) indicated that 4 should be an isomer of proposed compound 1. Unfortunately, we failed to grow a single crystal suitable for X-ray diffraction.
Interestingly, there are two notable extra methines at δH 4.34 (s, 1H) and 3.03 (s, 1H) and the corresponding carbon that the attached to appeared at dc 46.8 (CH) and dc 52.9 (CH) in the NMR of 4. Notably, both methines are singlet, and they have cross peaks in the 1H-1H COSY. This inicated they are two vicinal CHs. Both protons have long range correlations with the carbon at δC 164.7, which also has correlation with O-methyl at δC 54.0. Therefore, it is hypothesized that the quinoline ring was partly reduced to a 3, 4- disubstituted 3, 4-dihydroquinoline in the reaction.
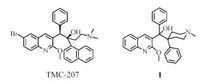
The correlations of H-80 and H-110 of piperidine to C-10 (δC 46.6) , C-20 (δC 142.6) in the HMBC spectrum supported the presence of the substructure A (Fig. 2). The correlations of H-200, 600 to the carbonyl C-700 (δC 195.1) confirmed the presence of the benzoyl B (Fig. 2).
|
Download:
|
| Figure 2. COSY, key HMBC correlations of compound 4. | |
The HMBC correlations of H-3 (δH 4.34) to C-2 (δC 164.7) , C-4 (δC 52.9) , C-9 (δC 124.0) and H-4 (δH 3.03) to C-2 (δC 164.7) , C-5 (δC 132.3) , C-9 (δC 124.0) , C-10 (δC 146.4) and OCH3 (δH 3.78, s) to C-2 (δC 164.7) supported the presence of 2-methoxy-3, 4-disubstituted 3, 4-dihydroquinoline skeleton C. In addition, the cross-peaks of H- 4 to C-10, 80, 110, 20, 700 and H-3 to C-700 in the HMBC indicated the three fragments A, B and C were connected via the linkages of C-4/ C-10 and C-3/C-700 . The absence of methine signals at δH 4.34 (s, 1H, H-3) and the debasement at δC 46.84 (C-3) in the deuterium exchange in NMR confirmed the linkages of C-3/C-700. Thus, the structure of compound 4 was confirmed as shown in Fig. 2. It is obvious that a rearrangement has occurred during the reaction. With the aid of 1D and 2D NMR experiments, all the 1H NMR and 13C NMR signals of compound 4 were assigned as shown in Table 1. But at room temperature, some signals (H-30 , H-70 , C-30, C- 70, C-40 , C-60) in the NMR spectra of compound 4 are broad or week due to the existence of rotamers. And clearer 1H NMR and 13C NMR were obtained when recorded at 276 K, which confirmed the structure of 4.
|
|
Table 1 NMR spectroscopic data of compound 4 in acetone-d6a. |
In an NOE experiment, when H-4 was irradiated, obvious NOE was observed at H-200 and H-600 signals that indicated H-3 and H-4 were in trans relationship (Fig. 3). Furthermore, H-3 and H-4 showed no coupling, indicating a dihedral angle of approximately 908 between them.

|
Download:
|
| Figure 3. Selected NOE correlations of compound 4. | |
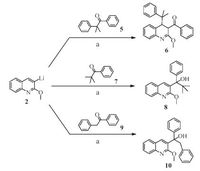
Highly inspired by this unexpected rearrangement, we were wondering if other similar structures could also undergo the rearrangement. The crowded environment around the carbonyl group of reagent 3 is very likely to favor the migration of 4- phenylpiperidinyl group. However, we are also interested in finding out the importance of the electro-stabilizing benzyl group. Compound 5 containing a 1, 1-dimethylbenzyl as a repelling group, and compound 7 with a tert-butyl group were used to replace reagent 3 to undergo the same procedure (Scheme 2). It was found that only the reaction of 5 with 2 provided the rearranged product 6, while compound 7 gave normal addition product 8. Therefore, electro-stabilizing factor is also important to promote the rearrangement. Then a benzyl phenyl ketone 9 that has an electro-stabilizing benzyl was introduced to the reaction, and it was found the only product observed was 10, and the rearrangement did not occur. Taken together, we can see that both steric factor and electro-stabilizing factor are important to achieve this 1, 3-rearrangement.
|
Download:
|
| Scheme. 2. Addition of 2-methoxyquinoline 3-lithium to different ketones to explore the rearrangement. | |
Based on the analysis above, a possible mechanism of a nucleophilic 1, 3-rearrangement was depicted in Scheme 3. Intermediate 1 was produced by the nucleophilic addition at the carbonyl group. In this intermediate, the 4-position of quinoline is electro-deficient due to the strong electron withdrawing effect of the 1-nitrogen. Migration of the electron-stabilized bulky group to the 4-position gave intermediates 11a and 11b. The more stable 3, 4-trans isomer 4 was generated as a pair of enantiomer after trapping a proton by the enol or enamine.
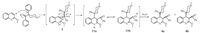
|
Download:
|
| Scheme. 3. Proposed mechanism of this 1, 3-rearrangement reaction. | |
4. Conclusion
In conclusion, we have found a new type of nucleophilic 1, 3- rearrangement reaction in which two new C-C bonds simultaneously formed at the 3- and 4-positions on a quinoline ring. The rearrangement is promoted by both steric and electron stabilizing effects. Given the simplicity of this one-step reaction, this novel 1, 3-rearrangement can be implemented in the synthesis of complex quinoline derivatives. This new 1, 3-carbon rearrangement will also inspire the synthesis of similar systems.
AcknowledgmentWe are grateful to "The National High-Tech Research and Development Program ("863"Program) of China" No. 2012AA020302) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.02.030.
| [1] | M. Ló pez-García, I. Alfonso, V. Gotor. Synthesis of (R)-3, , 4-diaminobutanoic acid by desymmetrization of dimethyl 3-(benzylamino)glutarate through enzymatic ammonolysis. J. Org. Chem. 68 (2002) 648–651. |
| [2] | M. Ochiai, T. Okada, N. Tada, A. Yoshimura, K. Miyamoto, M. Shiro, Difluoro-λ3-bromane-induced Hofmann rearrangement of sulfonamides: synthesis of sulfamoyl fluorides, J. Am. Chem. Soc. 131 (2009) 8392-8393. |
| [3] | A.B. Smith, I.G. Safonov, R.M. Corbett, Total syntheses of (+)-zampanolide and (+)-dactylolide exploitinga unifiedstrategy, J.Am.Chem.Soc.124(2002) 11102-11113. |
| [4] | A. Carrë r, J.C. Florent, E. Auvrouin, P. Rousselle, E. Bertounesque. Synthesis of, 3-aryl-2-arylamidobenzofurans based on the curtius rearrangement. J. Org. Chem. 76 (2011) 2502–2520. DOI:10.1021/jo102265b |
| [5] | D.G. Hilmey, L.A. Paquette, Promoter-dependent course of the Beckmann rearrangement of stereoisomeric spiro[4.4]nonane-1, 6-dione monoximes, Org. Lett. 7 (2005) 2067-2069. |
| [6] | Y. Furuya, K. Ishihara, H. Yamamoto. Cyanuric chloride as a mild and active Beckmann rearrangement catalyst. J. Am. Chem. Soc. 127 (2005) 11240–11241. DOI:10.1021/ja053441x |
| [7] | J.S. Kingsbury, E.J. Corey. Enantioselective total synthesis of isoedunol and β-araneosene featuring unconventional strategy and methodology. J. Am. Chem. Soc. 127 (2005) 13813–13815. DOI:10.1021/ja055137+ |
| [8] | K. Suzuki, H. Takikawa, Y. Hachisu, J.W. Bode. Isoxazole-directed pinacol rearrangement: stereocontrolled approach to angular stereogenic centers. Angew. Chem. Int. Ed. 46 (2007) 3252–3254. DOI:10.1002/(ISSN)1521-3773 |
| [9] | S. Braverman, M. Cherkinsky, E.V.K. Suresh Kumar, H.E. Gottlieb. Rearrangements of trihalomethyl ketones. Tetrahedron 56 (2000) 4521–4529. DOI:10.1016/S0040-4020(00) 00360-4 |
| [10] | A.S. Ionkin, W.J. Marshall, B.M. Fish. Highly sterically hindered olefins: a case of E- and Z-di-tert-butyl a, b-unsaturated acids. Org. Lett. 10 (2008) 2303–2305. DOI:10.1021/ol800808g |
| [11] | K. Andries, P. Verhasselt, J. Guillemont, H.W. Gohlmann, J.M. Neefs, H. Winkler, J. Van Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. de Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis, V. Jarlier. A diarylquinoline drug active on the ATP synthase of mycobacterium tuberculosis. Science 307 (2005) 223–227. DOI:10.1126/science.1106753 |
| [12] | J. Guillemont, C. Meyer, A. Poncelet, X. Bourdrez, K. Andries, Diarylquinolines, synthesis pathways and quantitative structure-activity relationship studies leading to the discovery of TMC207, Future Med. Chem. 3 (2011) 1345-1360. |
| [13] | A.Matteelli, A.C.C. Carvalho, K.E.Dooley, A. Kritski, TMC207: the first compound of a new class of potent anti-tuberculosis drugs, Future Microbiol. 5 (2010) 849-858. |
| [14] | C.X. He, H. Meng, X. Zhang, H.Q. Cui, D.L. Yin. Synthesis and bio-evaluation of phenothiazine derivatives as new anti-tuberculosis agents. Chin. Chem. Lett. 26 (2015) 951–954. DOI:10.1016/j.cclet.2015.03.027 |
| [15] | M. Yutaka, T. Sakae, N. Yoshiharu, H. Masatomo, Reactions of 3-substituted quinoline 1-oxides with acylating agents, Heterocycles 32 (1991) 1579-1586. |
 2016, Vol. 27
2016, Vol. 27