b Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, China ;
c Laboratory of Advanced Materials, Fudan University, Shanghai, 200433, China
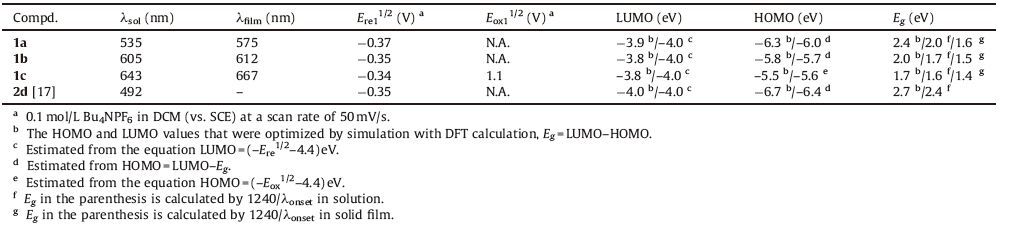
Organic semiconductors (OSCs) have been widely used in organic field-effect transistors (OFETs), organic photovoltaics (OPVs) and organic light-emitting diodes (OLEDs) because of OSCs’ low-cost and large-area film-forming properties, flexibility and tunable molecular energy levels [1-5]. OSCs can be divided into n-type (electron transport), p-type (hole transport) and ambipolar ones (both electron and hole transport), and some high performance p-type and n-type OSCs for OFETs have been achieved recently [6]. In order to achieve high performance OSCs, one of the most issues is to control their HOMO and LUMO energy levels [7]. For instance, the energy levels of HOMO and LUMO should be close to the work function of the electrode for hole and electron charge carrier injection [8]. And the construction of the push-pull structure is one of the most successful approaches to tune molecular orbital energy levels [9]. In fact, different types of OSCs can transform into each other by tuning the molecular orbital energy levels [10]. The p-type oligothiophenes-based OSCs could be turned into n-type ones by the end-capped pentafluorobenzene groups [11], and the diketopyrrolopyrrole-based derivatives (ntype) can be changed to ambipolar OSCs by incorporating thiophene units [12]. Naphthalene diimides (NDIs) are one of the best n-type OSCs [13, 14]. Gao et al. have developed a series of symmetric core-expanded NDIs fused with sulfur heterocycles and end-capped with electron-withdrawing groups (such as NDIDTYM2) for high performance n-type OSCs [14]. Besides these high performance symmetric OSCs, several asymmetric conjugated molecules with high field-effect mobility have been reported [15, 16]. The FET mobilities of asymmetric n-alkyl oligofluorenethiophene molecules are as high as their symmetric counterparts [16b]. Very recently, asymmetric core-expanded NDI molecules NDI-DTYM (2a and 2d) with low-lying LUMO level (≤-4.0 eV) have been developed by our group [17].Weenvision that a series of OSCs based on the molecular backbone of NDI-DTYM could be synthesized from the reactive compound 2a. Herein, our molecular design strategy is that attaching different numbers of thiophene units to the π-core of NDI-DTYM to fine-tune the molecular energy levels. The target molecules (1a-c) with thiophene units from 1 to 3 are shown in Fig. 1. Their spectroscopic, electrochemical, and field-effect behaviors were investigated, and the relationship between molecular structure and molecular energy levels were discussed to give some insights on the molecular design for ambipolar OSCs.
|
Download:
|
| Figure 1. Molecular structures of target compounds 1a-c and the reference compounds 2a and 2d. | |
2. Experimental
General information for chemicals, instruments and experiments, including 1H NMR and 13C NMR spectra, mass spectroscopy of compounds 1a-c, 2b, 3b, 2c and 3c can be seen in Supporting information.
2.1. 2-(2, 7-Bis(2-ethylhexyl)-1, 3, 6, 8-tetraoxo-4-(thiophen-2-yl)-1, 2, 3, 6, 7, 8-hexahydrobenzo[lmn][1, 3]dithiolo[4, 5-f][3, 8]- phenanthrolin-10-ylidene)malononitrile 3bTo a Schlenk tube equipped with a magnetic stir bar were added compound 2a [17] (200 mg, 0.283 mmol), thiophen-2-ylboronic acid 4 (112 mg, 0.875 mmol) and Pd (PPh3)4 (25.0 mg, 0.022 mmol) under N2 atmosphere. Anhydrous THF (12.00 mL) and potassium acetate (2.00 mL, 1.00 mol/L) were added to the tube. The reaction mixture was stirred at 60 ℃ overnight and then cooled to room temperature (Scheme 1). After removing the solvent under reduced pressure, the crude products were purified by column chromatography, using dichloromethane/petroleum (DCM/PE) (1:1, v:v) as the eluent. Compound 3b: 150 mg, yield 75%, red solid. 1H NMR (300 MHz, CDCl3): δ 8.80 (s, 1H), 7.63 (d, 1H, J= 5.1 Hz), 7.34 (d, 1H, J= 3.6 Hz), 7.24 (dd, 1H, J= 5.1, 3.6 Hz), 4.18(m, 4H), 2.03-1.85 (m, 2H), 1.40-1.25 (m, 16H), 0.91 (m, 12H). 13C NMR (100 MHz, CDCl3): δ 183.43, 163.14, 162.80, 161.84, 161.27, 144.8, 143.83, 142.00, 140.27, 137.33, 129.07, 127.84, 127.43, 125.54, 124.25, 122.42, 119.41, 119.02, 112.02, 70.22, 45.50, 37.99, 30.80, 29.84, 28.66, 24.14, 23.20, 14.18, 10.72. MS (MALDI-TOF) m/z: 711.5 (M + H+). Anal. Calcd. for C38N38N4O4S3: C 64.20, H 5.39, N 7.88; Found: C 63.90, H 5.41, N 7.69.

|
Download:
|
| Scheme. 1. The synthesis routes of compound 1a-c. | |
2.2. 2-(4-([2, 20-Bithiophen]-5-yl)-2, 7-bis(2-ethylhexyl)-1, 3, 6, 8- tetraoxo-1, 2, 3, 6, 7, 8-hexahydrobenzo[lmn][1, 3]dithiolo[4, 5-f][3, 8]- phenanthrolin-10-ylidene)malononitrile 3c
To a Schlenk tube equipped with amagnetic stir bar were added compound 2a (150 mg, 0.212 mmol), and Pd (PPh3)4 (12.8 mg, 0.011mmol) under N2 atmosphere. Anhydrous toluene (18.0 mL) and [2, 20-bithiophen]-5-yltributylstannane 5 (207 mg, 0.454mmol) were added to the tube. The reaction mixture was stirred at 80 ℃ overnight and then cooled to roomtemperature. Potassium fluoride solutionwas added to the mixture. After being stirred overnight, the mixture was extracted with DCM. The extract was concentrated. After removing the solvent under reduced pressure, the crude products were purified by column chromatography, using DCM/PE (1:1, v:v) as the eluent. 3c was obtained as a purple solid (115 mg, yield 68%). 1H NMR (300 MHz, CDCl3): δ 8.83 (s, 1H), 7.34 (d, 1H, J= 3.9 Hz), 7.31 (d, 1H, J= 5.1 Hz), 7.25 (d, 1H, J= 3.6 Hz), 7.24 (d, 1H, J= 3.6 Hz) 7.06 (dd, 1H, J= 3.9, 5.1 Hz), 4.15 (m, 4H), 2.03-1.88 (m, 2H), 1.32(m, 16H), 0.94-0.82(m, 12H). 13CNMR(100 MHz, CDCl3): δ 183.48, 163.12, 162.74, 161.82, 161.49, 144.87, 143.49, 141.97, 141.21, 138.70, 137.12, 136.59, 130.98, 128.25, 127.65, 125.85, 125.37, 124.89, 124.31, 121.47, 119.26, 118.96, 112.08, 70.07, 45.52, 37.99, 30.79, 29.84, 28.67, 24.13, 23.23, 14.22, 10.71.HRMS (MALDI) for C42N40N4O4S4 calcd. 792.1924 (M), found 792.1927.
2.3. 2-(4-(5-Bromothiophen-2-yl)-2, 7-bis(2-ethylhexyl)-1, 3, 6, 8-tetraoxo-1, 2, 3, 6, 7, 8-hexahydrobenzo[lmn][1, 3]dithiolo[4, 5-f][3, 8]- phenanthrolin-10-yidene)malononitrile 2b3b (150 mg, 0.211 mmol) was dissolved in chloroform (6.00 mL) and AcOH (6.00 mL). NBS (56.0 mg, 0.314 mmol) was added to the solution. The reaction mixture was stirred at 60 ℃ overnight and then cooled to room temperature then the mixture was extracted with water and DCM. The extract was concentrated. After removing the solvent under reduced pressure, the crude products were purified by column chromatography, using DCM/PE (2:3, v:v) as the eluent. 2b was obtained as a red-purple solid (145 mg, yield: 90%). MS (MALDI-TOF) m/z: 790.9 (M + H+). 1H NMR (300 MHz, CDCl3): δ 8.76 (s, 1H), 7.17 (d, 1H, J= 9.6 Hz), 7.16 (d, 1H, J= 9.6 Hz), 4.13 (m, 4H), 1.93 (m, 2H), 1.45-1.16 (m, 16H), 0.99-0.80 (m, 12H). 13C NMR (100 MHz, CDCl3): δ 183.22, 162.84, 162.48, 161.49, 161.08, 144.75, 143.69, 141.44, 140.40, 136.84, 130.43, 129.54, 127.16, 125.39, 124.28, 121.95, 119.13, 118.79, 116.43, 111.77, 69.90, 45.35, 37.76, 30.55, 28.43, 23.90, 23.09, 14.12, 10.55. HRMS (MALDI) for C38H37BrN4O4S3 calcd. 789.1253 (M + H+), found 789.1233.
2.4. 2-(4-(50-Bromo-[2, 20-bithiophen]-5-yl)-2, 7-bis(2-ethylhexyl)-1, 3, 6, 8-tetraoxo-1, 2, 3, 6, 7, 8-hexahydrobenzo[lmn][1, 3]dithiolo[4, 5- f][3, 8]-phenantrolin-10-ylidene)malononitrile 2cThe synthesis of 2c is carried out according to the synthesis of 2b. 2c was prepared as a blue-purple solid (yield: 87%). 1H NMR (300 MHz, CDCl3): δ 8.81 (s, 1H), 7.31 (d, 1H, J= 3.3 Hz), 7.16 (d, 1H, J= 3.3 Hz), 6.98 (d, 1H, J= 5.1 Hz), 6.97 (d, 1H, J= 5.1 Hz), 4.20 (m, 4H), 1.98-1.89 (m, 2H), 1.34-1.26 (m, 16H), 0.96-0.84 (m, 12H). 13C NMR (100 MHz, CDCl3): δ 183.28, 162.89, 162.52, 161.59, 161.26, 144.76, 143.47, 140.73, 140.36, 139.03, 137.85, 136.89, 130.94, 130.78, 127.39, 125.26, 124.69, 124.23, 121.46, 119.04, 118.76, 112.43, 111.88, 69.95, 45.35, 37.79, 30.59, 29.69, 28.48, 23.92, 23.08, 14.10, 10.56. HRMS (MALDI) for C42H39BrN4O4S4 calcd. 871.1125 (M + H+), found 871.1116.
2.5. General synthetic for compounds 1a-cTo a Schlenk tube equipped with a magnetic stir bar were added compound 2a, 2b or 2c (0.124 mmol), and Pd (PPh3)4 (12.8 mg, 0.011 mmol) under N2 atmosphere. Anhydrous toluene (8.00 mL) and 2-hexyl-5-(tri-n-butylstannyl) thiophene 6 (68.4 mg, 0.150 mmol) were added to the tube. The reaction mixture was stirred at 80 ℃ for 20 h and then cooled to room temperature. Then potassiumfluoride solution was added to deal with excess 6. After being stirred overnight, the mixture was extracted with DCM. The extract was concentrated. After removing the solvent under reduced pressure, the crude products were purified by column chromatography, using DCM/PE (1:1, v:v) as the eluent.
Compound 1a: Purple solid (yield: 52%). MS (MALDI-TOF) m/z: 795.3 (M + H+). 1H NMR (300 MHz, (CD3)2CO): δ 8.50 (s, 1H), 7.26 (d, 1H, J= 3.6 Hz), 6.85 (d, 1H, J= 3.6 Hz), 3.95 (m, 4H), 2.83 (m, 2H), 1.92 (m, 2H), 1.35-1.12 (m, 24H), 0.79 (m, 15H). 13C NMR (100 MHz, CDCl3): δ 183.46, 162.98, 162.63, 161.74, 161.31, 151.27, 144.42, 143.01, 142.21, 137.34, 129.75, 127.48, 125.04, 123.94, 121.12, 119.16, 118.81, 111.95, 69.71, 45.27, 37.79, 31.49, 30.60, 30.37, 29.68, 28.84, 28.49, 23.93, 23.05, 22.56, 14.06, 10.54 Anal. Calcd. for C44N50N4O4S3: C 66.47, H 6.34, N 7.05; found: C 66.56, H 6.49, N 6.92.
Compound 1b: Blue-green solid (yield: 80%). MS (MALDI-TOF) m/z: 877.2 (M + H+). 1H NMR (300 MHz, CDCl3): δ 8.80 (s, 1H), 7.33 (d, 1H, J= 4.5 Hz), 7.12 (d, 1H, J= 4.5 Hz), 7.03 (d, 1H, J= 3.6 Hz), 6.71 (d, 1H, J= 3.6 Hz), 4.15 (m, 4H), 2.82 (m, 2H), 2.03-1.88 (m, 2H), 1.33 (m, 24H), 0.99-0.85 (m, 15H). 13CNMR(100 MHz, CDCl3): δ 183.42, 162.96, 162.57, 161.69, 161.36, 147.18, 144.58, 143.07, 142.72, 141.17, 137.77, 136.99, 133.73, 131.06, 127.56, 125.12, 124.55, 124.07, 123.35, 120.90, 119.04, 118.76, 111.95, 69.75, 45.31, 37.80, 31.52, 30.61, 30.24, 29.68, 28.73, 28.50, 23.93, 23.07, 22.56, 14.07, 10.54. Anal. Calcd. for C48N52N4O4S4: C 65.72, H 5.97, N 6.39; found: C 65.72, H 5.84, N 6.33.
Compound 1c: Green solid (yield: 52%). MS (MALDI-TOF) m/z: 958.0 (M+). 1H NMR (400 MHz, CDCl3): δ 8.75 (s, 1H), 7.24 (m, 1H), 6.90 (m, 2H), 6.79 (m, 1H), 6.72 (m, 2H), 4.19 (m, 4H), 2.83 (m, 2H), 1.95 (m, 2H), 1.40-1.23 (m, 24H), 0.96-0.83 (m, 15H). 13C NMR (100 MHz, CDCl3): δ 183.19, 162.86, 162.45, 161.60, 161.28, 147.16, 144.59, 143.27, 141.10, 140.36, 138.60, 138.08, 136.53, 133.62, 131.19, 127.27, 125.34, 125.01, 124.52, 124.05, 122.99, 120.60, 118.71, 118.55, 111.96, 69.52, 45.29, 37.97, 37.75, 31.47, 30.62, 30.24, 28.85, 28.49, 23.93, 23.14, 22.59, 14.12, 10.58. Anal. Calcd. for C52N54N4O4S5: C 65.10, H 5.67, N 5.84; Found: C 64.91, H 5.55, N 5.82.
3. Results and discussionAs shown in Scheme 1, a series of NDI-DTYM and thiophenebased conjugated compounds 1a-c were synthesized. Compound 1a was achieved by a one-step Stille coupling reaction from compound 2a with a yield of 50%. Compounds 1b and 1c were obtained by two steps of coupling reactions (Suzuki and Stille coupling reactions for 1b and two-step Stille coupling reactions for 1c) with total yield of 54% and 26% (calculated from 2a), respectively. Compounds 1a-c were characterized by NMR spectra, mass spectroscopy and element analysis. These compounds are soluble in common organic solvents, such as chloroform and chlorobenzene, and can be processed by solution- based methods for OFETs.
As shown in Fig. S1 in Supporting information, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were carried out under nitrogen atmosphere to evaluate the thermal behaviors of compounds 1a-c. Three compounds have nearly the same thermostability with the 5% decomposition temperature at 386-391 ℃. Except that compound 1a only presents an irreversible endothermic peak at 152 ℃ in the first heating-cooling cycle, 1b and 1c present the reversible endothermic/exothermic peaks at 172/214 ℃ and 209/229 ℃.
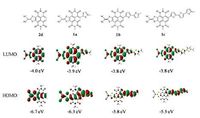
Density functional theory (DFT) calculations were utilized to estimate the frontier molecular orbital positions and energy levels of compounds 1a-c, using Gaussian 09 at the B3LYP/6-31G (d, p) level. To reduce the time about calculations, all alkyl chains were replaced by methyl groups. The molecular orbital energy levels are shown in Fig. 2. Obviously, the HOMO density distribution of NDIDTYMp- core got smaller with increasing the number of thiophene unit. Compared to compound 2d, the HOMO wave functions of 1a- c shift from the electron-deficient NDI-DTYM segment (acceptor moiety) to the electron-rich thiophene units (donor moiety). For compounds 1b and 1c, the HOMO wave functions are almost entirely localized on the thiophene units. So it can be concluded that HOMO energy distribution is dominated by the donor moiety. However, the LUMO density distribution of 1a-c and 2d are mainly localized on the NDI core, demonstrating that the LUMO energy level is mainly determined by the acceptor moiety. The energy levels of these compounds estimate by the DFT calculations are listed in the Table 1. In comparison with 2d, the HOMO energy levels of 1a-c were up-shifted with increasing the number of thiophene unit, with HOMO changed from -6.3 eV (1a) to -5.5 eV (1c). While the LUMO energy levels of 1a-c are almost unchanged (from -3.9 eV for 1a to -3.8 eV for 1c) with the deviation ≤ 0.1 eV, due to the strong electron-accepting property of NDI-DTYM. Therefore, the incorporation of NDI-DTYM with thiophene units can effectively elevate theHOMOenergy level, but has little impact on LUMO energy level, leading to the gradually decreased HOMOLUMO gaps of 1a-c (from 2.4 eV to 1.7 eV).
|
Download:
|
| Figure 2. Frontier molecular orbital and HOMO/LUMO levels of N, N-bis(methyl)- substituted model molecules of 1a-c and 2d, estimated by DFT calculations. | |
|
|
Table 1 Optical and electrochemical data for 1a-c and 2d. |
UV-vis measurement was performed to study the optical properties of compounds 1a-c. UV-vis spectra of these three compounds are shown in Fig. 3 and the optical data are shown in Table 1. The maximum peaks of long wavelength absorptions of compounds 2d and 1a-c in DCM solution are red-shifted from 492 to 643 nm. This remarkable red-shift of about 150 nm from 2d to 1c can be explained by the extension of thiophene units. Compare to the absorption in solution, the maximum peaks of long wavelength absorptions of 1a-c in solid film are all red-shifted, indicating that these three compounds formed the J-type stacking in solid state [18]. Optical gaps of 1a-c in solution/solid state have been estimated from edge of the absorption, and their optical gaps are 2.0/1.6 eV (1a), 1.7/1.5 eV (1b) and 1.6/1.4 eV (1c), demonstrating that increasing the length of thiophene units would decrease the optical band gap.

|
Download:
|
| Figure 3. Normalized UV–vis spectra of 1a–c in DCM solution (a) and in solid film (b). | |
Cyclic voltammetric (CV) measurements were carried out to investigate the electrochemical properties of compounds 1a-c. As shown in Fig. 4, these compounds all display two reversible reduction processes in DCM solution with similar half-wave potentials (Table 1, Ere 1/2 : 0.34-0.37 eV). The results indicated that attaching thiophene units to the NDI-DTYM core has a limited impact on the electrochemical reduction behavior. In addition, a reversible oxidation behavior with Eox 1/2 at 1.1 eV is observed for compound 1c when the number of thiophene units increased to three. This oxidation behavior is mainly attributed to the terthiophene unit. The electron-donating abilities of 1a-c are gradually enhanced by the extension of the p-electron system with the increased thiophene units. Therefore, the electrochemical reduction properties of 1a-c are dominated by the NDI-DTYM segment, and the thiophene units are mainly in charge of the electrochemical oxidation property. As shown in Table 1, the LUMO energy levels of 1a-c determined by CV results are almost the same at about -4.0 eV, which are consistent with the DFT calculated values (-3.8∼-3.9 eV). It should be noted that the reversible redox behavior of compound 1c (not only donate electron but also accept electron) make it potential ambipolar OSCs.

|
Download:
|
| Figure 4. Cyclic voltammograms of compounds 1a-c with 0.1 mol/L Bu4NPF6 in dry DCM at a scan rate of 50 mV/s (vs. SCE). | |
The thin film morphology and field-effect (FET) properties of compounds 1a-c have been studied by X-ray diffraction (XRD), atomic force microscopy (AFM) and OFET devices. OFET devices based on 1a-c were fabricated by spin-coating their respectively chloroform solutions onto the OTS-treated SiO2/Si substrates, affording a bottom-gate top-contact device configuration. XRD study (Fig. S3 in Supporting information) demonstrates that the thin films of 1a-c all exhibited good crystallinity after thermal annealing. Except that the thin film of 1a showed no FET behaviors, thin films of 1b and 1c both performed n-type FET characteristics, with electron motilities of up to 0.08 cm2 V-1 s-1 (Fig. S5 and Table S1 in Supporting information). XRD and AFM studies (Figs. S3 and S4) demonstrate that thin films of 1b and 1c have better crystallinity and much enlarged grain size when annealed at 160 ℃, leading to the increased electron mobility (about 0.07- 0.08 cm2 V-1 s-1) and the decreased threshold voltage (∼ 10 V). The DFT calculations and CV measurements have indicated that compound 1c has the proper HOMO and LUMO energy levels to inject and transport hole and electron charge carriers and would be potential ambipolar OSCs for OFETs. However, we could not observe the ambipolar FET features of 1c, which is probably due to the unfavorable molecular packing of 1c in solid state for hole transport. We believe that ambipolar OSCs based on NDI-DTYM and thiophene units would be achieved by further extension of the p-systems using more thiophene units and by the optimized OFET device structure and molecular self-assembly process.
4. ConclusionA series of organic semiconductors based on NDI-DTYM and thiophene (1a-c) were designed and synthesized, and their HOMO and LUMO energy levels were fine-tuned by varying the number of thiophene units. As the number of thiophene units increased, the HOMO energy levels go upward while the LUMO energy levels remain almost unchanged, resulting in the narrowed band gaps. OFETs based on 1b and 1c showed n-type charge transport characteristics, demonstrating that their LUMO energy levels are suit for electron injection and transport.We believe that ambipolar OSCs based on NDI-DTYM and thiophene would be realized by fine-tuning the HOMO energy levels and the molecular selfassembly property with more than three thiophene units attaching to the core of NDI-DTYM. The synthetic work for new NDI-DTYM and thiophene (or other donors)-based derivatives and their applications in OFETs and OPVs are currently underway in our lab.
AcknowledgmentsThis study was supported financially by the National Natural Science Foundation of China (Nos. 21302212 and 21522209) and the "Strategic Priority Research Program" (No. XDB12010100).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.05.003.
| [1] | C. Wang, H. Dong, W. Hu, et al. Semiconducting π-conjugated systems in fieldeffect transistors: a material odyssey of organic electronics. Chem. Rev. 112 (2012) 2208–2267. DOI:10.1021/cr100380z |
| [2] | (a) C.L. Chochos, N. Tagmatarchis, V.G. Gregoriou. Rational design on n-type organic materials for high performance organic photovoltaics. RSC Adv., 2013, 3: 7160-7181;(b) X. Zhang, X. Li. Effect of the position of substitution on the electronic properties of nitrophenyl derivatives of fulleropyrrolidines: fundamental understanding toward raising LUMO energy of fullerene electron-acceptor. Chin. Chem. Lett., 2014, 25: 501-504;(c) T. Jiang, Z. Wang, B. Du, et al., Theoretical characterization of hole mobility in BTBPD. Chin. Chem. Lett., 2013, 24: 945-948. |
| [3] | R.H. Friend, R.W. Gymer, A.B. Holmes, et al. Electroluminescence in conjugated polymers. Nature 397 (1999) 121–128. DOI:10.1038/16393 |
| [4] | G. Gelinck, P. Heremans, K. Nomoto, et al. Organic transistors in optical displays and microelectronic applications. Adv. Mater. 22 (2010) 3778–3798. DOI:10.1002/adma.200903559 |
| [5] | H. Usta, A. Facchetti, T.J.Marks. n-Channel semiconductor materials design for organic complementary circuits. Acc. Chem. Res. 44 (2011) 501–510. DOI:10.1021/ar200006r |
| [6] | (a) X. Gao, Z. Zhao. High mobility organic semiconductors for field-effect transistors. Sci. China Chem., 2015, 58: 947-968;(b) J. Dou, Y. Zheng, Z. Yao, et al., A cofacially stacked electron-deficient small molecule with a high electron mobility of over, 10 cm2 V-1 s-1 in air. Adv. Mater. 2015, 27: 8051-8055;(c) G. Xue, J. Wu, C. Fan, et al., Boosting the electron mobility of solution-grown organic single crystals via reducing the amount of polar solvent residues. Mater. Horiz., 2016, 3: 119-123. |
| [7] | L. Pandey, C. Risko, J.E. Norton, et al. Donor-acceptor copolymers of relevance for organic photovoltaics: a theoretical investigation of the impact of chemical structure modifications on the electronic and optical properties. Macromolecules 45 (2012) 6405–6414. DOI:10.1021/ma301164e |
| [8] | H. Usta, A. Facchetti, T.J. Marks. Air-stable, solution-processable n-channel and ambipolar semiconductors for thin-film transistors based on the indenofluorenebis( dicyanovinylene) core. J. Am. Chem. Soc. 130 (2008) 8580–8581. DOI:10.1021/ja802266u |
| [9] | (a) P. Sonar, S.P. Singh, P. Leclere, et al., Synthesis, characterization and comparative study of thiophene-benzothiadiazole based donor-acceptor-donor (D-A-D) materials, J. Mater. Chem. 2009, 19: 3228-3237;(b) Y. Cheng, S.H. Yang, C. Hsu. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev., 2009, 109: 5868-5923. |
| [10] | L. Bü rgi, M. Turbiez, R. Pfeiffer, et al. High-mobility ambipolar near-infrared light-Emitting polymer field-effect transistors. Adv. Mater. 20 (2008) 2217–2224. DOI:10.1002/(ISSN)1521-4095 |
| [11] | A. Facchetti, M.H. Yoon, C.L. Stern, et al. Building blocks for n-type organic electronics: regiochemically modulated inversion of majority carrier sign in perfluoroarene-modified polythiophene semiconductors. Angew. Chem. Int. Ed. 42 (2003) 3900–3903. DOI:10.1002/(ISSN)1521-3773 |
| [12] | B. Sun, W. Hong, Z.Q. Yan, et al. Record high electron mobility of, 6.3 cm2 V-1 s-1 achieved for polymer semiconductors using a new building block. Adv. Mater. 26 (2014) 2636–2642. DOI:10.1002/adma.v26.17 |
| [13] | H. Krü ger, S. Janietz, D. Sainova, et al. Hybrid supramolecular naphthalene diimide-thiophene structures and their application in polymer electronics. Adv. Funct. Mater. 17 (2007) 3715–3723. DOI:10.1002/(ISSN)1616-3028 |
| [14] | (a) X. Gao, C. Di, Y. Hu, et al., Core-expanded naphthalene diimides fused with, 2-(1, 3-dithiol-2-ylidene) malonitrile groups for high-performance, ambient-stable, solution-processed n-channel organic thin film transistors. J. Am. Chem. Soc. 2010, 132: 3697-3699;(b) Y. Hu, Y. Qin, X. Gao, et al., One-pot synthesis of core-expanded naphthalene diimides: enabling n-substituent modulation for diverse n-type organic materials. Org. Lett., 2012, 14: 292-295;(c) X. Gao, Y. Hu. Development of n-type organic semiconductors for thin film transistors: a viewpoint of molecular design. J. Mater. Chem. C., 2014, 2: 3099-3117. |
| [15] | S.L. Suraru, U. Zschieschang, H. Klauk, et al. A core-extended naphthalene diimide as a p-channel semiconductor. Chem. Commun. 47 (2011) 11504–11506. DOI:10.1039/c1cc15144d |
| [16] | (a) M.L. Tang, T. Okamoto, Z. Bao. High-performance organic semiconductors: asymmetric linear acenes containing sulphur. J. Am. Chem. Soc., 2006, 128: 16002-16003;(b) M.L. Tang, M.E. Roberts, J.J. Locklin, et al., Structure property relationships: asymmetric oligofluorene-thiophene molecules for organic TFTs. Chem. Mater., 2006, 18: 6250-6257. |
| [17] | B. Leng, D. Lu, X. Jia. Synthesis of monolateral and bilateral sulfur-heterocycle fused naphthalene diimides (NDIs) from monobromo and dibromo NDIs. Org. Chem. Front. 2 (2015) 372–377. DOI:10.1039/C4QO00252K |
| [18] | A. Mishra, R.K. Behera, P.K. Behera, et al. Cyanines during the, 1990s: a review. Chem. Rev. 100 (2000) 1973–2012. DOI:10.1021/cr990402t |
 2016, Vol. 27
2016, Vol. 27