b Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University , Tianjin 300071, China ;
c Changlu Engineering Research Center of New Chemical Materials, Tianjin 300160, China
Hexafluoropropylene oxide (HFPO) trimer (1,Fig. 1) is a byproduct of industrial HFPO polymerization process,which was used for producing fluoropolymer PFA. At least 100 tons of HFPO trimer was produced each year worldwide. Environmental Protection Agency of the United States reported that HFPO trimer is highly toxic (Table S1 in Supporting formation) [1, 2] and even trace amount cannot be metabolized or destroyed in human or animal bodies,which ends up accumulating and causes increase of liver weight [3]. Therefore,HFPO trimer was identified by öberg and Iqbal [4, 5] as one of 68 persistent organic pollutants (POPs) in 2012 [6],and its parameters exceed the screening criteria of the Stockholm Convention [7]. Meanwhile,other short chain HFPO oligomers (2,Fig. 1),which are also byproducts of HFPO polymerization manufacture,have the same problem of environmental pollution [8]. So it is very critical to consume HFPO oligomers safely to prevent them from contaminating the natural environment [9, 10].

|
Download:
|
| Figure 1. Structure of HFPO trimer and HFPO oligomers. | |
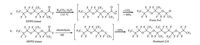
Currently,there are three major ways to consume HFPO trimer in industry: a) Heating HFPO trimer with K2CO3 in water to trigger decarboxylation to produce Freon E-2,a very simple and economic way developed by DuPont [11] (a,Scheme 1). Unfortunately,due to the restriction of Freon use,this effective method lost its commercial value and nearly faded away nowadays. b) Producing Hostinert 216,an industrial solvent,through electrolysis of HFPO trimer with HF in Germany [12] (b,Scheme 1). However,the massive use of HF and the low efficiency make the process highly environmental unfriendly. In addition,the market demands of Hostinert 216 have been dropping in recent years. Taking together,the route appears less attractive from both economic and environmental point of view. (c) Burning HFPO trimers in the combustion furnace,which is obviously problematic. As far as HFPO oligomers are concerned,there is no good ways to dispose of them and they are mainly used as surfactant as PFOA alternatives [13]. Given that the above situation,it is very urgent to seek new solutions to consume these harmful chemicals.
|
Download:
|
| Scheme. 1. Current commercial routes to consume HFPO trimer. | |
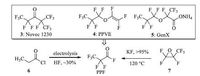
To the best of our knowledge,there was no report that HFPO oligomers were degraded to pentafluoropropionyl fluoride (PPF,Scheme 2),as the ether bond was difficult to break by nucleophilic substitution [14-18]. Meanwhile,PPF is an important industrial chemical used for the manufacture of Novec 1230 (3,Scheme 2) [19],Heptafluoropropyl trifluorovinyl ether (PPVE) (4,Scheme 2) [20],and HFPO dimer acid ammonium salt,namely GenX (5,Scheme 2) [21] etc. Currently,there are two major ways reported for the large-scale synthesis of PPF itself (Scheme 2): Electrolysis of propionyl chloride (6,Scheme 2) in HF [22] or rearrangement of hexafluoropropylene oxide (7,Scheme 2) catalyzed by KF [23]. They both have their own drawbacks: the electrolysis method is of low efficiency and uses HF as solvent whose leakage could be lethal and disaster to natural environment; while the rearrangement chemistry faces the high cost of starting material.
|
Download:
|
| Scheme. 2. Current reports on large-scale synthesis of PPF and representative chemicals manufactured from it. | |
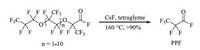
Herein,we report the degradation of HFPO trimer and oligomers to PPF using CsF/tetraglyme as catalytic system both under batch and autoclave conditions. These harmful chemicals can produce PPF under the optimized conditions at 160 ℃ with acceptable reaction rate in excellent yield (Scheme 3).
|
Download:
|
| Scheme. 3. Degradation of HFPO oligomers to PPF. | |
2. Experimental
NaF,KF,and CsF were dried at 200 ℃ under vacuum for 24 h. Tetraglyme and diglyme were dried at 160 ℃ with CaH2 under N2 protection for 24 h and vacuum distillated. Gas chromatographic data were obtained using Agilent 7820 series gas chromatograph. The reaction was monitored with an Agilent 7820 GC using a SE-30 capillary column. Temperature program was a hold at 40 ℃ and then taken to 300 ℃ at 10 ℃/min. Infrared spectra were obtained on a Shimadzu FTIR-8400S Spectrometer. NMR spectra were recorded on Bruker AM400 using neat 5 mm samples. CDCl3 is the references for the 1H and 19F NMR,respectively.
2.1. Degradation reaction performed in flaskA dry 250 mL flask equipped with a magneton,a thermocouple,a reflux condenser into a dry ice trap protected under nitrogen was set up. The equipment was dried by flame for three times under nitrogen protection. Fluoride salt,substrate,and solvent were quickly transferred into the flask. The mixture was heated to reflux until no more liquid was collected in the dry ice trap. Methanol was slowly dropped into dry ice trap to derive the product into corresponding methyl ester. The methyl ester derivative was washed three times with distilled water and then analyzed with gas chromatography.
2.2. Degradation reaction preformed in batch autoclaveA 1 L batch autoclave equipped with a mechanical agitator,a thermocouple,a heat booster was set up. It was dried by heating under vacuum. Fluoride salt,substrate,and solvent were quickly transferred into the autoclave. The mixture was heated until the pressure of the autoclave did not increase. The autoclave was cooled to r.t. Product was collected in a dry ice trap. Analysis of the product was performed in the same way as that in 2.1.
The detailed procedures are deposited in Supporting information.
3. Results and discussion 3.1. Degraduation of HFPO trimer to HFPO dimer and PPF in flaskFirst of all,we examined the degradation of HFPO trimer with different alkali metal fluoride in either diglyme or tetraglyme considering the host/guest effect of the solvents with alkali metal ion enhancing the nucleophilicity of the fluoride ion (Table 1). When NaF or KF was used as catalyst and diglyme as solvent,even at reflux temperature of 113 ℃ for 12 h,nothing was collected in the dry ice trap (entries 1,2). When the catalyst switched to CsF,under otherwise same as above conditions,small amount of liquid was collected in the dry ice trap,but the product distribution was complex and no major product could be identified (entry 3). However,while the solvent switched from diglyme to tetraglyme and the reaction was performed at reflux temperature of 121 ℃ for 8 h,large amount of liquid product was collected in the dry ice trap. After the liquid was transferred to methyl ester and analyzed using GC SE-30 column,65% PPF methyl ester,and 32% HFPO dimer methyl ester were identified (Fig. S1 in Supporting information),whose combined weight equaled to 97% weight of HFPO trimer.
|
|
Table 1 Degradation of HFPO trimer in the presence of different alkali metal fluoride and solvent combinationsa. |
3.2. Complete degradation of HFPO oligomers to PPF in batch
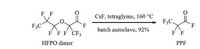
autoclave In order to degrade HFPO trimer to PPF completely without staying at the HFPO dimer stage,we examined the conditions to degrade HFPO dimer to PPF (Scheme 4). It turned out that when the reaction was performed in a batch autoclave at 160 ℃ under CsF/tetraglyme conditions,92% yield PPF was collected (Fig. S2 in Supporting information)! Encouraged by this exciting result,we examined the reaction condition with HFPO trimer and HFPO oligomers. Satisfyingly,both HFPO trimer and HFPO oligomers (up to n = 10) were degraded completely under the reaction conditions although slightly higher temperature was needed for oligomers to reach optimal yield of 92% (Fig. S3 in Supporting informaiton).
|
Download:
|
| Scheme. 4. Complete degradation of HFPO dimer to PPF in batch autoclave. | |
3.3. Degradation of HFPO trimer or oligomers on 200 g scale
In order to test the robustness of the new chemistry,we also performed the reactions on 200 g scale. Both HFPO trimer and oligomers were degraded completely to PPF in reasonable time frame remaining the high-yielding performance (Table 2). We are confident that this process has the potential to be applied in real industrial practice.
|
|
Table 2 Degradation of HFPO trimer or oligomers on 200 g scalea. |
3.4. Proposed reaction mechanism
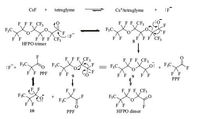
Inspired by the earlier report [24, 25],we have proposed a mechanism for the transformation (Scheme 5). It is reasoned that after the fluoride ion turns into a more aggressive nucleophile owing to the host/guest effect of Cs+ and tetraglyme,it attacks the rather electrophilic carbonyl group to make an intermediate 8. Intermediate 8 then could undergo an intramolecular fluoride migration to break the ether linkage to release first PPF molecule and intermediate 9; Intermediate 9 repeats the above process to release another molecule of PPF and intermediate 10,which further produces the third molecule of PPF and gives away a fluoride ion to close the catalytic cycle. Alternatively,intermediate 9 can collapse to produce HFPO dimer,which explains the formation of this side product.
|
Download:
|
| Scheme. 5. Proposed mechanism for degradation of HFPO trimer to PPF. | |
4. Conclusion
The method of degrading HFPO oligomers to PPF was firstly reported in this work. A combination of CsF and tetraglyme and using a batch autoclove are the key factors for this degradation process to go to completion. Under the optimal conditions,largescale reaction on 200 g was tested and no deduction of efficiency was observed. Industrial application of this process on producing PPF while consuming the harmful HFPO oligomers is currently under further investigation in our laboratories.
Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.04.026.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21372127,21572104),Program for New Century Excellent Talents in University,and Changlu Engineering Research Center of New Chemical Materials for financial support.
| [1] | EPA Data of HFPO Trimer, see: http://yosemite.epa.gov/oppts/epatscat8.nsf/by+Service/E91FE2961720F6DC85256930004F2372/$File/88940000083.pdf |
| [2] | HFPO Trimer Toxicology Study data see: http://www.siri.org/msds/tox/tf/q112/q689.html. |
| [3] | C. Lu, Y.L. Shi, Z. Zhou, et al. Perfluorinated compounds in blood of textile workers and barbers. Chin. Chem. Lett. 25 (2014) 1145–1148. |
| [4] | T. Oberg, M.S. Iqbal. The chemical and environmental property space of REACH chemicals. Chemosphere 87 (2012) 975–981. |
| [5] | M. Scheringer, S. Strempel, S. Hukari, et al. How many persistent organic pollutants should we expect?. Atmos. Pollut. Res. 3 (2012) 383–391. |
| [6] | M. Sha, P. Xing, B. Jiang. Strategies for synthesizing non-bioaccumulable alternatives to PFOA and PFOS. Chin. Chem. Lett. 46 (2015) 491–498. |
| [7] | UNEP, Stockholm Convention on Persistent Organic Pollutants. http://www.pops. int/documents/convtext/convtext_en.pdf |
| [8] | FluoroCouncil. Global regulatory activity on long chain perfluorochemicals and fluoropolymers, in: Spring Fluoropolymers Division Conference, Miami Beach, FL. 2012. |
| [9] | O. Kysilka, M. Rybá čková, M. Skalický, M. Kvíčalová, J. Cvačka. Fluorous imidazolium room-temperature ionic liquids based on HFPO trimer. J. Fluor. Chem. 130 (2009) 629–639. |
| [10] | J. Lapčík, O. Gimello, V. Ladmiral, C.M. Friesen, B. Ameduri. A new oligo(hexafluoropropylene oxide)-b-oligo(ethylene oxide) diblock surfactant obtained by radical reactions. Polym. Chem. 6 (2015) 79–96. |
| [11] | S. Jr, C. E. Tamborski. Fluorinated aliphatic polyalkylether lubricant with an additive composed of an aromatic phosphine substituted with perfluoroalkylether groups. US, 4, 443, 349, 1984. |
| [12] | F. Richard. Dielectric liquids, US, 5, 159, 527 A. 1991. |
| [13] | P.D. Brothers, S.V. Gangal. Aqueous polymerization of fluorinated monomer using polymerization agent comprising fluoropolyether acid or salt and short chain fluorosurfactant. US B2 (2010) 7705074. |
| [14] | L. Robert, Burwell Jr.. The cleavage of ethers. Chem. Rev. 54 (1954) 615–685. |
| [15] | M.V. Bhatt, S.U. Kulkarni. Cleavage of ethers. Synthesis 4 (1983) 249–282. |
| [16] | G. Drivera, K.E. Johnsona. 3-Methylimidazolium bromohydrogenates(I): a roomtemperature ionic liquid for ether cleavage. Green Chem. 5 (2003) 163–169. |
| [17] | M. Yamashita, A. Tani, F. Kawai. Cloning and expression of an ether-bond-cleaving enzyme involved in the metabolism of polyethylene glycol. J. Biosci. Bioeng. 98 (2004) 313–315. |
| [18] | A. Atesin, N. Ray, P. Stair, T. Marks. Etheric C-O bond hydrogenolysis using a tandem lanthanide triflate/supported palladium nanoparticle catalyst system. J. Am. Chem. Soc. 134 (2012) 14682–14685. |
| [19] | A.K. Kim, G.P. Crampton. Performance of Novec1230 in electronic facility fire protection, fire suppression and detection-A technical working conference (Proceedings of SUPDET, 2010): 16 February 2010, Orlando. Florida. |
| [20] | B.M. Dwight, F.R. Victor, M.R. Alan. Process for treating melt-processible tetrafluoroethylene/perfluoro(alkyl vinyl)-ether copolymers. EP, 0226668 B1, 1992. |
| [21] | DuPontTM GenX processing aid for making f luoropolymer resins; DuPont, 2010 http://www2.dupont.com/Industrial_Bakery_Solutions/en_GB/assets/downloads/DuPont_GenX_Brochure_Final_07July2010.pdf. |
| [22] | R.M. Flynn, M.G. Costello, D.R. Vitcak. Hydrofluoroether compounds and processes for their preparation and use. WO:, 2008070606, 2008. |
| [23] | G. Siegemun, R. Franz. Process for the preparation of perfluoropropionyl fluoride,US. (1997) 5684193. |
| [24] | S.V. Kostjuk, E. Ortega, F. Ganachaud, B. Amé duri, B. Boutevin. Anionic ringopening polymerization of hexafluoropropylene oxide using alkali metal fluorides as catalysts: a mechanistic study. Macromolecules 42 (2009) 612–619. |
| [25] | Paul H. Kasai. Perfluoropolyethers: intramolecular disproportionation. Macromolecules 25 (1992) 6791–6799. |
 2016, Vol. 27
2016, Vol. 27 




