b Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Soochow University, Suzhou 215123, China ;
c Testing and Analysis Center, Soochow University, Suzhou 215123, China
Mercury is the third most frequently found and second most toxic heavy metal according to the research results of the Agency for Toxic Substances and Disease Registry (ATSDR) of the USA Department of Health and Human Services [1]. Due to easy absorptivity,high bioaccumulation and persistence,all elemental, inorganic and organic mercury can cause human health problems such as serious congnitive and motion disorders,kidney,central nervous system and prenatal brain damages [2]. Hg2+ is the most common species of mercury pollutants. Hence,monitoring the presence of Hg2+ is very important.
Fluorescent sensors attract great attention in the field of heavy metal ion detection owing to being highly selective,highly sensitive,maneuverable,low-cost,and suitable for on-site and real-time signaling [3, 4]. A large number of 1,8-naphthalimide [5-7],rhodamine [8-12],BODIPY [13-15],coumarin [16, 17],and dansyl-based [18, 19] Hg2+ fluorescent sensors have been reported. However,some of them could only respond to Hg2+ in organic media [9, 11, 13],others suffered from poor sensing properties including selectivity,sensitivity,interference immunity and practicability [8, 12, 20]. The overall performance of the sensors still remains to improve and the relationship between the structure and the performance of the sensor has not been well established. So the study of new fluorescent Hg2+ sensors is of great significance.
Rhodamine dyes have the advantages of high photostability, long excitation and emission wavelength,easily modified parent structure,as well as remarkable and vivid color changes when triggered by the analyte [3, 21, 22]. N and S atoms are most effective coordination sites for Hg2+ [9, 23-25]. Therefore,herein we employed rhodamine B as reporter,thiourea as receptor,and polyethylenepolyamines as linker to construct novel Hg2+ sensors with pleasant overall performance such as aqueous working medium,favorable working pH,high sensitivity and selectivity, wide linear Hg2+ concentration range,strong interference immunity, and good availability.
2. ExperimentalThe novel fluorescent Hg2+ sensors with two,three and four thiourea receptors were prepared by rhodamine B (RB) and phenyl isothiocyanate (PITC) linked with diethylenetriamine (DETA), triethylenetetramine (TETA) and tetraethylenepentamine (TEPA) respectively,as shown in Scheme 1. Their structures were fully characterized by FTIR,1H NMR,13C NMR,LC-MS and elementary analysis.

|
Download:
|
| Scheme. 1. Synthetic route of the fluorescent Hg2+ sensors. | |
These three fluorescent Hg2+ sensors are: 1-(2-(3',6'-bis(diethylamino)- 3-oxospiro[isoindoline-1,9'-xanthen]-2-yl)ethyl)-3-phenyl- 1-(2-(3-phenylthioureido)ethyl)thiourea (RDTU),1-(2-(3',6'- bis(diethylamino)-3-oxospiro[isoindoline-1,9'-xanthen]-2- yl)ethyl)-3-phenyl-1-(2-(3-phenyl-1-(2-(3-phenylthioureido) ethyl) thioureido)ethyl)thiourea (RTTU) and 1-(2-(3',6'-bis(- diethylamino)-3-oxospiro[isoindoline-1,9'-xanthen]-2-yl)ethyl)-3- phenyl-1-(2-(3-phenyl-1-(2-(3-phenyl-1-(2-(3-phenylthioureido) ethyl)thioureido)-ethyl)thioureido)ethyl)thiourea (RPTU).
The detailed procedures and characterization of the new compounds,and the materials,apparatus and methods were described in the Supporting information (Figs. S1-S12).
3. Results and discussionThe novel fluorescent Hg2+ sensors were easily prepared by two step reactions between rhodamine B,polyethylenepolyamine (PEPA) and phenyl isothiocyanate (PITC). Through the quasi Click reaction between the -NH2 and -NH groups in PEPA and the -NCS group in PITC,multiple phenylthiourea units were introduced into the sensors by a one-pot reaction under very mild conditions, which made the synthetic process high efficiency and energy saving.
The sensing properties of RDTU,RTTU and RPTU to Hg2+ was investigated through adding various metal ions,Na+,K+,Ca2+,Mg2+, Fe3+,Cu2+,Zn2+,Cr3+,Pb2+,Ni2+,Fe2+,Mn2+,Co2+,Cd2+ and Hg2+ (120 mmol/L),into the CH3CN/HEPES buffer (pH 7.2) solutions of the sensors (20 mmol/L),and recording the absorption and fluorescence spectra of the solutions. It was found that the absorption spectra of the solutions showed no significant changes (Figs. S13-S15 in Supporting information),but the fluorescence of the solutions was enhanced dramatically by Hg2+ over other metal ions (Fig. 1). The results indicated that RDTU,RTTU and RPTU may be potential highly selective and sensitive turn-on fluorescent sensors for Hg2+. Further study showed that the optimal media for RDTU,RTTU and RPTU was CH3CN/HEPES buffer (pH 7.2) 1:1,9:1 and 10:1 (v/v) respectively.

|
Download:
|
| Figure 1. Fluorescence spectra of the sensors in the absence and presence of various metal ions. Solvent: CH3CN/HEPES buffer (pH 7.2, 1:1, 9:1 and 10:1 (v/v) for RDTU, RTTU and RPTU, respectively). Concentration: 20 mmol/L for sensors, 120 mmol/L for metal ions. λex: 520 nm, slit width: 10 nm. Other ions: Na+, K+, Mg2+, Ca2+, Fe3+, Cu2+, Zn2+, Cr3+, Pb2+, Ni2+, Fe2+, Mn2+, Co2+, and Cd2+. | |
The dependence of the fluorescence intensity of the sensor/Hg2+ solutions on the concentration of Hg2+ was studied. The results showed that the fluorescence intensity of the sensor/Hg2+ solutions first increased then level off with the rising of Hg2+ from 0 to 220 mmol/L (Fig. 2). The solutions of RDTU,RTTU and RPTU (20 mmol/L) with 140 mmol/L of Hg2+ got 7.7,15.5 and 17.6-fold fluorescence enhancement,respectively. The maximal fluorescence intensity and the concentration of Hg2+ ([Hg2+]) showed good linear relationship in the range of 0-80,0-100 and 0-140 mmol/L of [Hg2+] with the detection limits of 512,66.2 and 37.6 ppb,respectively. The results showed that not only RDTU, RTTU and RPTU could be used to quantitatively detect Hg2+,but also the linear working concentration range was broadened,and the sensitivity was raised along with the increase of the number of the NCH2CH2 and the thiourea units.

|
Download:
|
| Figure 2. The relationship between the maximal fluorescence intensity and the concentration of Hg2+. Solvent: CH3CN/HEPES buffer (pH 7.2, 1:1, 9:1 and 10:1 (v/v) for RDTU, RTTU and RPTU, respectively). Concentration: 20 mmol/L for sensors. 0–220 mmol/L for Hg2+. λex: 520 nm; slit width: 10 nm. | |
To examine the effects of coexistent metal ions on the detection of Hg2+,the environmentally and biologically relevant ions,Na+,K+, Ca2+,Mg2+,Fe3+,Cu2+,Zn2+,Cr3+,Pb2+,Ni2+,Fe2+,Mn2+,Co2+ and Cd2+ (120 mmol/L),were added to the sensor (20 mmol/L)/Hg2+ (120 mmol/L) CH3CN/HEPES buffer solutions (pH 7.2) and the fluorescence spectrawere tested. As shownin Fig. 3,the competitive ions showed no pronounced influence on the fluorescence intensity of the sensor/Hg2+ solutions. Therefore RDTU,RTTU and RPTU have excellent anti-interference ability for Hg2+ detection and they are reliable Hg2+ fluorescent sensors.

|
Download:
|
| Figure 3. Effects of coexisting ions on the fluorescence maxima of the sensor/Hg2+ solutions. a, c and e: Sensor + Hg2++Mn+, whereMn+: 1-Na+, 2-K+, 3-Ca2+, 4-Mg2+, 5- Fe3+, 6-Cu2+, 7-Zn2+, 8-Cr3+, 9-Pb2+, 10-Ni2+, 11-Fe2+, 12-Mn2+, 13-Co2+, 14-Cd2+. b, d and f: Sensor + Hg2+. Solvent: CH3CN/HEPES buffer (pH 7.2, 1:1, 9:1 and 10:1 (v/v) for RDTU, RTTU and RPTU respectively). Concentration: 20 mmol/L for sensors, 120 mmol/L for metal ions. λex: 520 nm; slit width: 10 nm. | |
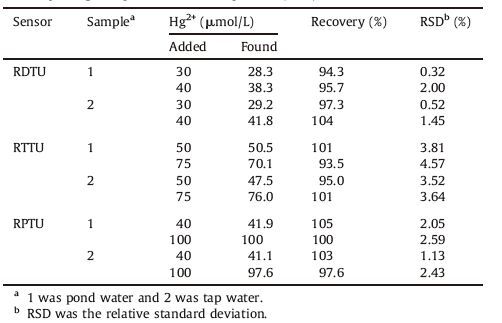
To know their practicability,the sensors were used for the determination of Hg2+ in pond water and tap water. The results were displayed in Table 1. The detected concentration of Hg2+ was close to that of the added Hg2+. The recovery was 94.3%-104%, 93.5%-101% and 97.6%-105%,and the relative standard deviation (RSD) of three measurements was less than 2.00%,4.57% and 2.59% for RDTU,RTTU and RPTU,respectively. Therefore the sensors can be applied in monitoring Hg2+ in environmental water.
|
|
Table 1 Recovery of Hg2+ in pond water and tap water (n = 3). Sensor Samplea Hg2+ (mmol/L) Recovery |
The molar fractions of Hg2+ (0.67,0.75 and 0.8) corresponding to the maximal fluorescent increment of the three sensor solutions in Job’s plot (Fig. 4) showed that 1:2 RDTU/Hg2+,1:3 RTTU/Hg2+ and 1:4 RPTU/Hg2+ complexes formed with the association constants of 5.30 × 108 L2 mol-2,9.88× 1011 L3 mol-3 and 1.00 × 1016 L4 mol-4,respectively. The number of the bound Hg2+ expectedly equaled to that of the thiourea units,indicating that the S atom in thiourea other than the N atom in NCH2CH2 acted as the receptor. The reason may lie in the sulphophile affinity of the Hg2+. Moreover,excess EDTA could not quench the fluorescence of the sensor/Hg2+ solutions (Fig. 5),indicating the irreversible coordination of the sensor with Hg2+.

|
Download:
|
| Figure 4. Job’s plot for sensor versus Hg2+. Total concentrations: [RDTU] + [Hg2+] (40 mmol/L); [RTTU] + [Hg2+] (90 mmol/L); [RPTU] + [Hg2+] (100 mmol/L). F0 and F: fluorescence maxima before and after addition of Hg2+, respectively. λex: 520 nm; slit width: 10 nm. | |

|
Download:
|
| Figure 5. Reversibility of the fluorescent detection of Hg2+ with the sensors. 1: sensor, 2: sensor +Hg2+, 3: sensor + Hg2+ + EDTA. Solvent: CH3CN/HEPES buffer (pH 7.2, 1:1, 9:1 and 10:1 (v/v) for RDTU, RTTU and RPTU respectively). Concentration: 20 mmol/ L for the sensors, 120 mmol/L for Hg2+. λex: 520 nm; slit width: 10 nm. | |
These results could not be explained by the reversible sensing mechanism through amino/oxo/thioxo donor coordination [9, 25, 26],the irreversible sensing mechanism based on the hydrolysis [27, 28] or cyclization reaction [29-31],and the sandwich-stacking binding mode [32] reported in the references. Therefore,we conjectured that a new interaction way between Hg2+ and the sensor might occur.We currently could suppose Hg2+ was bound to the S atom in the thiourea receptor,leading to the formation of the sensor/Hg2+ complex aswell as the conversion of the rhodamine spirolactam to the ring-opened amide form,as shown in Scheme 2.

|
Download:
|
| Scheme. 2. Supposed sensing mechanism of the sensors for Hg2+. | |
4. Conclusion
In conclusion,we designed and synthesized three novel turn-on fluorescent sensors for Hg2+. They are highly selective and sensitive to Hg2+ in water matrix with excellent anti-interference ability, and can be applied in monitoring Hg2+ in environmental water samples effectively. As expected,each thiourea unit binds one Hg2+,resulting in the linear working ranges of the sensors broadened,and the sensitivity rose along with the increase of the thiourea receptor’s numbers. Subsequent work such as the further sensing mechanism exploration is undergoing in our laboratory.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21074085),the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Graduate Student Innovation Training Project of Jiangsu Province (No. KYLX_1241),and the State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.03.027.
| [1] | Y.L. Liu, X. Lv, Y. Zhao, et al. , A naphthalimide-rhodamine ratiometric fluorescent probe for Hg2+ based on fluorescence resonance energy transfer. Dyes Pigments 92 (2012) 909–915. |
| [2] | S.Y. Yu, S.P.Wu. A highly selective turn-on fluorescence chemosensor for Hg(II) and its application in living cell imaging. Sens. Actuators B: Chem. 201 (2014) 25–30. |
| [3] | D.T. Quang, J.S. Kim. Fluoro-and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 110 (2010) 6280–6301. |
| [4] | X.H. Qian, Z.C. Xu. Fluorescence imaging of metal ions implicated in diseases. Chem. Soc. Rev. 44 (2015) 4487–4493. |
| [5] | Z.Y. Zhang, S.Z. Lu, C.M. Sha, et al. , A single thiourea-appended, 1,8-naphthalimide chemosensor for three heavy metal ions: Fe3+, Pb2+, and Hg2+. Sens. Actuators B: Chem. 208 (2015) 258–266. |
| [6] | S. Huang, R. Han, Q. Zhuang, et al. , New photostable naphthalimide-based fluorescent probe for mitochondrial imaging and tracking. Biosens. Bioelectron. 71 (2015) 313–321. |
| [7] | Y. Ding, W.P. Zhu, Y.F. Xu, et al. , A small molecular fluorescent sensor functionalized silica microsphere for detection and removal of mercury, cadmium, and lead ions in aqueous solutions. Sens. Actuators B: Chem. 220 (2015) 762–771. |
| [8] | Y. Mao, M.M. Hong, A.F. Liu, D.M. Xu. Highly selective and sensitive detection of Hg(II) from HgCl2 by a simple rhodamine-based fluorescent sensor. J. Fluoresc. 25 (2015) 755–761. |
| [9] | N. Wanichacheva, P. Praikaew, T. Suwanich, et al. "Naked-eye" colorimetric and "turn-on" fluorometric chemosensors for reversible Hg2+ detection. Spectrochim. Acta, Part A 118 (2014) 908–914. |
| [10] | C.J. Li, K.Q. Xiang, Y.C. Liu, et al. , A novel colorimetric chemosensor for Cu2+ with high selectivity and sensitivity based on Rhodamine B. Res. Chem. Intermed. 41 (2015) 10169–10180. |
| [11] | M. Kumar, N. Kumar, V. Bhalla. Rhodamine appended thiacalix[4]arene of, 1,3-alternate conformation for nanomolar detection of Hg2+ ions. Sens. Actuators B 161 (2012) 311–316. |
| [12] | F.Y. Yan, M. Wang, D.L. Cao, et al. , New fluorescent and colorimetric chemosensors based on the rhodamine detection of Hg2+ and Al3+ and application of imaging in living cells. Dyes Pigments 98 (2013) 42–50. |
| [13] | T.K. Khan, M. Ravikanth. 3-(Pyridine-4-thione)BODIPY as a chemodosimeter for detection of Hg(II) ions. Dyes Pigments 95 (2012) 89–95. |
| [14] | H.D. Xiao, J.H. Li, K.T. Wu, et al. , A turn-on BODIPY-based fluorescent probe for Hg(II) and its biological applications. Sens. Actuators B: Chem. 213 (2015) 343–350. |
| [15] | E. Karakus, M. Ucuncu, M. Emrullahoğlu. A rhodamine/BODIPY-based fluorescent probe for the differential detection of Hg(II) and Au(III). Chem. Commun. 50 (2014) 1119–1121. |
| [16] | S. Yang, W. Yang, Q. Guo, et al. , A highly selective and ratiometric fluorescence probe for the detection of Hg2+ and pH change based on coumarin in aqueous solution. Tetrahedron 70 (2014) 8914–8918. |
| [17] | B. Gao, W.T. Gong, Q.L. Zhang, et al. , A selective "turn-on" fluorescent sensor for Hg2+ based on "reactive", 7-hydroxycoumarin compound. Sens. Actuators B: Chem. 162 (2012) 391–395. |
| [18] | M.H. Lee, H.J. Kim, S. Yoon, et al. , Metal ion induced FRET off-on in tren/dansylappended rhodamine. Org. Lett. 10 (2008) 213–216. |
| [19] | J. Isaad, A.E. Achari. Azathia crown ether possessing a dansyl fluorophore moiety functionalized silica nanoparticles as hybrid material for mercury detection in aqueous medium. Tetrahedron 69 (2013) 4866–4874. |
| [20] | J.H. Hu, J.B. Li, J. Qi, J.J. Chen. Highly selective and effective mercury(II) fluorescent sensors. New J. Chem. 39 (2015) 843–848. |
| [21] | Q.P. Hu, Y.L. Liu, Z.Q. Li, et al. , A new rhodamine-based dual chemosensor for Al3+ and Cu2+. Tetrahedron Lett. 55 (2014) 4912–4916. |
| [22] | X.Q. Chen, T. Pradhan, F. Wang, et al. , Fluorescent chemosensors based on spiroring-opening of Xanthenes and related derivatives. Chem. Rev. 112 (2012) 1910–1956. |
| [23] | Z. Liu, W. He, M.S. Pei, G.Y. Zhang. A fluorescent sensor with a detection level of pM for Cd2+ and nM for Cu2+ based on different mechanisms. Chem. Commun. 51 (2015) 14227–14230. |
| [24] | J.H. Huang, Y.F. Xu, X.H. Qian. A rhodamine-based Hg2+ sensor with high selectivity and sensitivity in aqueous solution: a NS2-containing receptor. J. Org. Chem. 74 (2009) 2167–2170. |
| [25] | H.L. Chen, Z.F. Guo, Z.L. Lu. Controlling ion-sensing specificity of N-amidothioureas: from anion-selective sensors to highly Zn2+ selective sensors by tuning electronic effects. Org. Lett. 14 (2012) 5070–5073. |
| [26] | B. Bag, A. Pal. Rhodamine-based probes for metal ion-induced chromo-fluorogenic dual signaling and their selectivity towards Hg(II) ion. Org. Biomol. Chem. 9 (2011) 4467–4480. |
| [27] | A.F. Liu, L. Yang, Z.Y. Zhang, et al. , A novel rhodamine-based colorimetric and fluorescent sensor for the dual-channel detection of Cu2+ and Fe3+ in aqueous solutions. Dyes Pigments 99 (2013) 472–479. |
| [28] | W. Shi, H.M. Ma. Rhodamine B thiolactone: a simple chemosensor for Hg2+ in aqueous media. Chem. Commun. 16 (2008) 1856–1858. |
| [29] | J.S. Wu, I.C. Hwang, K.S. Kim, et al. , Rhodamine-based Hg2+-selective chemodosimeter in aqueous solution: fluorescent off-on. Org. Lett. 9 (2007) 907–910. |
| [30] | S. Angupillai, J.Y. Hwang, J.Y. Lee, et al. , Efficient rhodamine-thiosemicarbazidebased colorimetric/fluorescent ‘turn-on' chemodosimeters for the detection of Hg2+ in aqueous samples. Sens. Actuators B: Chem. 214 (2015) 101–110. |
| [31] | F.H. Wang, C.W. Cheng, L.C. Duan, et al. , Highly selective fluorescent sensor for Hg2+ ion based on a novel rhodamine B derivative. Sens. Actuators B: Chem. 206 (2015) 678–683. |
| [32] | G.K. Wang, Q.L. Mi, L.Y. Zhao, et al. , A pyrene derivative for Hg2+-selective fluorescent sensing and its application in in vivo imaging. Chem. Asian J. 9 (2014) 744–748. |
 2016, Vol. 27
2016, Vol. 27