b Beijing Engineering Research Center of Food Environment and Public Health, Minzu University of China, Beijing 10008l, China ;
c Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, China
Polysaccharide is made up of monosaccharide through glycosidic bonds and exists in the bodies of plants,animals and microbes. In recent decades,published data indicate that plants polysaccharides which existed in many Chinese herbs possess antioxidative,immunobiological,anti-viral,anti-tumor and other biological activity. Therefore,polysaccharides from herbs have become a hot topic in the research field of chemistry and biology.
Halenia elliptica D. Don ,name in Chinese is "heijicao" or "quhedoulaguoma" in the Tibetan language,belongs to the Gentianaceae plant family,and is mainly distributed in southwest China,including Tibet,Qinghai,Sichuan,and Gansu Province. It grows in forests or steep,at an altitude of 2500-4400 m,and prefers wet-warm environments but is adaptable [1]. H. elliptica D. Don is a popularly used as ethnodrug from Tibet for treating diseases of the hepatobiliary system,and is generally termed "zangyinchen". Herbs are sweet with bitter taste and cold in nature. According to traditional Chinese medical practice,this plant removes liver heat and cholagogue,clears heat and toxic substances,and used in treating acute icteric hepatitis,acute pyelonephritis (AP),influenza,and abnormally high condition of body heat caused by cholelithiasis and furunculosis [2].
Several scientific investigations,involving extraction and pharmacological studies of ketones,terpenes and alkaloids [3] from this herb,demonstrate the significant bioactivities. However,the polysaccharides from H. elliptica D. Don have been seldom reported. Polysaccharides have many biological activities which are closely related to their chemical structures. By using H. elliptica D. Don plant material,the authors extracted the herb followed by isolating and purifying the polysaccharides in order to identify and determine its molecular structure. This research provides preliminary data for an advanced study of pharmacological activity in the utilization of H. elliptica D. Don.
2. ExperimentalThe aerial part of H. elliptica D. Don was identified by Dr. Lin Yang at Minzu University of China. Plant material was provided by a Tibetan medicine factory (Lhasa of Tibet,China). All samples were sliced and ground into fine powder in a mill before extraction. DEAE-Sephadex A-25,Sepharose CL-4B,and MW standards of T-series Dextran were purchased from Amersham Pharmacia Biotech (China) Ltd. Pure monosaccharide standards of D-mannose (Man),L-rhamnose (Rha),D-ribose (Rib),D-galactose (Gal),D-xylose (Xyl),D-arabinose (Ara),L-fucose (Fuc),D-glucose (Glc) and Dgalacturonic acid (GalA) were obtained from Sinopharm Chemical Reagent Co.,Ltd. ( Shanghai). All other reagents used were of analytical grade.
2.1. General experimental proceduresV absorption spectra were recorded with a UV-Jasco53 spectrophotometer between 190 and 290 nm. IR spectra (KBr) were recorded with a Fourier transform infrared spectrometer of Magna-IR 750 in a range of 400-4000 cm-1. The hydrolysates were determined by GC (GC-2010) equipped with Rtx-225 capillary column (30 m×0.32 mm×0.25 μm) and a flame-ionization detector. Identification of acetate sugar was carried out from the retention time relative to standard sugars. GC-MS analyses were performed on a GC-MS-QP2010A instrument equipped with a OV- 210 capillary column (30 m×0.32 mm×0.25 μm). For a solution of HM41 (2%) in D2O,NMR spectra were recorded at 25 ℃ with a Bruker Avance 400 type magnetic resonance instrument. X-ray diffraction of HM41 was determined by a Japan D/Max-YB type Xray diffraction instrument. Uronic acids were determined colorimetrically using a method described in the literature [4].
2.2. Isolation and purificationried and ground H. elliptica D. Don (10 g) was previously defatted with light petroleum and 95% EtOH under reflux conditions,and successively extracted with hot water. The supernatant was collected,condensed to 300 mL with a rotary evaporator,and mixed with 95% (v/v) EtOH (4 volumes). The mixture was left for 24 h at room temperature. The mixture was then centrifuged (3000 r/min,15 min) to separate the supernatant and the precipitate. The precipitate was dried under vacuum at 37 ℃ to afford the crude polysaccharide (HMc,yield: 2.3%,based on the original dried material). The crude polysaccharide was deproteinated by a combination of proteinase (37 ℃,3 h) and the Sevage method (1:4,n-butanol: chloroform,v/v) [5]. The deproteinated extract was dialyzed using a regenerated cellulose membrane tube (MW cutoff 1000) against H2O for 48 h and distilled with H2O for 24 h. The concentrated nondialysate was precipitated with 5 volumes of dehydrated EtOH. The precipitate was washed with absolute EtOH,acetone and ether,respectively. The washed precipitate was collected as the crude polysaccharide and named as HM. The deproteined polysaccharide HM was fractionated by acidic EtOH,to obtain the fraction containing HM4. The polysaccharide HM4 was further purified on DEAESephadex A-25 column (2.5 cm×90 cm) and eluted with 0.1 mol/L NaCl at a flow-rate of 1.1 mL/min. The eluent was monitored by the phenol-sulfuric acid method [6, 7]. The 0.1 mol/L NaCl eluent was collected,concentrated,dialyzed in flowing tap-H2O (24 h) and distilled H2O (24 h) to remove salts. The retentate was lyophilized to obtain the purified polysaccharide termed HM41 for further study.
2.3. Homogeneity and molecular mass determination of HM41he homogeneity and molecular weight of HM41 were identified by high performance liquid chromatography equipped with a OHpak SB-805-HQ series gel column (molecular weight <4×106 ,8×1300 mm) and an RI2041 parallactic refraction detector (KNAUER Germany) [8]. The elution phase was 0.1 mol/ L NaNO3 and the flow rate was 0.8 mL/min. The polysaccharide sample was dissolved in the elution solvent and centrifuged. A 20 mL of the supernatant was injected in each run. The mean molecular weight was estimated according to the calibration curve made with the Dextran T-series standards (molecular weight 5000,10,000,20,000,40,000,70,000,110,000 and 500,000). During the process of experiment,the column was kept at 35 ℃. The molecular weight of HM41 was estimated with reference to the calibration curve prepared above.
2.4. Monosaccharide analysishe polysaccharide HM41 (1 mg) was hydrolyzed with 1 mL 4.0 mol/L TFA at 100 ℃ in a sealed tube for 4 h. After that,the removal of the excess amount of TFA was accomplished by coevaporation at reduced pressure with methanol added after reaction. The hydrolyzates were concentrated and dried,followed by successive reduction with NaBH4 and acetylation with Ac2Opyridine (1:1,v/v,1 mL) at 100 ℃ for 30 min. The resulting alditol acetates obtained were analyzed by GC as indicated above and identified by their typical retention time and electron impact profiles [9].
2.5. Periodate oxidation and smith degradationM41 (50 mg) was mixed with 0.04 mol/L NaIO4 (50 mL),kept at 4 ℃ for 8 days in the dark [10],and 0.1 mL aliquots were withdrawn at 3-6 h intervals,diluted to 25 mL with distilled water and read in a spectrophotometer at 223 nm. Excess periodate was decomposed by the addition of ethylene glycol (2 mL). The solution of periodate product (2 mL) was sampled to calculate the yield of formic acid by 0.01 mol/L NaOH. The rest was dialyzed against distilled H2O for 24 h. The solution was concentrated and reduced with NaBH4 (60 mg),and the mixture was left for 24 h at room temperature,neutralized to pH 6.0-7.0 with 50% acetic acid,dialyzed as described above,and was concentrated to a final volume (10 mL). The solutions were added to the same volume of 1 mol/L sulfuric acid,maintained for 40 h at 25 ℃,neutralized to pH 6.0 with barium carbonate,and filtered. The filtrate was dialyzed as mentioned previously,and the content out of the sack was desiccated for GC analysis; the content inside was diluted with ethanol,and after centrifugation,the supernatant and precipitate were also dried out for the GC analysis [11].
2.6. Methylation analysis of HM41he vacuum-desiccated HM41 (6 mg) was dissolved in anhydrousDMSO (2 mL),and methylated withMeI and anhydrous NaOH as a catalyst according to a modified Hakomori procedure [12],repeating the whole process twice. The fully methylated product showed noOHabsorption bands in the region of 3600-3300 cm-1 in the IR spectrum. The methylated HM41 was first hydrolyzed with 90% formic acid for 6 h at 100 ℃ and then with 2 mol/L TFA for 3 h at the same temperature. The hydrolyzate was then reduced with NaBH4,and the alditol acetate was prepared as usual [13]. The alditol acetate of the methylated sugar was analyzed by GC-MS using a DB-5MSfused silica capillary column(30m- 0.25 mm,film thickness: 0.25 mm). Partially methylated alditol acetates were identified by their fragment ions in EIMS,and the molar ratios were estimated from the peak areas and response factors [14].
2.7. NMR analysisor NMR measurements HM41 was dried in a vacuum over P2O5 for several days,and then exchanged with deuterium by lyophilizing with D2O several times [15]. The deuteriumexchanged polysaccharide (10 mg) was put in a 5-mm NMR tube and dissolved in 0.5 mL of 99.96% D2O. The 1 H NMR and 13C NMR spectra were recorded at room temperature with a Bruker AV-400 spectrometer.
2.8. Assay of hydroxyl radical scavenging activityenton reaction [16-18] was used to determine scavenging ability of hydroxyl radical by polysaccharide. The reaction mixture,containing salicylic acid (9 mmol/L,0.5 mL),Fe+(9 mmol/L,1 mL) and H2O2 (8.8 mol/L,0.5 mL). The HM and HM41 prepared were added to the above reaction mixture to scavenge the generated hydroxyl radical at 37 ℃ for 30 min,and then the absorbance value at 510 nm (Ax) was detected. The absorbance at 510 nm was measured against the blank (water instead of polysaccharide,A0). The scavenging percentage of hydroxyl radical was calculated according to the following equation:

|
(1) |
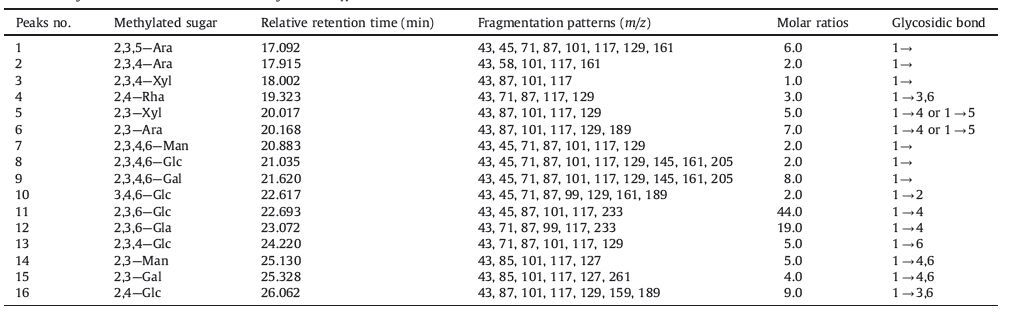
he crude polysaccharides HM from H. elliptica D. Don was obtained by extraction,deproteination,dialysis and precipitation. In order to obtain HM4 the deproteined polysaccharide HM was fractionated by acidic EtOH. The polysaccharide HM4 was further purified through DEAE-Sephadex A-25 column,leading to the isolation of a polysaccharide fraction,HM41. HM41 was obtained as a white powder soluble in water and insoluble in both acetone and ethanol solvents. The molecular weight distribution of HM41 was analyzed using high performance liquid chromatography (HPLC),and a typical HPLC profile is shown in Fig. 1. A single symmetrical narrow peak with elution time of about 12 min was observed,indicating good homogeneity for this polysaccharide. The HPLC analysis showed that the average molecular weight of HM41 was approximately 1.17×104 ,according to the molecular weight calibration curve [6].
|
Download:
|
| Figure 1. The HPLC profile of HM41. | |
3.2. Spectra analysis of HM41
lack of ultraviolet absorption at 280 nm confirmed that HM41 contained no proteins. HM41 showed absorption bands (Fig. S1 in Supporting information) at 3411,2975,1641,1300,1175-1040,891,and 761 cm-1 in the IR spectrum. The broad band at 1641 cm-1 was associated with absorbed water [6, 19]. The bands between 1175 and 1040 cm-1 were dominated by the glycosidic linkage (C-O-C) and (C-O-H) contributions. A band at 1300 cm-1 corresponded to a C-O stretch and C-H or OH bending. The weak band at 891 cm-1 was ascribed to b-pyranoid-type glycosidic linkages [4, 5]. X-ray diffraction measurement of HM41 show that the dispersion peak area is nearly amorphous except for having a small peak at 2u of 328. HM41 is in a polycrystalline amorphous state [20].
3.3. Monosaccharide analysis of HM41onosaccharide composition of HM41 was analyzed by gas chromatograph. Sugar analysis of HM41 revealed the presence of rhamnose,arabinose,xylose,mannose,galactose and glucose,at a molar ratio of 1.0:5.5:1.8:3.0:9.4:21,compared with sugar standards. The sulfate-carbazole method showed the absence of uronic acid.
3.4. Periodate oxidation and Smith degradation of HM41he periodate oxidation experiment showed that 1 mol of sugar residue consumed 1.16 mol of NaIO4 and produced 0.208 mol of formic acid. The consumption of NaIO4 was more than 2 times,the amount of formic acid produced indicating that HM41 not only contained linkages which can produce formic acid such as (1→)- and (1→6)-linked glycoside,but also contained some amount of a linkage type which can only consume NaIO4 such as (1→2)-,(1→4)-,(1→2,6)- and (1→4,6)-linked glycoside.
After Smith degradation,glycerol and erythritol were found by GC,indicating that HM41 contained linkages such as 1→,(1→2)-,(1→2,6)-,(1→6)-,(1→4,6)- and (1→4)-linked glycoside.
GC analysis for Smith degradation indicated that there was small amounts of glucose,demonstrating that HM41 contained small amounts of (1!3)-linked glucose. The results for periodate oxidation and Smith degradation was ambiguous and needs to be further investigated by another structural analysis.
3.5. Methylation and GC-MS analysiso further confirm the linkage of sugars,HM41 was methylated,reductively cleaved,and then the partially methylated alditol acetates were analyzed by GC-MS to show sixteen peaks (Fig. S2 in Supporting information).
The fragmentation patterns of these peaks were identified and the molar ratios were estimated (Table 1).
The GC-MS results (Table 1) indicated that HM41 had a branched structure. The main chain was mainly made up of b- (1→4)-linked-D-Gal,b-(1→4)-linked-D-Glc and b-(1→6)- linked-D-Glc. b-(1→4)-linked-D-Gal were substituted at 6-O and on average there were 14 branches among 23 main chain residues; b-(1→4)-linked-D-Glc had no branch; b-(1→6)-linked- D-Glc were substituted at 3-O and on average there were 9 branches among 14 main chain residues; The side chain was composed of (1→3,6)-Rha,(1→4) or (1→5)-Ara,(1→4) or (1→5)-Xyl,(1→4,6)-Man and (1→2)-Glc. The terminal residue was composed of Ara,Xyl,Man,Gal,and Glc. This was also in accordance with the results of the periodate oxidation and Smith degradation.
|
|
Table 1 GC–MS analysis of alditols derived from methylated HM41. |
3.6. Nuclear magnetic resonance spectroscopy of HM 41
n the anomeric region of the 1 H NMR spectrum of HM41 four signals occurred atδ 4.8-5.0. The 1H NMR chemical shifts were less than 5.0 ppm. This confirmed that b-pyranoid-type glycosidic linkages existed in HM41 (Fig. S3 in Supporting information).
In the 13CNMR spectrumofHM41 four carbon resonanceswere greater thanδ 101. This confirmed that b-pyranoid-type glycosidic linkages existed in HM41. This was also in accordance with the results of the 1H NMR spectrum and IR Spectrum. Four 13C NMR signals of HM41 appeared atδ 67-70,suggesting that substitution occurred at C6. Five signals appeared atδ 78-85,indicating that the substitution occurred at C2,C3,C4 [6, 21]. This was also in accordancewith the results of the periodate oxidation,Smith degradation and methylation (Fig. S4 in Supporting information).
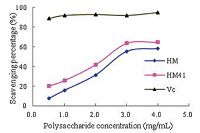
3.7. Scavenging activity on hydroxyl radical of HM and HM41ydroxyl radical is one of the most active free radical that attacks all the biological molecules (including lipids,proteins,carbohydrate and DNA) by setting off free radical chain reactions [22]. Fig. 2 shows the scavenging activity of hydroxyl radical by HM and HM41. At low concentration (0.5 mg/mL),a poor scavenging effect was shown for HM (7.49%) and HM41 (20.25%). Following an increase in concentration,the scavenging activity became more pronounced,reaching 58.06% for HM and 64.81% for HM41 at 4 mg/mL. However,the scavenging activity of these polysaccharides against the hydroxyl radical was less than that of Vc at the same concentrations. According to Zdenka et al. [23] the antioxidant ability of polysaccharide might be related to monosaccharide component,molecular weight,and conformation.
|
Download:
|
| Figure 2. The scavenging effect of polysaccharide HM and HM41 on OH. | |
4. Conclusion
A new water-soluble polysaccharide (HM41) had been successfully fractionated using DEAE-Sephadex A-25 chromatographic techniques. This new polysaccharide had a mean Mw value of about10 kDa,which composed mainly of glucose and galactose in the molar ration of 21:9.4. The backbone chain was b-(1→4)Gal,b-(1→4)Glc and b-(1!6)Glc. b-(1→4)Gal which were substituted at 6-O and there were 14 branches among 23 main chain residues on average; (1→4)Glc had no branch; (1!6)Glc were substituted at 3-O and there were 9 branches among 14 main chain residues on average; the branched chains were composed of (1→3,6)-Rha,(1→4) or (1→5)-Ara,(1→4) or (1→5)-Xyl,(1→4,6)-Man and (1→2)-Glc. Then,we preliminarily speculate HM and HM41 had antioxidant potential for using medical or health-care food based on the free radical scavenging assay.
| [1] | H.L. Yang, J.Q. Liu. Seven constituents in nine species used as Tibetan medicine "Zangyinchen". China Tradit. Herb Drugs 36 (2005) 1233–1237. |
| [2] | D. Zhang, Y.F. Zhu, S.K. Lin. Identification of new chemical constituents of Tibetan medicinal herb Halenia elliptica. China Tradit. Herb Drugs 34 (2003) 9–11. |
| [3] | X.Y. Qin, L. Yang, L.M. Shao, S.L. Liu. Inducement of the Callus of Halenia elliptica D. Don in Sichuan Province. Li Shi Zhen Med. Materia Med. Res. 19 (2008) 35–39. |
| [4] | Y. Liu, J. Zhao. Preliminary study on the physicochemical properties of the polysaccharide from Portobello Mushroom. Hubei Agric. Sci. 51 (2012) 981–983. |
| [5] | Y.J. Liu, H.Y. Zhu. Optimization of de-protein process for polysaccharide of Hippophae rhamnoides. J. Henan Agric. Sci. 3 (2008) 84–87. |
| [6] | N.S. Li, C.Y. Yan, D.H. Hua, et al. Isolation, purification, and structural characterization of a novel polysaccharide from Ganoderma capense. Int. J. Biol. Macromol 57 (2013) 285–290. |
| [7] | M. Dubois, K.A. Gilles, J.K. Hamilton, et al. , Colorimetric method for determination of sugars and related substances. Anal. Chem. 28 (1956) 350–360. |
| [8] | T. Li, Q.H. Zhang, W.B. Zhang. High Performance Liquid Chromatography (HPLC) Instrument System. Chemical Industry Press, Beijing (2009) 204–215. |
| [9] | G.Y. Xu, R.X. Cheng, L.W. Chang. Component analysis of monosaccharides in polysaccharides by capillary gas chromatography. J. Instrumental Anal. 19 (2000) 71–73. |
| [10] | Q. Dong, X. Liu, J. Yao. Structural characterization of a pectic polysaccharide from Nerium indicum flowers. Phytochemistry 71 (2010) 1430–1437. |
| [11] | Q. Li, Y. Xie, J.W. Su, et al. , Isolation and structural characterization of a neutral polysaccharide from the stems of Dendrobium densiflorum. Int. J. Biol. Macromol. 50 (2012) 1207–1211. |
| [12] | R.A. Kalyan, B.T. Paul. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 203 (1992) 101–108. |
| [13] | A. Linker, L.R. Evans, G. Impallomeni. The structure of a polysaccharide from infectious strains of Burkholderia cepacia. Carbohydr. Res. 335 (2001) 45–54. |
| [14] | G.L. Sassaki, P.A.J. Gorin, L.M. Souza, et al. , Rapid synthesis of partially O-methylated alditol acetate standards for GC-MS: some relative activities of hydroxyl groups of methyl glycopyranosides on Purdie methylation. Carbohydr. Res. 340 (2005) 731–739. |
| [15] | M.T. Duenas-Chasco, M.A. Rodriguez-Carvajal, P.T. Mateo, et al. , Structural analysis of the exopolysaccharides produced by Lactobacillus spp. G-77, Carbohydr. Res. 307 (1998) 125–133. |
| [16] | S.L. Wu, S.C. Xie, L. Yang, et al. , An experimental study on the production efficiency of hydroxyl radicals by several photocatalysis reaction systems. Photogr. Sci. Photochem. 2 (2006) 75–78. |
| [17] | D.R. Grymonpre, A.K. Sharma, W.C. Finney. The role of Fenton's reaction in aqueous phase pulsed streamer corona reactors. Chem. Eng. J. 82 (2001) 189–207. |
| [18] | F.X. Liu, Z.D. Li, N. Li, et al. , Determination techniques introduction on hydroxyl radical in Fenton method. Guangdong Chem. Ind. 23 (2007) 35–38. |
| [19] | Y.L. Wu, Y.J. Pan, C.R. Sun. Structural analysis of an alkali-extractable polysaccharide from the seeds of Retama raetamssp. Gussonei. Nat. Prod. 69 (2006) 1109–1112. |
| [20] | B. La, K. Xie, C.X. Hu. Studies on X-ray Fourier spectrum of Halenia elliptica D. Don. Qinghai Med. J. 34 (2004) 53–54. |
| [21] | Y.L. Sun, F. Shan, W.W. Cui, et al. , NMR analysis of polysaccharide ASP3 from Angelica sinensis (Oliv.) Diels and it's hydrolysis products. Chem. J Chin. Univ. 30 (2009) 1739–1743. |
| [22] | Z. Wu, H. Li, D.W. Tu, et al. , Extraction optimization, preliminary characterization, and in vitro antioxidant. Ind. Crop. Prod. 44 (2013) 145–151. |
| [23] | H. Zdenka, S.P. Berit, P. Martin, et al. , Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohydr. Polym. 93 (2013) 22–30. |
 2016, Vol. 27
2016, Vol. 27