b Chemistry Department, Qaemshahr Branch, Islamic Azad University, P.O. Box 163, Qaemshahr, Iran
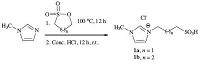
Ionic liquids (ILs),known as molten salts (consisting only of cations and anions) with melting point under 100-150 ℃ or room temperature,have attracted an increasing attentions in the context of green synthesis in recent years. ILs were introduced as an alternative green media for their unique properties,such as low vapour pressure being non-volatile,high thermal stability (up to about 300 ℃),wide liquid temperature range,low volatility,highly polar,non-flammability,large electrochemical window,miscible with certain organic solvents and/or water and good solubility of organic and inorganic materials,chemically inert,etc. [1-4]. In addition,the ionic liquids are readily recycled and tunable to specific chemical tasks. One type is Brønsted-acidic task-specific ionic liquids (BAILs). Also,ILs in the recent years have become more attractive in other fields such as formation of metal nanostructures [5],bioanalytical chemistry [6],catalysis [7],in basic electrochemical studies of organic compounds and inorganic compounds [8],analytical chemistry [9],sensors [10],and for electrochemical biosensors [11]. β-Azido alcohols are compounds of interest in organic synthesis,because they are precursors of vicinal amino alcohols or in the chemistry of carbohydrates,nucleosides,lactames,and oxazolines [12]. Also,b-azido alcohols are versatile intermediates in organic synthesis and increasingly important in drugs and pharmaceutics [13, 14]. A common method for the synthesis of a β-azido alcohols involves the regioselective ring opening of epoxide with sodiumazide under various reaction conditions. If the epoxide is unsymmetrical,azidolysis occurs via a bimolecular reaction at the least substituted carbon atom[15]. Several catalysts have been used to promote azidolysis of epoxides in the presence of sodium azide,including NaN3/3D-network polymer based on calix[4]resorcinarene [16],NaN3/CAN [17],NaN3/halohydrin dehalogenase [18],NaN3/(TBA)4PFeW11O39-3H2O [19],NaN3/[bmim]BF4/H2O [20],NaN3/SiO2-OPEG(300) [21],NaN3/PVA or PAA,poly(vinylamine) or poly(allylamine) [22],NaN3/CeCl3 [23],and NaN3/oxone [24]. It is worthmentioning some of thesemethods suffer fromdisadvantages such as long reaction times,difficulty in work-up and isolation of products,require high temperatures,using expensive catalysts and difficulty in preparation of catalysts,and low regioselectivity. Therefore,it seemsthat there is still aneedfordevelopment ofnewer methods that proceed under green and ecofriendly conditions and economically appropriate conditions. In this study,the Brønsted acidic ionic liquids 1a and 1b were prepared by condensation of methyl imidazole with 1,3-propane or 1,4-butane sultone and acidification of the zwitterion with concentrated HCl as shown in Scheme 1 [25].
|
Download:
|
| Scheme. 1. Synthesis of Brønsted acidic ionic liquids (1a, b) | |
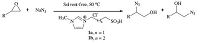
In continuation of our ongoing program to develop environmentally benign methods using ionic liquids as new promoters [26-31],we report herein an efficient and simple method for the ring opening of epoxide with sodium azide and consequently the corresponding β-azido alcohols under solvent-free conditions using 1-(1-alkylsulfonic)-3-methylimidazolium chloride as a reusable Brønsted acid catalyst (Scheme 2).
|
Download:
|
| Scheme. 2. Regioselective azidolysis of epoxides using Brønsted acidic ionic liquid (1a, b). | |
2. Experimental
Products were separated and purified by different chromatographic techniques and were identified by the comparison of their IR,NMR,and melting point with those reported for the authentic samples. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Avance (400 MHz) spectrometer using CDCl3 as the solvent and TMS as an internal standard. All chemical shifts are given asδ value with reference to Tetra methyl silane (TMS) as an internal standard. IR spectra were recorded on a Frontier Fourier transform infrared (FTIR) (Perkin Elmer) spectrometer using a KBr disk. Melting points were taken on an electrothermal capillary melting point apparatus and are uncorrected. The progress of reaction was followed with thin-layer chromatography (TLC) using silica gel SILG/UV 254 and 365 plate. All the solvents and reagents were purchased from Aldrich and Merck with high-grade quality and used without any purification.
2.1. Preparation of 1-(1-alkylsulfonic)-3-methylimidazolium chloride1-(1-Propylsulfonic)-3-methylimidazolium chloride was prepared via the condensation of 1-methylimidazole with 1,3-propanesultone and acidification of the resulting salt with concentrated HCl,according to the literature procedure [25]. To approximate the Brønsted acidity of the ILs,UV-vis spectroscopic measurement was used to determine the Hammett acidity.
2.2. Reactions of epoxides with NaN3 catalyzed Brønsted acidic ionic liquids 1a and 1bo a mixture of various epoxides (1mmol) in NaN3 (1.1mmol),BAIL 1a or 1b (5 mol%) was added; these were placed in a round bottomflask and stirredunder solvent-free conditions at 80 ℃. After complete consumption of epoxide monitored by TLC (using n-hexane/ethylacetate (5:1) as eluent),the catalyst was recovered for reuse by simple filtration,and the product was extracted with ethyl acetate (3×5mL2). The extract was dried over anhydrous Na2SO4,and concentrated under vacuum to obtain β-hydroxyazide in 85%-95% isolated yields. For styrene oxide,further purification was achieved by preparative TLC or by silica gel column chromatography. The spectral data of all the productswere identical with those of the authentic samples. The spectral data (1H NMR,13C NMR,and IR) of some representative compounds are given below,and the spectra are deposited in Supporting information.
1-Azido-3-phenoxy-2-propanol (Table 3,entry 1): IR vmax/ cm-1: 3420,3067,3036,2930,2878,2103,1599,1496,1291,1244,and 1045; 1H NMR (400 MHz,CDCl3):δ 3.45-3.54 (m,2H),3.89 (m,1H),3.97-4.03 (m,2H),4.18 (s,1H),6.95-7.00 (m,2H),7.02-7.06 (m,1H),and 7.27-7.36 (m,2H); 13C NMR (100 MHz,CDCl3):δ 53.5,69.2,69.3,114.3,121.1,129.4,and 158.3.
|
|
Table 3 Synthesis of b-azido alcohols using Brønsted acidic ionic liquids 1a and 1b. |
2-Azido-2-phenyl-1-ethanol (Table 3,entry 2): IR vmax/cm-1: 3373,3051,3025,2926,2102,1453,and 1070; 1H NMR (400 MHz,CDCl3):δ 3.37 (1H,s),3.74 (2H,m),4.65-4.69 (1H,m),and 7.34-7.44 (5H,m); 13C NMR (100 MHz,CDCl3):δ 66.37,68.03,127.49,128.46,128.61,and 136.47.
1-Azido-3-butoxy-2-propanol (Table 3,entry 11): IR vmax/ cm-1: 3445,2960,2932,2872,2102,1459,and 1121; 1H NMR (400 MHz,CDCl3):δ 0.87 (t,3H,J = 7.38 Hz),1.31-1.35 (m,2H),1.50-1.53 (m,2H),3.14 (s,1H),3.30-3.32 (m,2H),3.39-3.44 (m,4H),and 3.87 (m,1H); 13C NMR (100 MHz,CDCl3):δ 13.78,19.16,30.74,53.52,69.74,70.59,and 70.71.
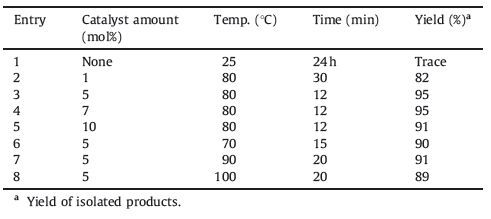
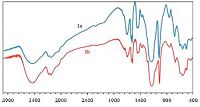
3. Results and discussionHerein we wish to report an extremely convenient and efficient method for regioselective ring opening of various epoxides using 1-(1-alkylsulfonic)-3-methylimidazolium chloride as a new,efficient,and recyclable catalyst under solvent-free conditions at 80 ℃. The molecular structure of the Brønsted acidic ionic liquids 1a and 1b were characterized via FT-IR analysis (Fig. 1). All the data of characterization are in accordance with the expected compositions andstructures. The O=S=O asymmetric and symmetric stretching modes of the sulfonic acid functional groups were found in the ranges 1227-1235 cm-1. Also,respectively one absorption is visible at 1400 cm-1 for C=C and 1637 for C=N cm-1 group. Also showed a broad OH stretching absorption around 3448 cm-1. After characterization of 1-(1-propylsulfonic)-3-methylimidazolium chloride [PSMIM]Cl 1a,as a model reaction,the reaction of phenyl glycidyl ether (1 mmol) with NaN3 (1.1 mmol) was tested using different amounts of [PSMIM]Cl at range of 70-100 ℃ in the absence of solvent. The results are summarized in Table 1,where it can be seen that this reaction was strongly influenced by the amount of catalyst. No product was obtained in the absence of the catalyst even after 24 h (Table 1,entry 1) indicating that the catalyst is necessary for the reaction. The best results were obtained when amount of the catalyst was 5 mol% at 80 ℃,and give the product in excellent yield and in short reaction time (Table 1,entry 3). Moreover,the product yield was not changed by increasing the amount of the catalyst (Table 1,entries 4 and 5). On the other hand,increasing the reaction temperature did not improve the results (Table 2,entries 7 and 8).
|
Download:
|
| Figure 1. The IR spectrum of Brønsted acidic ionic liquids 1a and 1b | |
|
|
Table 1 Effect of the catalyst amount and temperature on the reaction between phenyl glycidyl ether (1 mmol) with NaN3 (1.1 mmol). |
|
|
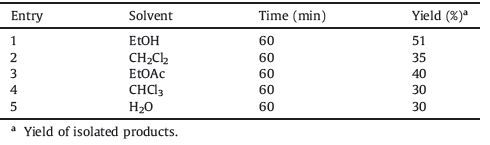
Table 2 Effect of various solvents on the reaction of phenyl glycidyl ether (1mmol) with NaN3 (1.1mmol) in the presence of [PSMIM]Cl (5 mol%). |
To improve the yield of the target product versus the solventfree procedure,the reaction between phenyl glycidyl ether (1 mmol) with NaN3 (1.1 mmol) using [PSMIM]Cl (5 mol%) was checked in various solvents (5 mL) at 80 ℃ and the results are presented in Table 2. As can be seen from Table 2,low yields of the product were obtained in solution conditions even after elongated reaction times.
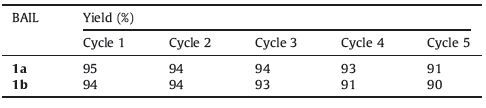
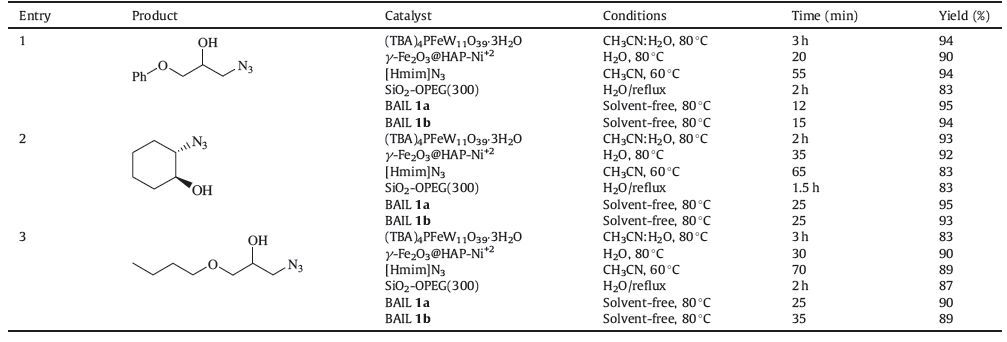
After optimization of the reaction conditions,various aromatic and aliphatic epoxides (1 mmol) reacted smoothly with sodium azide (1.1 mmol) in the presence of BAIL 1a and 1b at 80 ℃ under similar reaction conditions to produce the corresponding β-azido alcohols in excellent yields and short reaction time (Table 3). In general,Table 3 shows the results that clarify the fact that the reaction proceeds very efficiently in all cases. Various epoxides underwent ring opening easily in the presence of BAIL 1a and 1b at 80 ℃. Bicyclic epoxides such as cyclopentene,cyclohexene,and cyclooctene epoxides also underwent cleavage with sodium azide to produce β-azido alcohols in high yields. The products were formed in high yields (85%-95%) in short reaction time (12-35 min) for BAIL 1a and high yield (85%-95%) in short reaction time (15-35 min) for BAIL 1b. Environmental-friendly ionic liquid afforded a valuable alternative to promote numerous efficient catalytic systems that have already been proposed for the ring opening of epoxides. The products obtained were purified by silica gel column chromatography and characterized by spectroscopic (1H NMR,13C NMR,and IR). The infrared spectra of the 1-azido-3- phenoxy-2-propanol exhibit a band at 3420 cm-1 assigned to O-H and 2103 cm-1 assigned to N3. The good reusability of a catalyst is important aspect of green chemistry and environmental points of view. So the potential for recovery of BAIL 1a and 1b were investigated. The activities of the recovered catalyst were carefully investigated through the reaction of phenyl glycidyl ether (1 mmol) with NaN3 (1.1 mmol) using BAIL 1a and 1b at 80 ℃. After completion of the reaction,the product was extracted in to ethyl acetate; catalyst from aqueous layer was separated by simple filtration,washed with MeOH,dried and reused. The catalyst was reused for five times without any significant changes in the yields and the reaction times Table 4. To show the merit of the present work in comparison with reported results in the literature,we compared results of BAIL 1a and 2b with (TBA)4PFeW11O39-3H2O [19],[bmim]BF4/H2O [20],SiO2-OPEG(300) [21],and [Hmim]N3 [32],for the preparation of β-azido alcohols derivatives. As shown in ,Table 5,the present methodology offers several advantages,such as excellent yields,a simple procedure,short reaction times,easy synthesis,simple work-up and greener conditions,in contrast with other existing methods.
|
|
Table 4 The reusability of BAIL 1a and 1b for the preparation of 1-azido-3-phenoxy-2- propanol. |
|
|
Table 5 Comparison of β-azido alcohols of some epoxides with different catalysts. |
4. Conclusion
In conclusion,a new 1-(1-alkylsulfonic)-3-methylimidazolium chloride was synthesized and its application as an efficient,green,and reusable Brønsted acid catalyst for regioselective ring opening of epoxides to β-azido alcohols by azide anion under solvent-free conditions at 80 ℃. The advantages of the present protocol,such as being an environmentally friendly alternative,the short reaction times,high yield of products,simple work-up procedure,and excellent regioselectivity. Also,the catalyst was recyclable and has been reused for five successive runs with little loss of the catalytic activities.
| [1] | J.S. Wilkes. Properties of ionic liquid solvents for catalysis. J. Mol. Catal.: A 214 (2004) 11–17. |
| [2] | T. Welton. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 99 (1999) 2071–2084. |
| [3] | K.N. Marsh, J.A. Boxall, R. Lichtenthaler. Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilib. 219 (2004) 93–98. |
| [4] | A. Zare, A. Hasaninejad, A. Parhami, et al. , Ionic liquid, 1-butyl-3-methylimidazolium bromide (. J. Serb. Chem. Soc. 75 (2010) 1315–1324. |
| [5] | A.L. Bhatt, A.Mechler, L.L.Martin, A.M. Bond. Synthesis of Ag and Au nanostructures in an ionic liquid: thermodynamic and kinetic effects underlying nanoparticle, cluster and nanowire formation. J. Mater. Chem. 17 (2007) 2241–2250. |
| [6] | S. Park, R.J. Kazlauskas. Biocatalysis in ionic liquids—advantages beyond green technology. Curr. Opin. Biotechnol. 14 (2003) 432–437. |
| [7] | T. Welton. Ionic liquids in catalysis. Coord. Chem. Rev. 248 (2004) 2459–2477. |
| [8] | M.C. Buzzeo, R.G. Evans. Non-haloaluminate room-temperature ionic liquids in electrochemistry—a review. Chem. Phys. Chem. 5 (2004) 1106–1120. |
| [9] | J.F. Liu, J.A. Jonsson, J.B. Jiang. Application of ionic liquids in analytical chemistry. Trends Anal. Chem. 24 (2005) 20–27. |
| [10] | R. Wang, T. Okajima, F. Kitamura, T. Ohsaka. A novel amperometric O2 gas sensor based on supported room-temperature ionic liquid porous polyethylene membrane-coated electrodes. Electroanalysis 16 (2004) 66–72. |
| [11] | Y. Liu, L. Shi, M. Wang, et al. , A novel room temperature ionic liquid sol-gel matrix for amperometric biosensor application. Green Chem. 7 (2005) 655–658. |
| [12] | S.W. Chen, S.S. Thakur, W. Li, et al. , Efficient catalytic synthesis of optically pure, 1,2-azido alcohols through enantioselective epoxide ring opening with HN3,. J. Mol. Catal. 259 (2006) 116–120. |
| [13] | J.S. Yadav, B.V.S. Reddy, A.K. Basak, A.V. Narsaiah. Bmim]BF4 ionic liquid: a novel reaction medium for the synthesis of β-amino alcohol. Tetrahedron Lett 44 (2003) 1047–1050. |
| [14] | B.T. Smith, V.Gracias, J. Aube. Regiochemical studies of the ring expansion reactions of hydroxy azides with cyclic ketones. J. Org. Chem. 65 (2000) 3771–3774. |
| [15] | M. Chini, P. Crotti, F. Macchia. Efficient metal salt catalyzed azidolysis of epoxides with sodium azide in acetonitrile. Tetrahedron Lett. 31 (1990) 5641–5644. |
| [16] | A. Mouradzadegun, A.R. Kiasat, P. Kazemian Fard. 3D-network porous polymer based on calix[4]resorcinarenes as an efficient phase transfer catalyst in regioselective conversion of epoxides to azidohydrines. Catal. Commun 29 (2012) 1–5. |
| [17] | N. Iranpoor, F. Kazemi. Regioselective azidolysis of epoxides catalyzed with Ce(IV). Synth. Commun. 29 (1999) 561–566. |
| [18] | J.H.L. Spelberg, J.E.T.H. Vlieg, L. Tang, D.B. Janssen, R.M. Kellogg. Highly enantioselective and regioselective biocatalytic azidolysis of aromatic epoxides. Org. Lett. 3 (2001) 41–43. |
| [19] | B. Yadollahi, H. Danafar. A facile synthesis of, 1,2-azidoalcohols by (TBA)4PFe-W11O39·3H2O catalyzed azidolysis of epoxides with NaN3. Catal. Lett. 113 (2007) 120–123. |
| [20] | J.S. Yadav, B.V.S. Reddy, B. Jyothirmai, M.S.R. Murty. Ionic liquids/H2O systems for the reaction of epoxides with NaN3: a new protocol for the synthesis of, 2-azidoalcohols. Tetrahedron Lett. 46 (2005) 6559–6562. |
| [21] | A.R. Kiasat, M. Fallah-Mehrjardi. An efficient catalyst-free ring opening of epoxides in PEG-300: a versatile method for the synthesis of vicinal Azidoalcohols. J. Iran. Chem. Soc. 6 (2009) 542–546. |
| [22] | B. Tamami, M. Kolahdoozan, H. Mahdavi. Regioselective azidolysis of epoxides in water using poly(vinylamine) and poly(allylamine) as new polymeric cosolvents. Iran. Polym. J. 13 (2004) 21–28. |
| [23] | G. Sabitha, R.S. Babu, M. Rajkumar, J.S. Yadv. Cerium(III) chloride promoted highly regioselective ring opening of epoxides and aziridines using NaN3 in acetonitrile: a facile synthesis of, 1,2-azidoalcohols and 1,2-azidoamines. Org. Lett. 4 (2002) 343–345. |
| [24] | G. Sabitha, R.S. Babu, M.S.K. Reddy, J.S. Yadv. Ring opening of epoxides and aziridines with sodium azide using oxone® in aqueous acetonitrile: a highly regioselective azidolysis reaction. Synthesis 15 (2002) 2254–2258. |
| [25] | A.S. Amarasekara, O.S. Owereh. Hydrolysis and decomposition of cellulose in Brønsted acidic ionic liquids under mild conditions. Ind. Eng. Chem. Res. 48 (2009) 10152–10155. |
| [26] | S. Rezayati, R. Hajinasiri, Z. Erfani. Microwave-assisted green synthesis of, 1,1-diacetates (acylals) using selectfluorTM as an environmental-friendly catalyst under solvent-free conditions. Res. Chem. Intermed. DOI, 10.1007/s11164-015-2168-1. |
| [27] | S. Rezayati, F. Sheikholeslami-Farahani, F. Rostami-Charati, S. Afshari Sharif Abad. One-pot synthesis of coumarine derivatives using butylenebispyridinium hydrogen sulfate as novel ionic liquid catalyst. Res. Chem. Intermed. Doi: 10.1007/s11164-015-2261-5. |
| [28] | H.S. Haeri, S. Rezayati, E. Rezaee Nezhad, H. Darvishi. Fe2+ supported on hydroxyapatite-core-shell-γ-Fe2O3 nanoparticles: efficient and recyclable green catalyst for the synthesis of, 14-aryl-14H-dibenzo[a,j]xanthenederivatives. Res. Chem. Intermed. Doi:10.1007/s11164-015-2318-5. |
| [29] | S. Rezayati, S. Sajjadifar. Recyclable boron sulfonic acid as an environmentally benign catalyst for the one-pot synthesis of coumarin derivatives under solventfree condition. J. Sci. I. Repub. Iran. 25 (2014) 329–337. |
| [30] | S. Rezayati, Z. Erfani, R. Hajinasiri. Phospho sulfonic acid as efficient heterogeneous Brønsted acidic catalyst for one-pot synthesis of, 14-dibenzo. Chem. Pap. 69 (2015) 536–543. |
| [31] | S. Sajjadifar, S. Rezayati. Synthesis of, 1,1-diacetates catalysed by silica-supported boron sulfonic acid under solvent-free conditions and ambient temperature. Chem. Pap. 68 (2014) 531–539. |
| [32] | F. Heidarizadeh, A. BeitSaeed, E. Rezaee Nezhad. Synthesis of, 1,2-azidoalcohols from epoxides using a task-specific protic ionic liquid: 1-hydrogen-3-methylimidazolium azide. C.R. Chim. 17 (2014) 450–453. |
 2016, Vol. 27
2016, Vol. 27