The prevalent structure of quinoline derivatives is fundamental in heterocyclic compounds,and these key structural units have been shown to have pharmaceutical applications [1-5]. Especially 1,2-dihydroquinolines,which can be transformed to 1,2,3,4- tetrahydroquinolines through the reduction reaction [6, 7],were widely synthesized due to their special biological activity and the characteristics of the drug intermediates. To date,catalysts including transition metals [8-16],Brønsted acids [17],Lewis acids [18] and iodine [19],etc. have been reported for achieving 1,2- dihydroquinoline derivatives. In 2001,Shibasaki and co-workers first reported that a bifunctional Lewis acid was able to catalyze the asymmetric addition of cyanide to various substituted quinolones (isoquinoline) to give the correspondingReissert compounds [20]. In 2003,Hamada et al. used N-protected o-aminobenzaldehydes and α,β-unsaturated carbonyl compounds for the preparation of 1,2-dihydroquinolines inthe presence of a quaternaryammonium salt [21]. In 2007,Co´ rdova et al. demonstrated the asymmetric aza- Michael/aldol reaction between 2-aminobenzaldehydes and a,bunsaturated aldehydes for the synthesis of 1,2-dihydroquinolidines using a chiral amine catalyst [22]. Subsequently,several similar approaches for the synthesis of dihydroquinoline by chiral amine catalysts or bifunctional thiourea catalysts were independently reported [23-25]. Good yields and ee were reported utilizing chemical catalysis. In consideration of the great significance of 1,2- dihydroquinolidines,development of new methods with environmentally friendly and sustainable catalysts to form this important structure is still desired.
Enzymes,as a kind of green catalyst for modern organic synthesis,have attracted increased attention. Enzyme catalytic promiscuity is the functional property of an enzymeto catalyze an otherwise unnatural reaction,using the same active site responsible for its natural activity. Enzyme catalytic promiscuity widens the scope of enzyme use in organic synthesis and allows for the discovery of new synthetic methods [26, 27]. Continuing research has shown that many enzymes exhibit catalytic promiscuity [28]. Some examples of the use of enzyme promiscuity, such as enzyme-catalyzed aldol [29-34],Henry [35-37], Mannich [38-42],Povarov [43] and domino reactions [44, 45],etc. have been reported.
Pepsin,a kind of hydrolase,belongs to the family of aspartic acid protease [46, 47] and is present during chemical digestion of protein. In the 1930s,Northrop crystallized swine pepsin supplying convincing evidence for its identity as a protein. The purified pepsin provided the needed evidence for confirming its peptide structure which is characteristic of proteins [48]. Pepsin-catalyzed aldol reactions have been developed [32, 33]. Herein,we report a novel example of enzyme catalytic promiscuity using pepsin from porcine gastric mucosa to catalyze the domino aza-Michael/aldol reaction for the synthesis of 1,2-dihydroquinolidines.
2. ExperimentalPepsin from porcine gastric mucosa [EC 3.4.23.1,P7000-25g,Lot #050M1304V,powder,49.0% protein (UV),920 units/mg protein, and P7125-100g,Lot #SLBD7698V,powder,18.0% protein (UV), 721 units/mg protein; one unit will produce a ΔA280 nm of 0.001 per min at pH 2.0 at 37 ℃,measured as TCA-soluble products using hemoglobin as substrate. (Final vol. = 16 mL. Light path = 1 cm)] were purchased from Sigma-Aldrich. Recombinant pepsin expressed in E. coli was purchased from Hangzhou Biosci Biotech Co.,Ltd. Other chemical reagents and solvents were purchased from commercial vendors,and used without any further purification unless otherwise stated.
Flash column chromatography was carried out using 200-300 mesh silica gel at increased pressure. The NMR spectra were recorded with TMS as the internal standard in CDCl3 on a Bruker Avance 600 Spectrometer (600 MHz 1H,150 MHz 13C) at room temperature. In each case,the enantiomeric excess was determined by chiral HPLC analysis on Chiralpak AD-H,IA and Chiralcel OD-H in comparison with authentic racemates. Highresolution mass spectra were obtained using an ESI ionization source (Varian 7.0T FTICR-MS). All reactions were monitored by thin-layer chromatography (TLC) with Haiyang GF254 silica gel plates.
General procedure for the pepsin-catalyzed domino aza- Michael/aldol reactions: To a mixture of 2-aminobenzaldehyde (0.30 mmol),α,β-unsaturated aldehyde (0.26 mmol),pepsin (12.3 kU) and DMF (0.5 mL),deionized water (0.3 mL) was added. The resultant mixture was stirred for the specified time at 40 ℃, and monitored by TLC analysis. The reaction was terminated by filtering the enzyme. Ethyl acetate was employed to wash the residue on the filter paper to assure that products obtained were all dissolved in the filtrate. The filtrate was washed with saturated brine three times,and the combined organic layers were dried over anhydrous Na2SO4,and concentrated under vacuum. The residue was purified by flash column chromatography on silica gel using a mixture of petroleum ether and ethyl acetate ratio 3:1-20:1 as eluent.
3. Results and discussionThrough a large number of screenings,we found that pepsin from porcine gastric mucosa could catalyze aza-Michael/aldol reaction of 2-aminobenzaldehyde and cinnamaldehyde. Thus,this reaction was used as a model to investigate the influence of different parameters on the pepsin-catalyzed aza-Michael/aldol reaction. In view of the fact that the reaction medium plays an important role in the enzymatic reactions [49],different solvents were screened (Table 1,entries 1-15). Based on the experimental data,pepsin showed certain catalytic activity,not only in polar solvents,but also in nonpolar solvents in the model reaction. The highest yield of 32% was obtained in 1,4-dioxane (Table 1,entry 1). The best enantioselectivity of 16% ee was observed in DMF,ethanol, and methanol,respectively (Table 1,entries 2,7 and 9). Among them,the yield of 31% was obtained in DMF. Considering both yield and selectivity,DMF was selected as a suitable solvent for further investigation.
|
|
Table 1 Solvent screening and control experiments.a |
Next,to confirm the specific catalytic effect of pepsin on the aza- Michael/aldol reaction,some control experiments were performed (Table 1,entries 16-21). The blank experiment was conducted and only a trace amount of the desired product observed (Table 1,entry 16). To exclude the possibility that the catalytic activity of the pepsin for the aza-Michael/aldol reaction could arise from the catalysis of an unspecific amino acid residue on the surface of the enzyme [50], albumin from chicken egg white,representing a protein without an enzymatic function,was used as a catalyst in the model reaction, and only gave the product in 2% yield without ee (Table 1,entry 17). Therefore,it can be assumed that the protein surface of pepsin is predominately catalytically inactive in the process. Enzymes maintain their native tertiary structures mainly through a combination of coordinated hydrogen bonding,hydrophobic, electrostatic,steric,and other interactions [51]. Heavy metal ions, ascommon denaturationagents,can inactivate enzymes by reacting with some structural groups (e.g.,-SH groups) resulting in irreversible damage,or interacting with some amino acid residues causing changes in three-dimensional structure. Thus,metal ions Ag+ and Cu2+ were employed to pretreat the pepsin,separately, and then the pretreated pepsin was used to catalyze the model reaction,which gave only trace amounts of product (Table 1,entries 18 and 19). Another denaturation agent,guanidine hydrochloride (GuHCl),also can change the conformational structure of enzymes, and ultimately denature the enzyme; the reaction with GuHCl pretreated pepsin only gave a trace amount of product (Table 1, entry 20). These control experiments indicated that the threedimensional structure of the enzyme was responsible for its catalytic activity in the domino reaction. Moreover,according to the literature [52],the active site of pepsin from porcine gastric mucosa contains Asp32 and Asp215 residues. Carbonyldiimidazole (CDI),an irreversible inhibitor of aspartic acid,which can bond covalently with the carbonyl of aspartic acid was used to pretreat pepsin,and the reaction with CDI pretreated pepsin only gave the product in a low yield of 4% without ee (Table 1,entry 21). This result implied that the enzyme-catalyzed domino aza-Michael/aldol reaction may occur at the active site of the enzyme,or in close proximity. The above experiments demonstrated that inhibition and denaturation of pepsin caused a nearly complete disruption of the catalytic activity of the enzyme. As a consequence, it can be concluded that pepsin has the ability to catalyze the domino aza-Michael/aldol reaction with a certain degree of enantioselectivity,and the native structure of the enzyme is responsible for its activity and aspartic acids may play a key role in this catalysis event.
To rule out the possibility of catalysis by the presence of some impure proteins,we purchased the pepsin from porcine gastric mucosa,recombinant,expressed in E. coli for comparison. The purity of this protein was checked by SDS-PAGE (for the SDS-PAGE image,please see the Supporting information),which showed a clear pepsin band with a molecular weight of 35 kDa and that the protein was quite pure. Then,recombinant pepsin was used to catalyze the model domino reaction providing yields of 45% and 14% ee (Table 1,entry 22). This result clearly confirmed that pepsin indeed has the ability to catalyze the reaction.
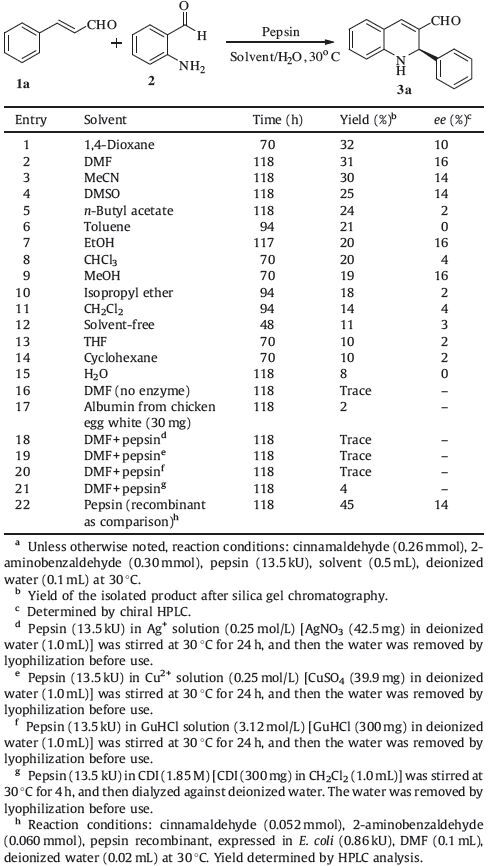
Usually enzymes require water molecules to maintain their optimum spatial structure via hydrogen bonding and other noncovalent interactions,thus,a small amount of water is often required for enzymatic reactions in non-aqueous medium. Consequently,the influence of water content on pepsin-catalyzed domino reaction in DMF was investigated (Fig. 1). It can be seen that the yield of the product tended to rise as the addition of water increased from 0 to 0.3 mL in DMF (0.5 mL),and further increases of water additions caused a decrease in yield. The best yield of 41% with 16% ee was obtained when 0.3 mL of water was added to the reaction system. The addition of water had slight effects on the enantioselectivity of the reaction. Thus 0.3 mL of water in DMF (0.5 mL) was chosen as the optimal reaction medium for the following study.
Hereto,our supply of the enzyme preparation (pepsin from porcine gastric mucosa,Sigma-Aldrich,P7000-25g,Lot #050M1304V) used for the above investigations (Table 1 and Fig. 1) was exhausted. We then continued the study using another enzyme preparation (Pepsin from porcine gastric mucosa, Sigma-Aldrich,P7125-100g,Lot #SLBD7698V) for the following investigation.
|
Download:
|
| Figure 1. Influence of water addition on the pepsin-catalyzed domino reaction. | |
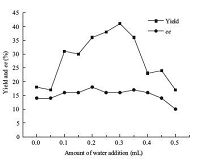
The influence of catalyst loading on the pepsin-catalyzed domino reaction of 2-aminobenzaldehyde and cinnamaldehyde was surveyed (Fig. 2). The best yield of 35% with 18% ee was obtained when 12.3 kU of enzyme was used in the reaction system, which was similar to the results obtained with the former enzyme preparation (13.5 kU of enzyme,product yield 41% with 16% ee, Fig. 2). On the other hand,these results demonstrated that this procedure was not only applicable to a specific enzyme preparation, but also to other preparations of the same enzyme. Thus, 12.3 kU of enzyme was selected as a suitable catalyst loading for the reaction system discussed.
|
Download:
|
| Figure 2. Influence of enzyme loading on the pepsin-catalyzed domino reaction. | |
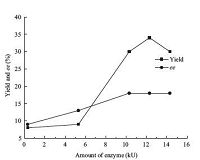
Temperature has effects on the selectivity and rate of the reaction,and also on the stability of the enzyme. Thus,the influence of temperature on the pepsin-catalyzed model domino reaction was examined (Fig. 3),demonstrating that a lower temperature was beneficial to improve the enantioselectivity and 18% ee was obtained at 30-40 ℃. A higher temperature was beneficial to improve the yield,and a yield of 45% was received at 40-50 ℃. Therefore,40 ℃ was chosen as the optimal temperature for the pepsin-catalyzed domino reaction.
|
Download:
|
| Figure 3. Influence of temperature on the pepsin-catalyzed domino reaction | |
After having established the optimal reaction conditions,the scope generality of this pepsin-catalyzed domino aza-Michael/aldol reaction was explored (Table 2). As documented in the table,a variety of aliphatic and aromatic substituents in the α,β-unsaturated aldehydes were well tolerated,leading to the corresponding products in moderate to good yields with low enantioselectivity (Table 2,entries 1-13). Different electron-donating and electronwithdrawing aromatic α,β-unsaturated aldehydeswere used to test the influence of electronic effects of substituents on the reaction (Table 2,entries 2-7),and some aromatic α,β-unsaturated aldehydes with substituents in a different position of the benzene ring were also investigated to examine the steric effects of substituents on the reaction (Table 2,entries 5,8 and 10). Both electronic and steric effects of aromatic α,β-unsaturated aldehydes had no obvious impact on the yield and enantioselectivity of the reaction. The reactions with aliphatic α,β-unsaturated aldehydes, trans-2-hexenal and trans-2-heptenal,gave the products in yields of 57% and 45% with 22% and 24% ee,respectively (Table 2,entries 11 and 12). It was notable that trans-4-oxo-2-butenoate was successfully utilized in the reaction providing the desired product in 97% yield with 6% ee (Table 2,entry 13).
In summary,we developed a biocatalytic strategy to synthesize 1,2-dihydroquinoline derivatives. Pepsin from porcine gastric mucosa has the ability to promote the enantioselective domino aza-Michael/aldol reaction of α,β-unsaturated aldehydes and 2-aminobenzaldehyde. The desired products were obtained in yields of 38%-97% with [3TD$DIF]6%-24% ee. Although the yields and stereoselectivities were not thoroughly satisfactory in comparison with those reported by chemical catalysis, this is the first reported study utilizing pepsin to catalyze the aza-Michael/aldol reaction to afford 1,2-dihydroquinoline derivatives. The enzyme-catalyzed domino reaction showed the comprehensive advantages of biocatalysis,such as mild reaction conditions and reduced toxicity to humans and the environment. As a proof of the concept,this work provides a novel case of a promiscuous enzyme-catalyzed enantioselective domino reaction. Meanwhile,this finding broadens the application of pepsin in organic synthesis.
| [1] | P. Bohn, F. Gourand, C. Papamicaël, et al. , Dihydroquinoline carbamate derivatives as "bio-oxidizable" prodrugs for brain delivery of acetylcholinesterase inhibitors:[11C] radiosynthesis and biological evaluation. ACS Chem. Neurosci. 6 (2015) 737–744. |
| [2] | M.A. Cawthorne, E.D. Palmer, J. Green. Effect of, 6-ethoxy 2,2,4-trimethyl-1,2-dihydro-quinoline (ethoxyquin) on carbon tetrachloride metabolism in the rat. Biochem. Pharmacol. 22 (1973) 783–788. |
| [3] | E.A. Couladouros, A.T. Strongilos. Generation of libraries of pharmacophoric structures with increased complexity and diversity by employing polymorphic scaffolds. Angew. Chem. Int. Ed. 41 (2002) 3677–3680. |
| [4] | Z. Gan, P.T. Reddy, S. Quevillon, S. Couve-Bonnaire, et al. , Stereocontrolled solidphase synthesis of a, 90-membered library of indoline-alkaloid-like polycycles from an enantioenriched aminoindoline scaffold. Angew. Chem. Int. Ed. 44 (2005) 1366–1368. |
| [5] | R. Pougeois, M. Satre, P.V. Vignais. N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline, a new inhibitor of the mitochondrial F1-ATPase. Biochemistry 17 (1978) 3018–3023. |
| [6] | M. Anzini, A. Cappelli, S. Vomero, et al. Novel, potent, and selective 5-HT3 receptor antagonists based on the arylpiperazine skeleton: synthesis, structure, biological activity, and comparative molecular field analysis studies. J. Med. Chem. 38 (1995) 2692–2704. |
| [7] | J.V. Johnson, B.S. Rauckman, D.P. Baccanari, et al. , X-ray analyses of aspartic proteinases: II. Three-dimensional structure of the hexagonal crystal form of porcine pepsin at, 2.3Åresolution. J. Med. Chem 32 (1989) 1942–1949. |
| [8] | J. Barluenga, M. Fañanás-Mastral, F. Aznar, et al. [1,5]-Hydride transfer/cyclizations on alkynyl Fischer carbene complexes: synthesis of 1,2-dihydroquinolinyl carbene complexes and cascade reactions. Angew. Chem. Int. Ed. 47 (2008) 6594–6597. |
| [9] | P. Kothandaraman, S.J. Foo, P.W.H. Chan. Gold-catalyzed intramolecular allylic amination of, 2-tosylaminophenylprop-1-en-3-ols. A concise synthesis of (±)-angustureine. J. Org. Chem 74 (2009) 5947–5952. |
| [10] | G. Lu, J.L. Portscheller, H.C. Malinakova. Palladacycles with a metal-bonded sp3-hybridized carbon as intermediates in the synthesis of, 2,2,3,4-tetrasubstituted 2H-1-benzopyrans and 1,2-dihydroquinolines. Effects of auxiliary ligands and substitution at a palladium-bonded tertiary carbon. Organometallics 24 (2005) 945–961. |
| [11] | Y. Luo, Z. Li, C.J. Li. A silver-catalyzed domino route toward, 1,2-dihydroquinoline derivatives from simple anilines and alkynes. Org. Lett. 7 (2005) 2675–2678. |
| [12] | N. Purkait, S. Blechert. Synthesis of bi-and tricyclic, 1,2-dihydroquinoline derivatives from arylamines and alkynes by a consecutive zinc-ammonium salt catalysis. Adv. Synth. Catal. 354 (2012) 2079–2083. |
| [13] | M. Rueping, J. Dufour, L. Bui. Convergent catalysis: asymmetric synthesis of dihydroquinolines using a combined metal catalysis and organocatalysis approach. ACS Catal. 4 (2014) 1021–1025. |
| [14] | S. Schach, B. Tshisuaka, S. Fetzner, et al. Quinoline 2-oxidoreductase and 2-oxo-1,2-dihydroquinoline 5,6-dioxygenase from comamonas testosteroni 63. Eur. J. Biochem 232 (1995) 536–544. |
| [15] | H. Yanai, H. Mimura, K. Kawada, et al. , Convenient synthesis of fluorinated quinoline,, 1,2-dihydroquinoline, and 1,2,3,4-tetrahydroquinoline derivatives. Tetrahedron 63 (2007) 2153–2160. |
| [16] | X. Zeng, G.D. Frey, R. Kinjo, et al. , Synthesis of a simplified version of stable bulky and rigid cyclic (alkyl)(amino)carbenes, and catalytic activity of the ensuing gold(I) complex in the three-component preparation of, 1,2-dihydroquinoline derivatives. J. Am. Chem. Soc. 131 (2009) 8690–8696. |
| [17] | M. Lachia, S. Iriart, M. Baalouch, et al. , Ethyl-2-(2-chloroethyl)acrylate: a new very versatile α-cyclopropylester cation synthon. Efficient synthesis of cyclopropane ester derivatives by Michael addition-induced cyclization reaction,. Tetrahedron Lett 52 (2011) 3219–3222. |
| [18] | Z. Wang, S. Li, B. Yu, et al. FeCl3·6H2O-catalyzed intramolecular allylic amination: synthesis of substituted dihydroquinolines and quinolines. J. Org. Chem 77 (2012) 8615–8620. |
| [19] | B.-Q. Zhang, Y. Luo, Y.-H. He, et al. , Highly efficient synthesis of polysubstituted, 1,2-dihydroquinolines via cascade reaction of a-ketoesters with arylamines mediated by iodine. Tetrahedron 70 (2014) 1961–1966. |
| [20] | M. Takamura, K. Funabashi, M. Kanai, et al. , Catalytic enantioselective Reisserttype reaction: development and application to the synthesis of a potent NMDA receptor antagonist (-)-L-689,560 using a solid-supported catalyst. J. Am. Chem. Soc. 123 (2001) 6801–6808. |
| [21] | K. Makino, O. Hara, Y. Takiguchi, et al. , Efficient construction of, 1,2-dihydroquinoline and 1,2,3,4-tetrahydroquinoline rings using tandem Michael-aldol reaction. Tetrahedron Lett. 44 (2003) 8925–8929. |
| [22] | H. Sundén, R. Rios, I. Ibrahem, et al. , A highly enantioselective catalytic domino aza-Michael/aldol reaction: one-pot organocatalytic asymmetric synthesis of, 1,2-dihydroquinolidines. Adv. Synth. Catal. 349 (2007) 827–832. |
| [23] | C. Bhanja, S. Jena, S. Nayak, et al. , Organocatalytic tandem Michael addition reactions: a powerful access to the enantioselective synthesis of functionalized chromenes, thiochromenes and, 1,2-dihydroquinolines. Beilstein J. Org. Chem. 8 (2012) 1668–1694. |
| [24] | X. Liu, Y. Lu. Bifunctional thiourea-promoted cascade aza-Michael-Henry-dehydration reactions: asymmetric preparation of, 3-nitro-1,2-dihydroquinolines. Org. Biomol. Chem. 8 (2010) 4063–4065. |
| [25] | Y.F. Wang, W. Zhang, S.-P. Luo, et al. , One-pot organocatalytic asymmetric synthesis of, 3-nitro-1,2-dihydroquinolines by a dual-activation protocol. Chem. Asian J. 4 (2009) 1834–1838. |
| [26] | A. Aharoni, L. Gaidukov, O. Khersonsky, et al. The ‘evolvability' of promiscuous protein functions. Nat. Genet 37 (2005) 73–76. |
| [27] | K. Hult, P. Berglund. Enzyme promiscuity: mechanism and applications. Trends Biotechnol. 25 (2007) 231–238. |
| [28] | Z. Guan, L.Y. Li, Y.H. He. Hydrolase-catalyzed asymmetric carbon-carbon bond formation in organic synthesis. RSC Adv. 5 (2015) 16801–16814. |
| [29] | C. Branneby, P. Carlqvist, A. Magnusson, et al. , Carbon-carbon bonds by hydrolytic enzymes. J. Am. Chem. Soc. 125 (2003) 874–875. |
| [30] | J.P. Fu, N. Gao, Y. Yang, et al. , Ficin-catalyzed asymmetric aldol reactions of heterocyclic ketones with aldehydes. J. Mol. Catal. B: Enzym. 97 (2013) 1–4. |
| [31] | C. Li, X.W. Feng, N. Wang, et al. , Biocatalytic promiscuity: the first lipase-catalysed asymmetric aldol reaction. Green Chem. 10 (2008) 616–618. |
| [32] | C. Li, Y.J. Zhou, N. Wang, et al. , Promiscuous protease-catalyzed aldol reactions: a facile biocatalytic protocol for carbon-carbon bond formation in aqueous media. J. Biotechnol. 150 (2010) 539–545. |
| [33] | L.Y. Li, D.C. Yang, Z. Guan, et al. , Pepsin-catalyzed direct asymmetric aldol reactions for the synthesis of vicinal diol compounds. Tetrahedron 71 (2015) 1659–1667. |
| [34] | Y. Yuan, Z. Guan, Y. He. Biocatalytic direct asymmetric aldol reaction using proteinase from Aspergillus melleus. Sci. China Chem. 56 (2013) 939–944. |
| [35] | N. Gao, Y.L. Chen, Y.H. He, et al. , Highly efficient and large-scalable glucoamylasecatalyzed Henry reactions. RSC Adv. 3 (2013) 16850–16856. |
| [36] | M. Gruber-Khadjawi, T. Purkarthofer, W. Skranc, et al. , Hydroxynitrile lyasecatalyzed enzymatic nitroaldol (Henry) reaction. Adv. Synth. Catal. 349 (2007) 1445–1450. |
| [37] | T. Purkarthofer, K. Gruber, M. Gruber-Khadjawi, et al. , A biocatalytic Henry reaction-the hydroxynitrile lyase from hevea brasiliensis also catalyzes nitroaldol reactions. Angew. Chem. Int. Ed. 45 (2006) 3454–3456. |
| [38] | S.J. Chai, Y.F. Lai, H. Zheng, et al. , A novel trypsin-catalyzed three-component Mannich reaction. Helv. Chim. Acta 93 (2010) 2231–2236. |
| [39] | Z. Guan, J. Song, Y. Xue, et al. , Enzyme-catalyzed asymmetric Mannich reaction using acylase from Aspergillus melleus. J. Mol. Catal. B: Enzym. 111 (2015) 16–20. |
| [40] | T. He, K. Li, M.Y. Wu, et al. , Utilization of biocatalytic promiscuity for direct Mannich reaction. J. Mol. Catal. B: Enzym. 67 (2010) 189–194. |
| [41] | K. Li, T. He, C. Li, et al. , Lipase-catalysed direct Mannich reaction in water: utilization of biocatalytic promiscuity for C-C bond formation in a "one-pot" synthesis. Green Chem. 11 (2009) 777–779. |
| [42] | Y. Xue, L.P. Li, Y.H. He, et al. , Protease-catalysed direct asymmetric Mannich reaction in organic solvent. Sci. Rep. 2 (2012) 761. |
| [43] | L.P. Li, X. Cai, Y. Xiang, et al. , The a-chymotrypsin-catalyzed Povarov reaction: one-pot synthesis of tetrahydroquinoline derivatives. Green Chem. 17 (2015) 3148–3156. |
| [44] | T. He, Q.Q. Zeng, D.C. Yang, et al. , Biocatalytic one-pot three-component synthesis of, 3,3'-disubstituted oxindoles and spirooxindole pyrans using α-amylase from hog pancreas. RSC Adv. 5 (2015) 37843–37852. |
| [45] | C.-H. Wang, Z. Guan, Y.-H. He. Biocatalytic domino reaction: synthesis of, 2H-1-benzopyran-2-one derivatives using alkaline protease from Bacillus licheniformis. Green Chem. 13 (2011) 2048–2054. |
| [46] | J.B. Cooper, G. Khan, G. Taylor, et al. , X-ray analyses of aspartic proteinases: II. Three-dimensional structure of the hexagonal crystal form of porcine pepsin at, 2.3Åresolution. J. Mol. Bio 214 (1990) 199–222. |
| [47] | X.L. Lin, R.N. Wong, J. Tang. Synthesis, purification, and active site mutagenesis of recombinant porcine pepsinogen. J. Biol. Chem 264 (1989) 4482–4489. |
| [48] | J.S. Fruton. A history of pepsin and related enzymes. Q. Rev. Biol. 77 (2002) 127–147. |
| [49] | A.M. Klibanov. Enzymatic catalysis in anhydrous organic solvents. Trends Biochem. Sci. 14 (1989) 141–144. |
| [50] | G.A. Strohmeier, T. Sovic′, G. Steinkellner, et al. , Investigation of lipase-catalyzed Michael-type carbon-carbon bond formations. Tetrahedron 65 (2009) 5663–5668. |
| [51] | J.C. Lewis. Artificial metalloenzymes and metallopeptide catalysts for organic synthesis. ACS Catal. 3 (2013) 2954–2975. |
| [52] | D.R. Dee, S. Filonowicz, Y. Horimoto, et al. , Recombinant prosegment peptide acts as a folding catalyst and inhibitor of native pepsin. BBA Proteins Proteom. 1794 (2009) 1795–1801. |
 2016, Vol. 27
2016, Vol. 27