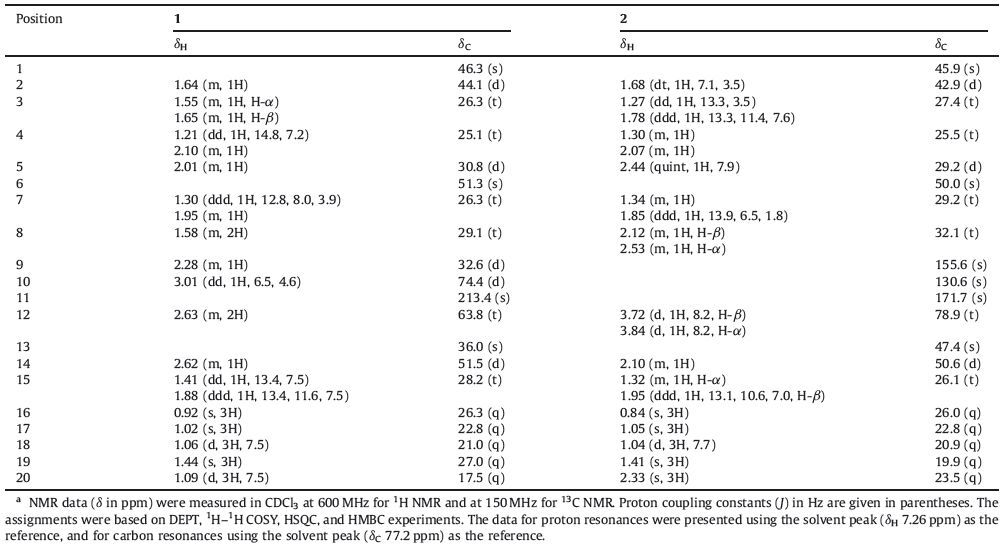
Trichoderma spp. are widely used as biocontrol microbes against plant pathogens for their production of a wide range of antibiotic substances [1, 2]. Their secondary metabolites exhibit multiple biological properties,such as immune system suppressor, plant growth promoter and regulator,anti-aging agents and so on [3]. Previously,an endophytic fungus was isolated and identified as Trichoderma sp. from a mangrove plant Xylocarpus granatum by our research group and a known harziane diterpene harzianone was isolated from the ethyl acetate (EtOAc) extract of the mycelia and filtrate [4]. Harzianone contained a unique tetracyclic scaffold with fused four-,five-,six-,and seven-membered carbon rings and exhibited 82.6% lethality in the brine shrimp toxicity assay [5]. To the best of our knowledge,there are only seven members of harziane tetracyclic diterpene family reported,including harzianone [5],harziandione [6],four harziane-related diterpenes [7] and a diterpenoid lactone [8]. Considering the structural novelty and biological activity of this kind of compounds,60 L fermentation of Trichoderma sp. Xy24 was performed for the search of this kind of compounds. Consequently,two new harziane diterpenoids, namely,(9R,10R)-dihydroharzianone (1) and harzianelactone (2) (Fig. 1) were obtained. Biogenetically,1 was the reductive product of harzianone and 2 was the Baeyer-Villiger monooxygenase catalyzed oxidation product of harzianone. Herein,we report the detailed isolation,structure elucidation and biological activity of the two new compounds.
|
Download:
|
| Figure 1. Structures of harzianone, (9R,10R)-dihydro-harzianone (1) and harzianelactone (2). | |
2. Experimental 2.1. General experimental procedures
Optical rotations were measured on a Perkin-Elmer Model-343 digital polarimeter. The CD and UV spectra were recorded on a JASCO J-815 spectropolarimeter. IR spectra were acquired on a Nicolet 5700 FT-IR microscope spectrometer (FT-IR Microscope Transmission). 1D and 2D NMR spectra were obtained at 600 MHz for 1H NMR and 150 MHz for 13C NMR on a VNOVA SYSTEM-600 spectrometer. Chemical shifts (δ ) are given in ppm,and coupling constants (J) are given in hertz (Hz). HR-ESI-MS data were measured using an Agilent Technologies 6520 Accurate Mass QTOF LC/MS spectrometer. Column chromatography (CC) was carried out with silica gel (200-300 mesh,Qingdao Marine Chemical Inc. Qingdao,PR China). Semi-preparative HPLC was performed on a Shimadzu HPLC instrument equipped with a Shimadzu RID-10A detector and a Grace Adsorbosphere C18 column (250 mm×10 mm,i.d.,5 mm) by eluting with mixtures of CH3OH and H2O or CH3CN and H2O. Analytical TLC was carried out on pre-coated silica gel GF254 plates (Qingdao Marine Chemical Industry,Qingdao,China),and spots were visualized under UV light or by spraying with10% H2SO4 in 90% EtOH followed by heating at 120 ℃.
2.2. Fungal material and fermentationThe fungal strain Trichoderma sp. Xy24 was isolated from the leaves,stems and peels of mangrove plant X. granatum collected in Sanya district,Hainan province of China. It was identified as Trichoderma sp. Xy24 according to the morphological and molecular (ITS1-5.8S-ITS2 rDNA sequence) analyses by our research group [9]. The strain was deposited at the Institute of Materia Medica,Chinese Academy of Medical Sciences.
The fungal strain was maintained on slants of modified potato dextrose agar (PDA) medium (potato 200 g,glucose 20 g,distilled water 1 L,KH2PO4 3 g,MgSO4 0.75 g,vitamin B1 10 mg,agar 8.0 g, pH 6.0; the media were autoclaved at 115 ℃ for 30 min) at 4 ℃. Seed cultures were performed in Erlenmeyer flasks (250 mL) containing 100 mL of PDA liquid medium on a shaker at 150 rpm at 25 ℃ for 2 days,after that 5 mL seed cultures were inoculated into each 1000 mL flask with 300 mL medium and cultivated for 14 days (150 rpm,25 ℃).
2.3. Extraction and isolationThe cultures (60 L) were filtered under reduced pressure to afford the filtrate and mycelia. The filtrate was applied to an Amberlite XAD-16 macroporous adsorbent resin column by eluting with H2O and 90% EtOH successively and then gained the residue (30.0 g) under reduced pressure. The dried mycelia were extracted with methanol by the ultrasonic extraction method to afford 92.0 g of residue. The residues of the two parts were combined for further separation based on their identical TLC profiles. The combined extract (122.0 g) was partitioned with petroleum ether. The petroleum ether extract was evaporated under reduced pressure to yield 30.0 g of residue,which was subjected to silica gel column chromatography (CC) eluting with a petroleum ether-EtOAc gradient (100:0,50:1,20:1,10:1,5:1,3:1,1:1,1:3,0:100) to give nine fractions based on TLC analysis.
Fraction 2 (5.2 g) was initially subjected to silica gel CC eluting with a detailed petroleum ether-EtOAc gradient (100:0,200:1, 100:1,50:1,10:1,3:1) to give six fractions (Fr2.1-Fr2.6). Fr2.2 (185.3 mg) was further separated by reversed-phase semipreparative HPLC with CH3CN/H2O (85:15,v/v) at 3 mL/min to give 1 (10.3 mg,tR 28.9 min). Fr2.3 (79.2 mg) was further separated by reversed-phase semi-preparative HPLC with CH3OH/H2O (90:10,v/v) at 3 mL/min to give 2 (2.4 mg,tR 21.8 min).
(9R,10R)-Dihydro-harzianone (1): colorless oil; [α]D 20-48:6 (c 0.19,MeOH); CD (MeOH) 304 (Δε- 1.04) nm; IR (cm-1): vmax 3021,2933,1775,1741,1461,1383,1198,1135,1007,979,937, 925; 1H NMR (CDCl3,600 MHz) and 13C NMR (CDCl3,150 MHz) data see Table 1; HR-ESI-MS m/z 289.2518 [M + H]+ (calcd. for C20H33O,289.2526).
|
|
Table 1 NMR spectroscopic data for compounds 1 and 2a. |
Harzianelactone (2): colorless solid,[α]D 20 þ7:5 (c 0.35,MeOH); IR (cm-1): vmax 3028,2933,2926,2896,1740,1649,1478,1382, 1254,1167,1031,976,910,779; UV (MeOH) lmax (log e): 233.0 (0.67) nm; 1H NMR (CDCl3,600 MHz) and 13C NMR (CDCl3, 150 MHz) data see Table 1; HR-ESI-MS m/z 303.2310 [M + H]+ (calcd. for C20H31O2,303.2319).
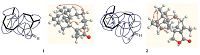
3. Results and discussionCompound 1 was obtained as colorless oil. Its molecular formula was determined to be C20H32O by HR-ESI-MS at m/z of 289.2518 [M + H]+ (calcd. for C20H33O,289.2526),corresponding to 5 degrees of unsaturation. The IR spectrum showed absorption band for carbonyl group at 1775 cm-1. The 1H NMR (Table 1) spectrum and HSQC data displayed three methyl singlets at δ H 0.92 (s,3H),1.02 (s,3H) and 1.44 (s,3H); two methyl doublets at δ H 1.06 (d,3H,J = 7.5 Hz) and 1.09 (d,3H,J = 7.5 Hz). The 13C NMR and DEPT spectra showed 20 carbon resonances (Table 1),which consisted of four quaternary carbons (δ C 213.4,51.3,46.3 and 36.0, including one carbonyl carbon),five methine carbons (δ C 74.4, 51.5,44.1,32.6 and 30.8),six methylene carbons (δ C 63.8,29.1, 28.2,26.3,26.3 and 25.1),and five methyl carbons (δ C 27.0,26.3, 22.8,21.0 and 17.5). The NMR data were closely related to those of harzianone,except for the replacement of a methyl singlet (δ H 2.07, s) in harzianone by a methyl doublet (δ H 1.09,d,J = 7.5 Hz) in compound 1,and the appearance of two additional methine protons at δ H 2.28 (m,1H) and δ H 3.01 (dd,1H,J = 6.3 Hz,4.8 Hz) in the 1H NMR spectrum of compound 1. The 13C NMR spectrum of compound 1 displayed two methine carbons at δ C 74.4 (appearing in lower field affected by carbonyl negative shielding effect,as well as C-12 at δ C 63.8) and 32.6 in the replacement of two olefinic carbons at δ C 150.2 (C-10) and δ C 146.6 (C-9) in harzianone. All the data above indicated that compound 1 was the reductive product of harzianone. The structure of compound 1 was supported by 2 D NMR 1H-1H COSY and HMBC correlations shown in Fig. 2.
|
Download:
|
| Figure 2. 1H–1H COSY (-), key HMBC (→) and NOESY (↔) correlations of (9R,10R)-dihydroharzianone (1) and harzianelactone (2). | |
The configuration of 1 was deduced by NOEs (Fig. S10 in Supporting information) and the CD spectrum (Fig. S13 in Supporting information). The irradiation of H-16 enhanced H-14 and H-17,the irradiation of H-20 enhanced H-14 and H-16,and the irradiation of H-19 enhanced H-5 and H-10. These observations indicated that H-16,H-17,H-14 and H-20 were syn-oriented,while H-19,H-5 and H-10 were on the opposite side. The negative Cotton curve at 304 nm arising from carbonyl n→π* transition in the CD spectrum of compound 1 suggested R configuration for C-10 by the ketone octant rule [10, 11]. Thus,the absolute configuration of 1 was assigned as 2S,5R,6R,9R,10R,13S,14S,and the structure of 1 was determined as (9R,10R)-dihydroharzianone,a reductive product of harzianone.
Compound 2 was obtained as colorless powder,and it gave a HR-ESI-MS ion peak at m/z of 303.2310 [M + H]+ (calcd. for C20H31O2,303.2319),which corresponded to a molecular formula of C20H30O2. The 1H NMR spectroscopic analysis of 2 were similar to those of harzianone except that H2-12 shifted downfield to δ H 3.84 (d,J = 8.2 Hz) and 3.72 (d,J = 8.2 Hz) from δ H 2.51 (d, J = 16.2 Hz) and2.36 (d,J = 16.2 Hz) in harzianone. In the 13CNMR spectrum,the carbon resonance of C-12 shifted downfield to δ C 78.9 from δ C 60.1 in harzianone and a ketone carbonyl at δ C 199.5 in harzianone was replaced by an ester carbonyl at δ C 171.7. All these data together with the biogenetic postulation suggested that compound 2 should be a lactone,which was biosynthesized from harzianone by a Baeyer-Villiger monooxygenase catalyzed oxidation reaction. The assignmentwas further supported by theHMBCcorrelations (Fig. 2) ofH-12/C-19,C-13,C- 14,C-10 and C-11.
The configuration of 2 was determined by NOEs (Fig. 2 and Fig. S21 in Supporting information) and the comparison of the optical rotation data. In the 1D NOESY experiments,the integration value of H-19 was enhanced when H-5 was irradiated,and H-14 was enhanced when H-16 was irradiated,which were consistent with those of harzianone [5]. Compound 2 gave a similar optical rotation,[α]D 20 +5.7° (c 0.35,MeOH),to that of the harzianone [5]. Thus,the absolute configurations at C-2,C-5,C-6,C-13,and C- 14 in 2 were proposed to be 2S,5R,6R,13S,and 14S,identical to those of harzianone. Therefore,the structure of compound 2 was determined as harzianelactone (Fig. 1).
Compounds 1 and 2 were biologically evaluated for in vitro cytotoxicity against four human cancer cell lines (HeLa,MCF-7, HCT-8,and Bel-7402) by using an MTT method [12, 13] with taxol as the positive control. Compound 1 exhibited selective activity against HeLa and MCF-7 cell lines with IC50 values of 30.1 mmol/L and 30.7 mmol/L,respectively; whereas 2 showed no activity against these cell lines at a concentration of 10 mmol/L.
4. ConclusionHarziane-type diterpenes belong to a scarce compound family containing a unique tetracyclic scaffold with fused four-,five-,six-, and seven-membered carbon rings. Currently,they are only reported from Trichoderma spp. In this communication,we described the isolation and identification of two new members of harziane-type diterpenoids (9R,10R)-dihydroharzianone (1) and harzianelactone (2) from a mangrove plant endophytic fungus Trichoderma sp. Xy24,which should be the reductive product and Baeyer-Villiger monooxygenase catalyzed oxidation product of harzianone,respectively. These results indicate the structural diversity of this type of compounds in nature.
| [1] | G.E. Harman, C.R. Howell, A. Viterbo, I. Chet, M. Lorito. Trichoderma species— opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2 (2004) 43–56. |
| [2] | J.L. Reino, R.F. Guerrero, R. Hernández-Galá n, I.G. Collado. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 7 (2008) 89–123. |
| [3] | C. Keswani, S. Mishra, B.K. Sarma, S.P. Singh, H.B. Singh. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl. Microbiol. Biotechnol. 98 (2014) 533–544. |
| [4] | M. Zhang, N. Li, R.D. Chen, et al. Two terpenoids and a polyketide from the endophytic fungus Trichoderma sp. Xy24 isolated from mangrove plant Xylocarpus granatum. J. Chin. Pharm. Sci. 23 (2014) 421–424. |
| [5] | F.P. Miao, X.R. Liang, X.L. Yin, G. Wang, N.Y. Ji. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org. Lett. 14 (2012) 3815–3817. |
| [6] | E.L. Ghisalberti, D.C.R. Hockless, C. Rowland, A.H. White, Harziandione, a new class of diterpene from Trichoderma harzianum. J. Nat. Prod. 55 (1992) 1690–1694. |
| [7] | E. Adelin, C. Servy, M.T. Martin, et al. , Bicyclic and tetracyclic diterpenes from a Trichoderma symbiont of Taxus baccata. Phytochemistry 97 (2014) 55–61. |
| [8] | Z.L. Xie, H.J. Li, L.Y. Wang, et al. Trichodermaerin, a new diterpenoid lactone from the marine fungus Trichoderma erinaceum associated with the sea star Acanthaster planci. Nat. Prod. Commun. 8 (2013) 67–68. |
| [9] | N. Li, F.Y. Ruan, Z.S. Wen, et al. , Diversity and in vitro antitumor activity of endophytic fungi from mangrove plants Xylocarpus. Chin. J. Chin. Mater. Med 38 (2013) 2282–2286. |
| [10] | W. Moffitt, R.B. Woodward, A. Moscowitz, W.T. Klyne, C. Djerassi. Structure and the optical rotatory dispersion of saturated ketones. J. Am. Chem. Soc. 83 (1961) 4013–4018. |
| [11] | A. Hassner, L.R. Krepski. Cycloadditions., 26. Regio-and stereochemistry of the cycloadditions of dichloroketene to 2-methyl-and 3-methyl-2-cholestene,. J. Org. Chem 44 (1979) 1376–1379. |
| [12] | T. Mosmann. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65 (1983) 55–63. |
| [13] | J. Carmichael, W.G. Degraff, A.F. Gazdar, J.D. Minna, J.B. Mitchell. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 47 (1987) 936–943. |
 2016, Vol. 27
2016, Vol. 27