Functionalized heterocyclic building blocks are of great importance in both medicinal and synthetic chemistry and development of new efficient synthetic methodologies for these scaffolds remains a great challenge in modern organic synthesis [1]. It is well known that several drugs exploit heterocyclic systems and are often nitrogen-containing with both five- and sixmembered rings.
For example,some derivatives of thiazolo[3,2-a]pyrimidines are known to exhibit versatile biological activity,which include anticancer [2],antitumor [3],antiinfl ammatory [4],antinociceptive [5],antiviral [6],and antibiofilm properties [7]. Owing to these remarkably broad pharmacological properties,a variety of synthetic methods have been reported for the preparation of thiazolo[3,2-a] pyrimidinone derivatives [6-10].
In addition,thienopyridine [11] and their annulated with heterocycles [12] have attracted widespread interest owing to their presence in natural products,and their biological and pharmacological activities.
The Pictet-Spengler reaction [13] has become one of the most prominent strategies for carbon-carbon bond formation in synthetic organic chemistry with excellent functional group tolerance,regio- and stereo-selectivity. From this perspective, the modified Pictet-Spengler reactions are attained considerable important for the synthesis of various products and novel heterocycles of biological interest [14].
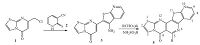
In view of the above observations and continuing our previous works on develop new methodologies for the preparation of fused heterocyclic compounds [15],herein we report the synthesis of some new condensed heterocyclic systems: pyrido[3",2":4',5'] thieno [3',2':2,3]pyrido[4,5:d][1, 3]thiazolo[3,2-a]pyrimidine-4- one derivatives by the application of Pictet-Spengler reaction (Scheme 1).
|
Download:
|
| Scheme. 1. Syntheses of fused thiazolopyrimidines | |
2. Experimental 2.1. Preparation of 2-(3-aminothieno[2,3-b]pyridin-2-yl)thiazolo [3,2-a]pyrimidin-5-one (3)
To a solution of 7-(chloromethyl)-5H-thiazolo[3,2-a]pyrimidin- 5-one 1 [16] (20.0 mmol) in DMF (25 mL) was added 3- cyanopyridine-2-thione 2 (4.11 g,30.0 mmol) and anhydrous potassium carbonate (5.52 g,40.0 mmol). The mixture was heated at 80℃ for 4 h. After cooling to room temperature,water (50 mL) was added and stirred for 20 min. The solid was filtered and recrystallized from HOAc to give 3 in 83% yield. Mp > 300℃. IR (KBr,cm-1):v 34265 (NH),3356 (NH),1686 (C=O). 1H NMR (400 MHz,DMSO-d6):δ 6.05 (s,1H),7.43-7.48 (m,3H),7.50 (d,1H, J = 4.8 Hz),8.01 (d,1H,J = 4.8 Hz),8.49 (d,1H,J = 7.6 Hz),8.61 (d,1H,J = 8.0 Hz). Anal. Calcd. for C13H8N4OS2: C 51.98,H 2.68, N 18.65. Found: C 52.05,H 2.76,N 18.73.
2.2. Preparation of pyrido[3",2":4',5'] thieno[3',2':2,3]pyrido-[4,5:d][1, 3]thiazolo[3,2-a]pyrimidine-4-one derivativesmixture of 2-(3-aminothieno[2,3-b]pyridin-2-yl)thiazolo[3,2-a]yrimidin-5-one 3 (1.0 mmol),aromatic aldehyde 4 (1.0 mmol) and sulfamic acid (SA) (10 mg,0.1 mmol) in DMF (15 mL) was heated for 7-16 h at 100℃. After the completion of the reaction judged by TLC analysis,the reaction mixture was cooled to room temperature. Water (50 mL) was added and the mixture was stirred for 30 min. The solid was filtered and recrystallized from DMF to afford the corresponding products (5a-l) [17].
3. Results and discussionIn this study,the key intermediate amine 2-(3-aminothieno[2,3-b]pyridin-2-yl)thiazolo[3,2-a]pyrimidin-5-one 3 was obtained by the condensation of 7-(chloromethyl)-5Hthiazolo[3,2-a]yrimidin-5-one 1 with 3-cyanopyridine-2-thione 2 via Thorpe-Ziegler isomerization [18] in good yield. Its structure was determined from the spectral data as well as elemental analysis.
In an initial endeavor,we selected benzaldehyde 4a as a model aldehyde to react with an equimolar of the intermediate amine 3a for the preparation of pyrido[3",2":4',5'] thieno[3',2':2,3]pyrido - [4,5:d][1, 3]thiazolo[3,2-a]pyrimidine-4-one 5a and investigated the optimal reaction conditions. The effects of solvents,catalysts and temperature were evaluated for this reaction,and the results are summarized in Table 1. It was found that the reaction could not proceed in DMF under catalyst-free conditions (entry 1). Later,the reaction was performed in the presence of catalytic amounts of sulfamic acid (H2NSO3H,SA,10 mol%),in the presence of different solvents,moderate yields of the product were obtained (entries 2-5). Notably,DMF was found to be the best solvent providing 74% of the product with in 10 h (entry 5). After obtaining the desired product in good yields,the amount of SA and the temperature required for this reaction were evaluated. The reaction was performed using 15 mol%,20 mol%,and 30 mol% of sulfamic acid in DMF at 100℃. It was found that while increasing the amount of SA, the yields increased from 86% to 85%,and 80%,respectively (entries 6-8). Later the reaction was conducted at different temperatures,it was observed that while increasing the temperatures from 110 to 120,and 130℃. The yields also increased from 84% to 82%,and 78%,respectively (entries 9-11). In addition,several other acids were then evaluated for their catalytic efficiency in this reaction. The results indicated that SA provided a superior catalytic effect to p-TsOH,TFA,and H2SO4 (entries 12-14). Therefore,it could be concluded that 15 mol% of sulfamic acid in DMF at 100℃ are optimum conditions for this transformation.
|
|
Table 1 Optimization of reaction conditions on the synthesis of pyrido[3",2":4',5'] thieno[3',2':2,3]pyrido [4,5:d][1,3]thiazolo[3,2-a]pyrimidine-4-one 5aa. |
Under the optimized conditions,a wide range of aromatic aldehydes 4 underwent this one-pot condensation with of 2-(3- aminothieno[2,3-b]pyridin-2-yl)thiazolo[3,2-a]pyrimidin-5-one 3 to give the corresponding fused thiazolo[3,2-a]pyrimidinones 5.
As shown in Table 2,this protocol could be applied not only to the aromatic aldehydes with either electron with drawing groups (such as halide groups) or electrondonating groups (such as alkyl, hydroxy groups),but also to heterocyclic aldehydes. Therefore,we concluded that the electronic nature of the substituents of aldehydes has no significant effect on this reaction. Furthermore, when an alphatic aldehyde was treated with 3,the desired products 5m and 5n were also obtained in 57% and 60%, respectively (entries 13,14).
|
|
Table 2 Synthesis of fused thiazolo[3,2-a]pyrimidinones 5. |
All the products were characterized by IR,1HNMR,13CNMRand elemental analysis. And all the data are consistent with the proposed structures.
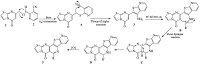
Based on these results,a plausible mechanism for the construction of fused thiazolo[3,2-a]pyrimidinones is proposed (Scheme 2). The formation of thioethers A occurs through S-alkylation of 7-(chloromethyl)-5H-thiazolo[3,2-a]pyrimidin-5- one 1 and 3-cyanopyridine-2-thione 2. Then,the thioether A occurred via Thorpe-Ziegler isomerization reaction to generate 2-(3-aminothieno[2,3-b][2TD$DIF]-pyridin-2-yl)thiazolo[3,2-a]pyrimidin- 5-one 3. Next,the intermediate amine 3 underwent a cationic p-cyclization with aldehyde under Pictet-Spengler cyclization to form D,which effects aromatisation to give pentacyclic product 5.
|
Download:
|
| Scheme. 2. A proposed mechanism for the formation of fused thiazolo[3,2-a]pyrimidinones 5. | |
4. Conclusion
In summary,we have developed an efficient method for the synthesis of pharmacologically important,functionalized fused thiazolo[3,2-a]pyrimidinones derivatives in two steps. The synthetic approach involves a Thorpe-Ziegler isomerization of 7-(chloro methyl)-5H-thiazolo[3,2-a]pyrimidin-5-one followed by a Pictet-Spengler cyclization of the corresponding 2-(3- aminobenzofuran-2-yl)thiazolo[3,2-a]pyrimidin-5-one with aromatic aldehydes in the presence of NH2SO3H. This method has the advantages of using utilizes easily accessible starting materials, mild reaction conditions,straightforward product isolation and good yields.
| [1] | A. Nefzi, J.M. Ostresh, R.A. Houghten. The current status of heterocyclic combinatorial libraries. Chem. Rev. 97 (1997) 449–472. |
| [2] | E.E. Flefel, M.A. Salama, M. El-Shahat. A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation, Phosphorus. Sulfur Silicon Relat. Elem. 182 (2007) 1739–1756. |
| [3] | A.A. Abu-Hashem, M.M. Youssef, H.A.R. Hussein. Synthesis, antioxidant, antituomer activities of some new thiazolopyrimidines, pyrrolothiazolo pyrimidines and triazolopyrrolothiazolopyrimidines derivatives. J. Chin. Chem. Soc. 58 (2011) 41–48. |
| [4] | B. Tozkoparan, M. Ertan, P. Kelicen, R. Demirdamar. Synthesis and anti-inflammatory activities of some thiazolo[3,2-a]pyrimidine derivatives. II Farmaco 54 (1999) 588–593. |
| [5] | O. Alam, S.A. Khan, N. Siddiqui, W. Ahsan. Synthesis and pharmacological evaluation of newer thiazolo[3,2-a]pyrimidines for anti-inflammatory and antinociceptive activity. Med. Chem. Res. 19 (2010) 1245–1258. |
| [6] | S.F. Mohamed, E.M. Flefel, A.E. Amra, D.N. Abd El-Shafy. Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur. J. Med. Chem. 45 (2010) 1494–1501. |
| [7] | B. Pan, R. Huang, L. Zheng, et al. , Thiazolidione derivatives as novel antibiofilm agents: design, synthesis, biological evaluation, and structure-activity relationships. Eur. J. Med. Chem. 46 (2011) 819–824. |
| [8] | I.V. Kulakov. Intramolecular cyclization of, 4-aryl-3,4-dihydropyrimidine-(1H)-2-thiones to give bicyclic thiazolo[3,2-a]pyrimidines. Chem. Heterocycl. Compd. 45 (2009) 1019–1021. |
| [9] | A.E. Abbas, Z. Mahdieh, R.F. Ali, H. Azizollah. An efficient regioselective synthesis of highly functionalized, 3-oxo-2,3-dihydro-5H-thiazolo[3,2-a] pyrimidines via an isocyanide-based three-component reaction. Tetrahedron Lett. 53 (2012) 1351–1353. |
| [10] | E.A. Abd El-Galil, S.S. Maigali, M.M. Abdulla. Synthesis, and analgesic and antiparkinsonian activities of thiopyrimidine, pyrane, pyrazoline, and thiazolopyrimidine derivatives from 2-chloro-6-ethoxy-4-acetylpyridine. Monatsh. Chem. 139 (2008) 1409–1415. |
| [11] | V.P. Litvinov, V.V. Dotsenko, S.G. Krivokolysko. The chemistry of thienopyridine. Adv. Heterocycl. Chem. 93 (2007) 117–178. |
| [12] |
(a) C.G. Dave, P.R. Shah, A.B. Shah, K.C. Dave, et al., Synthesis and biological activity of pyrido[3',2':4,5]thieno[3,2-d]pyrimidines. J. Indian Chem. Soc., 1989, 66: 48-50; (b) V.L. Ivanov, V.A. Artemov, L.A. Rodinovskaya, et al., New approaches to the synthesis of functionally substituted pyrido[3'2':4,5]thieno[3,2-b] pyridines and the structure of the product obtained. Chem. Heterocycl. Compd., 1996, 32: 105-111; (c) J.M. Quintela, C. Peinador, C. Veiga, et al., Synthesis and antiallergic activity of pyridothienopyrimidines. Bioorg. Med. Chem. Lett., 1998, 6: 1911-1925; (d) L.A. Rodinovskaya, A.M. Shestopalov, A.V. Gromova, et al., One-pot synthesis of diverse, 4-di(tri)fluoromethyl-3-cyanopyridine-2(1H)-thiones and their utilities in the cascade synthesis of annulated heterocycles. J. Comb. Chem. 2008, 10: 313-322; (e) A.K. Elansary, A.A. Moneer, H.H. Kadry, et al., Synthesis and anticancer activity of some novel fused pyridine ring system. Arch. Pharm. Res., 2012, 35: 1909-1917. |
| [13] | A Pictet, T.T Spengler. Über die bildung von isochinolin-derivaten durch einwirkung von methylal auf phenyl-thylamin, phenyl-alanin und tyrosin. Dtsch. Chem. Ges 44 (1911) 2030–2036. |
| [14] |
(a) S.W. Youn, The Pictet-Spengler reaction: efficient carboncarbon bond forming reaction in heterocyclic synthesis. Org. Prep. Proced. Int., 2006, 38: 505-591; (b) B. Kundu, P.K. Agarwal, S.K. Sharma, et al., Pictet-Spengler reaction revisited: engineering of tetherd biheterocycles into annulated polyheterocycles. Curr. Org. Synth., 2012, 9: 357-376. |
| [15] |
(a) D.L. Wang, Q.T. Cui, S.S. Feng, et al., A new synrhesis approch to azuleno[2,1-b]pyridine-4(1H)-ones. Heterocyles, 2012, 85: 697-704; (b) D.L. Wang, Z. Dong, Q.T. Cui, et al., Synthesis of some pyrazole-fused pyrido[3,2-a]azulenes. Heterocyles, 2013, 87: 2343-2350; (c) D.L. Wang, Z. Dong, Z. Liu, et al., Efficient one-pot synthesis of, 1,4-dihydropyridino[3,2-c]coumarins. Chin. J. Org. Chem. 2014, 34: 783-787; (d) D.L. Wang, J.Y. Wu, D. Wu, et al., An efficient synthesis of, 1-oxo-1, 2-dihydrobenzo[b][1,6]naphthyridine-4-carbonitriles. Chin. Chem. Lett. 2014, 25: 1555-1558; (e) D.L. Wang, D. Wu, W. Zhao, et al., An efficient synthesis of benzo[b]benzofurano[2,3-e][1,6]naphthyridine-8-ones. Chin. Chem. Lett., 2015, 26: 251-254. |
| [16] | S. Djekou, A. Gellisa, H. El-Kashef, P. Vanelle. Anefficient synthesis of new thiazolopyrimidinones under microwave irradiation. J. Heterocycl. Chem. 43 (2006) 1225–1229. |
| [17] | Physical and spectral (IR, NMR, Anal.) data: 5a: Mp > 300 ℃. IR (KBr, cm-1): v 1680 (C=O). 1H NMR (400 MHz, DMSO-d6): δ 7.49-7.53 (m,, 3H), 7.63-7.64 (m,, 1H), 7.68-7.71 (m,, 3H), 8.07 (d, J = 4.8 Hz, 1H), 8.77 (d, J = 7.6 Hz, 1H), 8.87 (d, J = 3.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6): δ 107.6, 115.5, 123.1, 123.2, 123.3, 127.8, 128.6, 128.9, 129.6, 131.5, 132.8,135.8, 141.9, 144.4, 153.5, 155.3, 162.2, 170.3. Anal. Calcd. for C20H10N4OS2: C, 62.16, H 2.61, N 14.50. Found: C, 62.24, H 2.72, N 14.59., 5b: Mp > 300 ℃. IR (KBr, cm-1): n 1686 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 2.39 (s, 3H),7.29-7.31 (m,, 2H), 7.41-7.49 (m,, 3H), 7.64-7.66 (m,, 1H), 8.02 (d, J = 4.8 Hz, 1H), 8.71 (d, J = 6.8 Hz, 1H), 8.81 (d, J = 4.0 Hz, 1H). 13C NMR (400 MHz, CF3CO2D): δ 19.5, 107.5, 123.1, 126.4, 127.9, 128.6, 129.6, 131.2, 135.3, 141.8, 141.9., 144.3, 145.1, 145.2, 153.4, 155.4, 155.5, 162.6, 170.2. Anal. Calcd. for C21H12N4OS2: C, 62.98, H 3.02, N 13.99. Found: C, 63.07, H 3.11, N 14.06., 5c: Mp > 300 ℃. IR (KBr, cm-1): v 1682 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 3.61 (s, 3H), 7.03-7.13 (m,, 2H), 7.24-7.35 (m,, 2H), 7.56-7.65 (m,, 1H), 8.11-8.21 (m,, 2H), 9.09-9.18 (m,, 1H), 9.55-9.63 (m,, 1H). 13C NMR (CF3CO2D): δ, 54.8, 108.9, 111.3, 111.4, 118.7, 121.1, 122.9, 123.0, 123.2, 128.5, 131.4., 134.7, 136.1, 141.8, 144.3, 153.1, 153.4, 155.0, 158.8, 159.9, 170.1. Anal. Calcd for C21H12N4O2S2: C, 60.56, H 2.90, N 13.45. Found: C, 60.67, H 2.96, N 13.54., 5d: Mp > 300 ℃. IR (KBr, cm-1): v 1682 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 3.61 (s, 3H), 7.03-7.13 (m,, 2H), 7.24-7.35 (m,, 2H), 7.56-7.65 (m,, 1H), 8.11-8.21 (m,, 2H), 9.09-9.18 (m,, 1H), 9.55-9.63 (m,, 1H). 13C NMR (100 MHz, CF3CO2D): δ 54.8, 108.9, 111.3, 111.4, 118.7, 121.1, 122.9, 123.0, 123.2, 128.5, 131.4., 134.7, 136.1, 141.8, 144.3, 153.1, 153.4, 155.0, 158.8, 159.9, 170.1. Anal. Calcd. for C21H12N4O2S2: C, 60.56, H 2.90, N 13.45. Found: C, 60.67, H 2.96, N 13.54., 5e: Mp > 300 ℃. IR (KBr, cm-1): v 1689 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 4.00 (s, 3H), 7.25 (d, J = 7.2 Hz, 1H), 7.33 (s, 1H), 7.38 (d, J = 8.4 Hz, 1H), 7.53 (d, J = 4.8 Hz, 1H), 7.61-7.65 (m,, 1H), 8.28-8.30 (m,, 2H), 9.26 (d, J = 4.4 Hz, 1H), 9.72 (d, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CF3CO2D): δ 55.3, 108.0, 113.5, 115.4, 117.4, 121.2, 123.4, 123.5, 128.8, 131.0, 131.2., 131.9, 136.0, 142.4, 144.7, 153.7, 153.8, 155.5, 159.0, 161.4, 170.7. Anal. Calcd. for C21H12N4O2S2: C, 60.56, H 2.90, N 13.45. Found: C, 60.64, H 2.98, N 13.56., 5f: Mp > 300 ℃. IR (KBr, cm-1): v 1685 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 4.02 (s, 3H), 7.22 (d, J = 8.4 Hz, 2H), 7.52 (d, J = 4.8 Hz, 1H), 7.66 (d, J = 8.4 Hz, 2H), 8.26-8.29 (m,, 2H), 9.23 (d, J = 5.6 Hz, 1H), 9.74 (d, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CF3CO2D): δ 55.2, 107.7, 115.0, 115.8, 122.5, 123.5, 123.6, 129.0, 131.0, 131.4., 136.1, 142.3, 144.6, 157.8, 153.9, 155.9, 162.2, 163.4, 170.4. Anal. Calcd. for C21H12N4O2S2: C, 60.56, H 2.90, N 13.45. Found: C, 60.64, H 2.97, N 13.54., 5g: Mp > 300 ℃. IR (KBr, cm-1): v 1679 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 3.96 (s, 3H), 4.07 (s, 3H), 7.23 (d, J = 8.4 Hz, 1H), 7.29 (s, 1H), 7.34-7.36 (m,, 1H), 7.54 (d, J = 4.8 Hz, 1H), 8.29-8.34 (m,, 2H), 9.26 (d, J = 5.6 Hz, 1H), 9.76 (d, J = 8.4 Hz, 1H). 13C NMR (100 MHz, CF3CO2D): δ 55.4, 55.8, 108.0, 111.7, 112.6, 115.9, 122.8, 123.3, 123.5, 123.6, 129.0, 131.7., 136.2, 142.4, 144.7, 148.8, 153.0, 153.9, 154.0, 155.7, 161.7, 170.6. Anal. Calcd. for C22H14N4O3S2: C, 59.18, H 3.16, N 12.55. Found: C, 59.26, H 3.25, N 12.74., 5h: Mp > 300 ℃. IR (KBr, cm-1): v 1682 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 7.51-7.62 (m,, 5H), 8.24-8.29 (m,, 2H), 9.24 (d, J = 4.8 Hz, 1H), 9.70 (d, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CF3CO2D): δ 107.9, 115.7, 123.4, 123.5, 123.9, 128.8, 129.5, 129.6, 131.8., 136.1, 140.5, 142.2, 144.7, 153.6, 153.7, 155.7, 161.2, 170.0. Anal. Calcd. for C20H9ClN4OS2: C, 57.07, H 2.16, N 13.31. Found: C, 57.14, H 2.25, N 13.38., 5i: Mp > 300 ℃. IR (KBr, cm-1): v 1688 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 7.29-7.31 (m,, 2H), 7.48-7.49 (m,, 1H), 7.64-7.65 (m,, 2H), 8.23-8.29 (m,, 2H), 9.22-9.28 (m,, 1H), 9.68-9.70 (m,, 1H). 13C NMR (100 MHz, CF3CO2D): δ 108.2, 116.6 (J =, 20.9 Hz), 116.9, 123.7, 123.8, 125.9, 129.1, 131.2, 131.7., 132.0 (J =, 8.3 Hz), 136.3, 142.4, 144.9, 154.0, 155.6, 164.9 (J =, 243.6 Hz), 167.5, 170.8. Anal. Calcd. for C20H9FN4OS2: C, 59.39, H 2.24, N 13.85. Found: C, 59.47, H 2.35, N 13.95., 5j: Mp > 300 ℃. IR (KBr, cm-1): v 1683 (C=O). 1H NMR (400 MHz, CF3CO2D): d 7.45-7.51 (m,, 1H), 7.79-86 (m, 2H), 8.10-8.12 (m,, 2H), 8.21-8.23 (m,, 2H), 9.18-9.25 (m,, 1H), 9.62-9.69 (m,, 1H). 13C NMR (100 MHz,CF3CO2D): δ 107.9, 123.1, 123.3, 123.8, 128.6, 129.5, 129.6, 132.0, 136.2., 136.4, 142.0, 144.6, 149.5, 153.4, 153.5, 154.6, 158.8, 170.5. Anal. Calcd. for C20H9N5O3S2: C, 55.68, H 2.10, N 16.23. Found: C, 55.75, H 2.17, N 16.30., 5k: Mp > 300 ℃. IR (KBr, cm-1): v 1689 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 6.67-6.89 (m,, 1H), 7.46 (d, J = 4.8 Hz, 1H), 7.93 (d, J = 1.6 Hz, 1H), 8.10-8.12 (m,, 1H), 8.32 (d, J = 4.8 Hz, 1H), 8.66 (d, J = 4.0 Hz, 1H), 9.14 (d, J = 4.2 Hz, 1H), 9.82-9.83 (m,, 1H). 13C NMR (100 MHz, CF3CO2D): δ 104.9, 115.1, 118.3, 122.8, 123.3, 128.5, 129.3, 129.4, 135.1, 141.9, 142.2., 143.9, 146.6, 150.4, 153.6, 153.7, 154.3, 169.4. Anal. Calcd. for C18H8N4O2S2: C, 57.44, H 2.14, N 14.88. Found: C, 57.52, H 2.23, N 14.95., 5l: Mp > 300 ℃. IR (KBr, cm-1): v 1680 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 7.25-7.26 (d, J =, 4.8 Hz, 1H), 7.46 (d, J = 4.8 Hz, 1H), 7.67 (d, J = 3.6 Hz, 1H), 7.88 (d, J = 4.8 Hz, 1H), 8.22-8.24 (m,, 1H), 9.17 (d, J = 4.0 Hz, 1H), 9.17 (d, J = 4.6 Hz, 1H), 9.89 (d, J = 8.4 Hz, 1H). 13C NMR (100 MHz, CF3CO2D): δ 107.6, 118.2, 123.1, 123.2, 127.9, 128.2, 128.4, 131.8, 133.8, 133.9., 135.7, 141.9, 144.2, 153.3, 153.4, 154.9, 155.4, 170.0. Anal. Calcd. for C18H14N4OS3: C, 55.08, H 2.05, N 14.28. Found: C, 55.15, H 2.13, N 14.35., 5m: Mp > 300 ℃. IR (KBr, cm-1): v 1678 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 1.11 (t, J = 6.4 Hz, 3H), 1.84-1.86 (m,, 2H), 3.71 (t, J = 6.8 Hz, 2H), 7.43 (d, J = 4.4 Hz, 1H), 8.21-8.24 (m,, 1H), 8.30 (d, J = 4.4 Hz, 1H), 9.13 (d, J = 4.4 Hz, 1H), 9.66 (d, J = 8.0 Hz, 1H). 13C NMR (100 MHz, CF3CO2D): d 12.3, 22.9, 36.1, 107.7, 115.1, 123.1, 123.2, 128.5, 130.7, 135.4, 141.7, 144.1, 153.3., 153.5, 154.7, 167.6, 169.9. Anal. Calcd. for C17H12N4OS2: C, 57.93, H 3.43, N 15.90. Found: C, 58.02, H 3.59, N 15.98., 5n: Mp. 269-271 ℃. IR (KBr, cm-1): v 1675 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 0.91 (t, J = 6.4 Hz, 3H), 1.54-1.56 (m,, 2H), 1.76-1.78 (m,, 2H), 3.74 (t, J = 6.8 Hz, 2H), 7.42 (d, J = 4.8 Hz, 1H), 8.21-8.23 (m,, 1H), 8.30 (d, J = 4.4 Hz, 1H), 9.12 (d, J = 3.2 Hz, 1H), 9.64 (d, J = 6.8 Hz, 1H). 13C NMR (100 MHz, CF3CO2D): δ 11.5, 22.3, 31.4, 34.3, 107.7, 115.0, 123.0, 123.2, 128.5, 130.6, 135.4, 141.3, 144.0, 153.2., 153.6, 154.7, 167.8, 169.9. Anal. Calcd. for C18H14N4OS2: C, 58.99, H 3.85, N 15.29. Found: C, 59.06, H 3.94, N 15.36. |
| [18] |
(a) A.M. Shestopalov, A.E. Fedorov, P.A. Belyakov. Study of the orientation of the Thorpe-Ziegler reaction. Chem. Heterocycl. Comp., 2000, 36: 609-610; (b) V. Gefenas,Ž. Stankevičūte, A. Malinauskas. Novel method for the synthesis of furo[2,3-d]pyrimidines by cyclization of, 4-(phenacyloxy)pyrimidine-5-carbonitriles. Chem. Heterocycl. Comp. 2010, 46: 372-374; (c) H.F. Zhang, Z.Q. Ye, G. Zhao. Enantioselective synthesis of functionalized fluorinated dihydropyrano[2,3-c]pyrazoles catalyzed by a simple bifunctional diaminocyclohexane-thiourea. Chin. Chem. Lett., 2014, 25: 535-540. |
 2016, Vol. 27
2016, Vol. 27 




