Asymmetric organocatalysis has emerged as a powerful, environmentally friendly,and mild methodology for the catalytic production of enantiomerically pure natural products,chiral drugs, building-blocks,and important molecules for materials chemistry [1]. Organocatalyst,that is,an organic compound which exhibits catalytic activities,does not contain heavy metal,and so is advantageous from an environmental as well as a resource standpoint. The easy reproducibility and flexible design of such a catalyst are additional advantages. In the past decade,urea and thiourea derivatives have been intensively investigated in the area of molecular recognition due to their strong hydrogen-bonding activity [2a-g]. Generally,thiourea based organocatalysts have been widely used in asymmetric catalysis due to their strong activation of carbonyl and nitro groups through efficient doublehydrogen- bonding interactions [3]. Since pioneering examples from Song [4a,4b],Wang [4c],Rassu [4d],Zhao [4e],Pan [4f],etc. groups were employed mono as well as bis-thiourea derivatives as catalysts has become a subject of considerable interest in asymmetric organocatalysis [5a-5m]. On account of the ubiquitous application of thiourea catalysts,the development of novel chiral catalysts for asymmetric synthesis continues to be one of the most challenging topics in modern synthetic organic chemistry because it provides the most efficient way to approach the synthesis of enantiopure compounds. Although significant number of chiral thiourea catalysts have been developed so far,camphor-derived thiourea catalysts are very limited as organocatalysts.
Camphor is one of nature’s privileged scaffolds,which is readily available in both enantiomeric forms. It undergoes a wide variety of chemical transformations which functionalize at first glance in the inactivated positions. In the light of the above camphor is a very desirable starting material for the preparation of a wide variety of compounds ranging from natural products to chiral auxiliaries and ligands in asymmetric synthesis [6]. In 2009,Reddy et al. has been prepared a novel class of camphor-derived carbene precursors and these were able to catalyze a formal [2 + 2] reaction of ketenes and aldehydes led to the corresponding chiral b-lactones in high yields and enatiomericexcesses [7]. In 2102,Bian et al. reported camphor sulfonyl ligands and assessed enantioselectivitives in the addition of dialkylzinc reagents to aldehydes in the presence of titanium teraisopropaxide [8]. Recently,Groselj and coworkers developed that 3,5-bis(trifluoromethyl)phenyl thiourea organocatalysts derived from (+)-camphor and they were tested in a model reaction of Michael addition of dimethyl malonate to trans-b-nitrostyrene [9]. Based on these results,we expected that a bis-thiourea organocatalyst derived from a chiral diamine bearing flexible structure might be desirable for wide applicability. In view of this hypothesis,we designed a new series of bis-thiourea/thiourea organocatalysts 3a-f,bearing camphor backbone,expecting that substrates would be captured in appropriate chiral environments by two thiourea parts through doublehydrogenbonding,irrespective of the types of substrates involved.
Since the biological and catalytic activities are dependent on the absolute configuration at the a-carbon,there have been many approaches to enantio-enriched a-aminophosphonates including resolution and auxiliary based approaches [10a-h]. However, catalytic asymmetric approaches have attracted tremendous attention in recent years [11a-d]. First,Jacobsen reported cyclohexyl bearing chiral thiourea for enantioselective α-aminophosphonates [12a]. Akiyama et al. [12b],List and coworkers [12c], and Pettersen et al. [12d] have also been reported the chiral aaminophosphonates by using various asymmetric organocatalysts via Kabachnik-Fields reaction in good yields and high ee’s. Given the importance of the valuable optically active a-amino phosphonate esters in both biological and chemical fields [12a-g],the development of a new catalytic asymmetric synthesis of these compounds remains vital. Herein,we report the novel thiourea catalysts derived from (1R,3S)-camphoric acid for Kabachnik- Fields reaction.
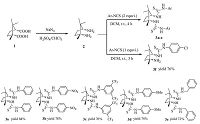
2. Experimental 2.1. General procedure for the synthesis of camphor-derived thiourea catalysts 3a-f(1R,3S)-1,3-Diamino-1,2,2-trimethylcyclopentane (2) was synthesized according to the procedure reported in the literature [7, 13].
Synthesis of 1,1'-((1R,3S)-1,2,2-trimethylcyclopentane-1,3- diyl)bis(3-phenylthiourea) 3a: To a solution of compound 2 (0.142 g,1.0 mmol,1 equiv.) in dry DCM,phenyl isothiocyanate (0.270 g,2.0 mmol,2 equiv.) was added at 0 ℃and the reaction mixture was stirred for 10 min. After warming to room temperature, the reaction mixture was stirred for 4 h. After completion of the reaction,the solvents were removed under reduced pressure and the residue was dissolved in 50 mL ethyl acetate. The organic layer was washed by water (2×30 mL) and brine (2×30 mL) after that dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure and the residue was purified by column chromatography on silica gel (100-200 mesh) using 50% ethyl acetate and petroleum ether as eluents to obtain the pure thiourea as a solid (3a) in 74% (0.288 g) yield. This procedure was applied successfully for the preparation of other thioureas 3b-e. Similarly the compound 3f was prepared by taking the equal amounts of camphor diamine and phenyl isothiocyanate.
2.2. General procedure for the synthesis of (+)-diphenyl(2-cyclopropylpyrimidin-4-yl) (phenylamino)methyl phosphonate (7a)A mixture of the 2-cyclopropyl pyrimidine 4-carbaldehyde (4) (0.148 g,1.0 mmol),aniline (5a) (0.093 g,1.0 mmol),and diphenylphosphite (6) (0.280 g,1.2 mmol) were taken into a 25 mL round bottom flask in dry toluene (10 mL) in the presence of 20 mol% of bis-thiourea 3c (0.0370 g,0.02 mmol) and the reaction mixture was stirred at -40℃ for 6 h. The progress of the reaction was monitored by TLC analysis. The reaction mass was evaporated under reduced pressure and the residue was purified by column chromatography on silica gel (100-200 mesh) using 30% ethyl acetate and petroleum ether as eluents to obtain the pure enantioselective a-aminophosphonate 7a as a colorless solid in 74% (0.338 g) yield. This procedure was applied successfully for the preparation of other compounds 7b-e.
Other experimental details are described in the Supporting information.
3. Results and discussionsInitially we developed the camphor-derived thiourea catalysts 3a-f using (1R,3S)-1,3-diamino-1,2,2-trimethylcyclopentane 2 which has prepared from readily available (1R,3S)-camphoric acid 1 via a Schmidt reaction [13]. Subsequently compound 2 reacted with various aryl isothiocyanates yielded the corresponding thiourea derivatives 3a-f as shown in Scheme 1. Further,these thiourea derivatives were examined for their ability to mediate the catalyst’s activity and enentioselectivity for the synthesis of enantioselective α-aminophosphonates via Kabachnik-Fields reaction [14].
The chemical structures of the newly synthesized thioureas were elucidated by IR,1H NMR,13C NMR and mass spectral analysis. In the IR spectrum,NH and C=S characteristic stretching vibrations appeared in the regions 3392-3362,and 1184- 1152 cm-1,respectively. In the 1H NMR spectra,the four NH protons of the thiourea derivatives (3a-f) were resonated as singlet in the region 10.1-6.0 ppm,these proton signals were confirmed by D2O exchange experiments. All the aromatic protons were observed in the expected regions. The aliphatic CH proton of all thioureas is attributed in the region 4.95-4.65 ppm. The remaining proton signals data and 13C NMR spectral data were listed in the experimental section. Further,high-resolution mass spectral data confirms that the structure of all synthesized thioureas.
At the outset,we studied the three-component Kabachnik- Fields reaction of 2-cyclopropylpyrimidin-4-carbaldehyde 4,aniline 5a,and diphenylphosphite 6 in the presence of camphorderived bis-thioureas 3a-f and the reaction observed as the model reaction at room temperature in DCM solvent toward this effort. Different reaction conditions were explored including catalyst loading,solvents,and reaction temperature without an additive as shown in Table 1.
|
Download:
|
| Scheme. 1. Synthesis of camphor-derived thiourea derivatives. | |
|
|
Table 1 Kabachnik–Fields reaction catalyzed by thiourea derivatives.a |
In the beginning we focused on the screening of suitable thiourea derivatives as organocatalysts in dichloromethane at room temperature. The results with bis-thiourea catalyst 3a gave 78% yield and poor enantioselectivity (5% ee) for 4 h (Table 1,entry 1). Another bis-thiourea 3b afforded the most promising catalytic ability in terms of reaction yield (82%),but received the less enantioselectivity (4%) for 4 h (Table 1,entry 2). The bulkier analogue,i.e. 3,5-bis-trifluoromethylphenyl groups bearing thiourea 3c displayed enantioselectivity (7%) however giving a higher yield (88%) for 2.5 h (Table 1,entry 3). Catalyst 3d showed low catalytic activity 60% yield and no ee were observed of this reaction (Table 1,entry 4). Similarly,bis-thiourea catalyst 3e furnished the product 7a with 80% yield but it does not show any enantioselectivity (Table 1,entry 5). Finally,when we carried out this reaction by using the mono thiourea catalyst 3f afforded the desired product 7a with 84% yield and 4% ee within 3.5 h (Table 1, entry 6). A set of experiments were carried out changing the amount of catalyst (Table 1,entry 7-10). It is remarkable to note that no improvements were observed in the case of catalytic activity and enantioselectivities by increasing the amount of catalyst from 20 mol% to 25 mol% (Table 1,entry 7). Further decreasing the amount of catalyst from 20 to 15,10,and 5 mol% caused a marginal drop in the yield,but with same enantiomeric excess (Table 1,entries 8-10).
The examination of solvent effects revealed that a variety of solvents such as toluene,xylene,acetonitrile (CH3CN),dichloroethane (DCE),and dimethylsulfoxide (DMSO) could be employed for the camphor-derived thiourea 3c catalyzed Kabachnik-Fields reaction. Among them,toluene is found to be the best solvent for the reaction,it gave the small excess of enantioselectivivity (10% ee) compare to DCM(7% ee),but the product yield was same in both cases (Table 1,entries 11-14). On the other hand dimethylsulfoxide (DMSO) gave very poor result in this reaction (Table 1,entry 15).
Encouraged by these results,we further examined the scope of 3c catalyzed three-component Kabachnik-Fields reaction in toluene by changing the reaction temperature,which disclosed that lowering the temperature from room temperature to 0 ℃resulted a slight improvement in enantioselectivity (13% ee) in 3.5 h,but the yield decreased (Table 1,entry 16). Subsequently, when the temperature was decreased to -10℃,the camphor thiourea 3c catalyzed Kabachnik-Fields reaction afforded the product 7a with same enantiomeric excess (13% ee) and 78% yield for 5 h (Table 1,entry 17). Similarly when we conducted the reaction at -20℃ gave 76% yield with 20% ee for 6 h (Table 1,entry 18). Same results were observed at -30℃ also (Table 1,entry 19). Interestingly,the reaction conducted at -40℃ to obtain the product with 74% yield and 35% ee for 6 h (Table 1,entry 20). At -70℃,even after 72 h,no desired product was observed. Complete conversion takes place at 50 ℃for 1 h,but no enantiomeric excess was observed (Table 1,entry 22). These studies prompted us to select the reaction conditions,toluene as solvent,temperature is -40℃ and catalyst is 20 mol% of 3c to probe the scope of the Kabachnik-Fields reaction.
With the optimized reaction conditions in hand,the camphorderived thiourea 3c (20 mol%) catalyzed asymmetric Kabachnik- Fields reaction in toluene at -40℃ was investigated for other substrates of amines 5b-e to furnish the corresponding enantioselective a-aminophosphonates (+)-7b-e in good yields and low enantioselectivites as shown in Scheme 2. The chemical structures 7a-e are characterized by (1H &13C &31P) NMR and mass spectral analysis,which for known compounds were found to be identical with those of racemates as described in the literature of our article [14].

|
Download:
|
| Scheme. 2. Synthesis of enantioselective a-aminophosphonates catalyzed by camphor-derived thiourea catalyst 3c | |
4. Conclusion
In summary,we have prepared enantiopure camphor-derived thioureas and applied in the field of organocatalysis. The present work demonstrates that the addition of phenyl isothiocyanates to suitable amino-functionalized camphor proceeds in high yields to give camphor-bearing thiourea-type structures. While these compounds show good catalytic activity but low to moderate enantioselectivities (up to 35% ee) have been achieved in the catalytic asymmetric Kabachnik-Fields reaction leading to an enantioselective α-aminophosphonate. In the future,we aim at expanding the scope of the chiral camphor-bearing thiourea framework and hope to useful more applications of these interesting molecules in asymmetric organocatalysis.
| [1] |
(a) L. Yu, P. Li, New simple primary amine-thiourea organocatalysts and their application in asymmetric conjugate addition, Tetrahedron Lett. 55 (2014) 3697-3700; (b) C.J. Wang, Z.H. Zhang, X.Q. Dong, X.J. Wu, Chiral amine-thioureas bearing multiple hydrogen bonding donors: highly efficient organocatalysts for asymmetric Michael addition of acetylacetoneto nitroolefins, Chem. Commun. 12 (2008) 1431-1433. |
| [2] |
(a) T.R. Kelly, M.K. Kim, Relative binding affinity of carboxylate and its isosteres: nitro, phosphate, phosphonate, sulfonate, and δ-lactone, J. Am. Chem. Soc. 116 (1994) 7072-7080; (b) F.P. Schmidtchen, M. Berger, Artificial organic host molecules for anions, Chem. Rev. 97 (1997) 1609-1646; (c) B.R. Linton, M.S. Goodman, A.D. Hamilton, Nitronate anion recognition and modulation of ambident reactivity by hydrogen-bonding receptors, Chem. Eur. J. 6 (2000) 2449-2455; (d) M.S. Sigman, E.N. Jocbson, Schiff base catalysts for the asymmetric Strecker reaction identified and optimized from parallel synthetic libraries, J. Am. Chem. Soc. 120 (1998) 4901-4902; (e) P.R. Schreiner, A. Wittkopp, H-Bonding additives act like Lewis acid catalysts, Org. Lett. 4 (2002) 217-220; (f) T. Okino, Y. Hoashi, Y. Takemoto, Enantioselective Michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts, J. Am. Chem. Soc. 125 (2003) 12672-12673; (g) Y. Sohtome, A. Tanatani, Y. Hashimoto, K. Nagasawa, Development of bisthioureα-type organocatalyst for asymmetric Baylis-Hillman reaction, Tetrahedron Lett. 45 (2004) 5589-5592. |
| [3] | J.F. Bai, X.Y. Xu, Q.C.Huang, L. Peng, L.X.Wang. Highly asymmetricMichael additions of α.α-disubstituted aldehydes to β-nitroalkenes promoted by chiral pyrrolidine-thiourea bifunctional catalysts. Tetrahedron Lett. 51 (2010) 2803–2805. |
| [4] |
(a) G. Bian, H. Fan, H. Huang, et al., Highly effective configurational assignment using bisthioureas as chiral solvating agents in the presence of DABCO, Org. Lett. 17 (2015) 1369-1372; (b) G. Bian, H. Fan, S. Yang, et al., A chiral bisthiourea as a chiral solvating agent for carboxylic acids in the presence of DMAP, J. Org. Chem. 78 (2013) 9137-9142; (c) J.Y. Fu, Q.L. Wang, Y.Y. Gui, L.X. Wang, Direct enantioselective amination of aketoester catalyzed by tertiary amine thiourea: a new approach to chiral ahydroxy-β-amino acid, Tetrahedron Lett. 56 (2015) 4220-4223; (d) G. Rassu, V. Zambrano, L. Pinna, et al., Direct and enantioselective vinylogous Michael addition of α-alkylidenepyrazolinones to nitroolefins catalyzed by dual Cinchona alkaloid thioureas, Adv. Syn. Catal. 356 (2014) 2330-2336; (e) S. Murammulla, J.A. Ma, J.C.G. Zhao, Michael addition of ketones and aldehydes to maleimides catalyzed by modularly designed organocatalysts, Adv. Syn. Catal. 355 (2013) 1260-1264; (f) Z. Duan, Z. Zhang, P. Qian, J. Han, Y. Pan, Asymmetric Morita-Baylis-Hillman reaction of isatins with a,β-unsaturated g-butyrolactam as the nucleophile, RSC Adv. 3 (2013) 10127-10130. |
| [5] |
(a) S.J. Connon, Organocatalysis mediated by (thio)urea derivatives, Chem. Eur. J. 12 (2006) 5418-5427; (b) A.G. Doyle, E.N. Jacobsen, Small-molecule H-bond donors in asymmetric catalysis, Chem. Rev. 107 (2007) 5713-5743; (c) S.J. Connon, Asymmetric catalysis with bifunctional cinchona alkaloid-based urea and thiourea organocatalysts, Chem. Commun. (2008) 2499-2510; (d) X. Yu, W. Wang, Hydrogen-bond-mediated asymmetric catalysis, Chem. Asian J. 3 (2008) 516-532; (e) H. Miyabe, Y. Takemoto, Discovery and application of asymmetric reaction by multi-functional thioureas, Bull. Chem. Soc. Jpn. 81 (2008) 785-795; (f) Z. Zhang, P.R. Schreiner, (Thio)urea organocatalysis-what can be learnt from anion recognition? Chem. Rev. 38 (2009) 1187-1198; (g) Y. Sohtome, K. Nagasawa, The design of chiral double hydrogen bonding networks and their applications to catalytic asymmetric carbon-carbon and carbon-oxygen bond-forming reactions, Synlett 1 (2010) 1-22; (h) Y. Takemoto, Development of chiral thiourea catalysts and its application to asymmetric catalytic reactions, Chem. Pharm. Bull. 58 (2010) 593-601; (i) W.Y. Siau, J. Wang, Asymmetric organocatalytic reactions by bifunctional amine-thioureas, Catal. Sci. Technol. 1 (2011) 1298-1310; (j) L.Q. Lu, X.L. An, J.R. Chen, W.J. Xiao, Dual activation in organocatalysis: design of tunable and bifunctional organocatalysts and their applications in enantioselective reactions, Synlett 4 (2012) 490-508; (k) Z. Chai, G. Zhao, Efficient organocatalysts derived from simple chiral acyclic amino acids in asymmetric catalysis, Catal. Sci. Technol. 2 (2012) 29-41; (l) S.N. Perumal, D.G. Rivera, R.C. Silva, M.W. Paixão, Terpene-derived bifunctional thioureas in asymmetric organocatalysis, Chem. Cat. Chem. 5 (2013) 2756-2773; (m) O.V. Serdyuk, C.M. Heckel, S.B. Tsogoeva, Bifunctional primaryamine-thioureas in asymmetric organocatalysis, Org. Biomol. Chem. 11 (2013) 7051-7071. |
| [6] | U. Grošelj, S. Ričko, J. Svete, B. Stanovnik. Synthesis of, 2-(3-(3,5-bis(trifluoromethyl) phenyl)thioureido)-3-((dimethylamino)methyl)camphor organocatalysts. 24 (2012) 412–419. |
| [7] | P.V.G. Reddy, S. Tabassum, A. Blanrue, R. Wilhelm. New enantiopure NHCs derived from camphor. Chem. Commun. (2009) (2009) 5910–5912. |
| [8] | G. Bian, H. Huang, H. Zong, L. Song. The syntheses of new camphorsulfonylated ligands derived from, 2-amino-2'-hydroxy-1,1'-binaphthyl and their enantioselectivities in the addition of dialkylzinc reagents to aldehydes. 24 (2012) 825–832. |
| [9] | S. Ričko, A. Golobič, J. Svete, B. Stanovnik, U. Grošelj. Synthesis of novel camphorderived bifunctional thiourea organocatalysts. Chirality 27 (2015) 39–52. |
| [10] |
(a) V.A. Alfonsov, Diastereoselective synthesis of enantiopure α-aminophosphonic acid derivatives: Pudovik reaction in stereoselective synthesis, Phosphorus Sulfur Silicon Relat. Elem. 183 (2008) 2637-2644; (b) O.I. Kolodiazhnyi, Asymmetric synthesis of organophosphorus compounds, Tetrahedron: Asymmetry 9 (1998) 1279-1332; (c) P. Lyzwa, M. Mikolajczyk, Chiral sulfinimines in asymmetric synthesis of enantiomeric aminophosphonic acids, Phosphorus Sulfur Silicon Relat. Elem. 189 (2014) 1174-1192; (d) P. Kaur, W. Wever, T. Rajale, G. Li, Asymmetric hydrophosphylation of chiral N-phosphonyl imines provides an efficient approach to chiral α-amino phosphonates, Chem. Biol. Drug. Des. 76 (2010) 314-319; (e) P. Lyzwa, M. Mikolajczyk, Asymmetric synthesis of aminophosphonic acids mediated by chiral sulfinyl auxiliary: recent advances, Pure Appl. Chem. 82 (2010) 577-582; (f) F. Palacios, T.K. Olszewski, J. Vicario, Diastereoselective hydrophosphonylation of imines using (R,R)-TADDOL phosphite. Asymmetric synthesis of α-amino phosphonic acid derivatives, Org. Biomol. Chem. 8 (2010) 4255-4258; (g) K.E. Metlushka, D.N. Sadkova, L.N. Shaimardanova, O.N. Kataeva, V.A. Alfonsov, Stereoselective synthesis of α-aminophosphonic acids using the Betti base as chiral auxiliary, Phosphorus Sulfur Silicon Relat. Elem. 186 (2011) 712-717; (h) V.A. Alfonsov, C.E. McKenna, E.V. Bayandina, et al., Diastereoselective synthesis of enantiopure cyclic α-aminophosphonic acids, Phosphorus Sulfur Silicon Relat. Elem. 183 (2008) 2647-2648. |
| [11] |
(a) M. Ordóňez, H. Rojas-Cabrera, C. Cativiela, An overview of stereoselective synthesis of α-aminophosphonic acids and derivatives, Tetrahedron 65 (2009) 17-49, For reviews, see:; (b) H. Groeger, B. Hammer, Catalytic concepts for the enantioselective synthesis of α-amino and α-hydroxy phosphonates, Chem. Eur. J. 6 (2000) 943-948; (c) P.S. Bhadury, H. Li, Organocatalytic asymmetric hydrophosphonylation/Mannich reactions using thiourea, cinchona and Brønsted acid catalysts, Synlett 23 (2012) 1108-1131; (d) M. Ordonez, J.L. Viveros-Ceballos, C. Cativiela, A. Arizpe, Stereoselective synthesis of α-aminophosphonic acids analogs of the 20 proteinogenic α-amino acids, Curr. Org. Synth. 9 (2012) 310-341. |
| [12] |
(a) G.D. Joly, E.N. Jacobsen, Thioureα-catalyzed enantioselective hydrophosphonylation of imines: practical access to enantiomerically enriched α-amino phosphonic acids, J. Am. Chem. Soc. 126 (2004) 4102-4103; (b) T. Akiyama, H. Morita, J. Itoh, K. Fuchibe, Chiral Brønsted acid catalyzed enantioselective hydrophosphonylation of imines: asymmetric synthesis of aamino phosphonates, Org. Lett. 7 (2005) 2583-2585; (c) X. Cheng, R. Goddard, G. Buth, B. List, Direct catalytic asymmetric threecomponent Kabachnik-Fields reaction, Angew. Chem. Int. Ed. 47 (2008) 5079-5081; (d) D. Pettersen, M. Marcolini, L. Bernardi, et al., Direct access to enantiomerically enriched α-amino phosphonic acid derivatives by organocatalytic asymmetric hydrophosphonylation of imines, J. Org. Chem. 71 (2006) 6269-6272; (e) P. Merino, E. Marques-Lopez, R.P. Herrera, Catalytic enantioselective hydrophosphonylation of aldehydes and imines, Adv. Synth. Catal. 350 (2008) 1195-1208; (f) D. Zhao, R. Wang, Recent developments in metal catalyzed asymmetric addition of phosphorus nucleophiles, Chem. Soc. Rev. 41 (2012) 2095-2108; (g) J.A. Ma, Catalytic asymmetric synthesis of α-and β-amino phosphonic acid derivatives, Chem. Soc. Rev. 35 (2006) 630-636. |
| [13] | D. Jaramillo, D.P. Buck, J.G. Collins. Synthesis, characterisation and biological activity of chiral platinum(II) complexes. Eur. J. Inorg. Chem (2006) 839–849. |
| [14] | P.S. Reddy, M.M. Reddy, P.V.G. Reddy. Phosphomolybdic acid promoted Kabachnik-Fields reaction: an efficient one-pot synthesis of α-aminophosphonates from, 2-cyclopropylpyrimidine-4-carbaldehyde. Tetrahedron Lett. 55 (2014) 3336–3339. |
 2016, Vol. 27
2016, Vol. 27 



