b Medical School, Hebei University, Baoding 071002, China
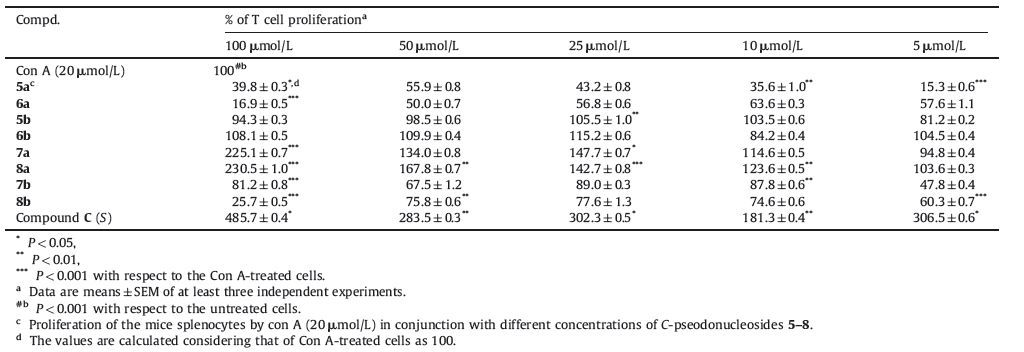
Immunomodulators,including immunostimulants and immunosuppressants, can help to regulate or normalize the immune systems that are out of balance,so they have been widely used in clinical treatments of immune-related diseases,such as cancer, AIDs,organ transplantation,rheumatoid arthritis,and so on [1-6]. Due to their potential value in clinical practice,it is of great significance to develop effective immunomodulators,which has also proven as one of the most prevalent areas in the development of new pharmaceuticals. Many chemical entities,either from natural sources or prepared by synthesis,are known to exert modulating activities on immune systems [7-10]. Among them, nucleosides,one of the hot topics in organic and medicinal chemistry,have offered more considerable promise in drug development [11]. For example,a group of substituted guanosines, like loxoribine (Fig. 1A),cause significant B and NK-cell stimulation by inducing interferon [12],while the aza-nucleosides,like immucillin-G (Fig. 1B),can block the T cell proliferation by inhibiting purine nucleoside phosphorylase (PNP) [13]. In our previous studies,a novel series of C-pseudonucleosides bearing N-phenyl thiazolidin-4-one (Fig. 1C) had been demonstrated to possess significant immunostimulatory properties by increasing IL-4 and IL-2 secretions [14]. It is of great interest and challenge to explore more such pseudonucleosides as immunomodulators.
n an effort to enhance the immunostimulant activity of this class of C-pseudonucleosides [15],in this paper,we report the design,synthesis,and the effects on T cell proliferation of the C-pseudonucleosides containing thiazolidin-4-one and phenyl connected by acetamide bond (Scheme 1). Acetamide bond formed by condensation of glysine was selected because it generally plays an important role in immune activation. For example,the imino group of the amide moiety in KRN7000 (a-galactosyl ceramide,D, Fig. 1) [16] which has become the most widely studied glycolipid antigen for specifically activating invariant natural killer T (iNKT) cells,is able to significantly form the hydrogen bonding in the combination between KRN7000 and its targeted protein CD1D. The absence of this H-bonding interaction will induce the markedly loss of the immunostimulating activity of KRN7000 [17]. Additionally, from a practical point of view,incorporating of the peptide bond would make a compound better compatibility with biomoleculars,facilitate easier synthesis,and more importantly, increase its’ biological activities with nontoxic [18]. Therefore, we performed structural modifications by introducing acetamide bond into our C-pseudonucleosides (Fig. 1C) that would maybe allow for an additional H-bonding interaction with its unknown target to improve their immunomodulatory activities.
|
Download:
|
| Figure 1. Some chemical structures of small molecule immunomodulating agents | |
|
Download:
|
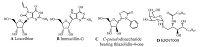
| Figure 1. The synthesis of C-pseudonucleosides 5-8. Reagents and conditions: 1 (1 mmol), 2 or 3 (1 equiv.), 4 (2 equiv.), DCC (1.2 equiv.), DMAP (0.2 equiv.), dry EtOH (3 mL), r.t., 2 h. | |
2. Experimental
Melting points were measured on an SGW®X-4 micro melting point apparatus and were uncorrected. Optical rotations were determined on an SGW®-1 automatic polarimeter. IR spectra were determined on a WQF-510 as KBr tablets for solid samples and were expressed in cm-1 scale. 1H NMR and 13C NMR spectra were measured on a RT-NMR Bruker AVANCE 600 MHz,NMR spectrometer using tetramethylsilane (Me4Si) as an internal standard. High Resolution Mass Spectra (HRMS) were carried out on a FTICR-MS (Ionspec 7.0T) mass spectrometer with electrospray ionization (ESI). The optical densities for examining the activities of immunological activities were measured on a BioRad Model 3550 microplate spectrophotometer. Thin-layer chromatography (TLC) was performed on precoated plates (Qingdao GF254) with detection by UV light or with phosphomolybdic acid in EtOH/H2O followed by heating. Column chromatography was performed using reverse silica gel (C18,50 mmol/L). Concanavalin A (type IV) was purchased from Sigma. Cell culture was carried out under sterile condition.
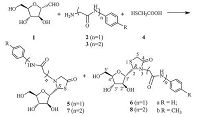
2.1. General procedure for the synthesis of compounds 5-8.he unprotected aldehyde 1 (0.16 g,1 mmol) was dissolved in 3 mL anhydrous EtOH. To the solution,anilines linked glysine 2 or 3 (a and b),mercaptoacetic acid 4 (0.14 mL,2 mmol) were added, followed by DMAP (0.2 equiv.) and DCC (1.2 equiv.). The mixture was stirred at room temperature for 2 h,then,was neutralized with solid K2CO3. Solvent was evaporated under reduced pressure to get a crude product,which was repeatedly purified using reverse silica (C18) gel column chromatography (H2O-MeOH,v/v = 90:10) to get the pure isomers 5 (7) and 6 (8),respectively.
2-((S)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-phenylacetamide (5a): White solid,yield 27%,mp 197-198 8℃,[α] D 25-384.6 (c 1.0, MeOH); 1H NMR (600 MHz,CD3OD:CDCl3 = 3:1):δ 3.58-3.63 (m, 2H,H-5' ,H-4),3.67 (dd,1H,J = 12.0 Hz,3.6 Hz,H-5),3.74 (dd,1H, J = 15.6 Hz,1.2 Hz,H-5'),3.96 (dd,1H,J = 8.4 Hz,4.8 Hz,H-5),4.00 (t,1H,J = 4.2 Hz,H-2'),4.05 (t,1H,J = 3.6 Hz,H-3'),4.15 (q,1H, J = 4.2 Hz,H-1'),4.38 (d,1H,J = 16.2 Hz,CH),4.52 (d,1H, J = 16.8 Hz,CH),5.04 (dd,1H,J = 8.4 Hz,1.2 Hz,H-2),7.10 (t,1H, J = 7.2 Hz,2CH),7.31 (t,2H,J = 7.2 Hz,2CH),7.54 (d,2H,J = 7.8 Hz, 2CH); 13C NMR (150 MHz,CD3OD:CDCl3 = 3:1):δ 30.9,61.8,62.7, 79.2,85.4,87.8,120.0,121.8,124.0,128.5,138.0,166.8,173.3; HRESIMS: Calcd. for C16H20N2O6SNa (M + Na)+: 391.0939,found: 391.0951.
2-((R)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-phenylacetamide (6a): Light yellow solid,yield 14%,mp 211-212 8C,[α] D 25 217.4 (c 1.0,MeOH); 1H NMR (600 MHz,CD3OD:CDCl3 = 3:1):δ 3.47 (d,1H, J = 15.6 Hz,H-5),3.60-3.65 (m,1H,H-5'),3.76-3.78 (m,1H,H-4'), 3.81 (d,1H,J = 16.2 Hz,H-5'),3.94-3.96 (m,1H,H-5),4.03 (t,1H, J = 7.2 Hz,H-2'),4.06-4.11 (m,2H,H-3' ,H-1'),4.18 (d,1H, J = 6.6 Hz,CH),4.48 (d,1H,J = 15.8 Hz,CH),5.04 (s,1H,H-2), 7.12 (t,1H,J = 7.2 Hz,2CH),7.32 (t,2H,J = 7.8 Hz,2CH),7.56 (d,2H, J = 7.8 Hz,2CH); 13C NMR (150 MHz,CD3OD:CDCl3 = 3:1):δ 31.7, 37.6,61.5,64.6,76.2,81.0,83.8,119.9,124.1,128.5,137.9,166.4, 173.6; HRESIMS: Calcd. for C16H20N2O6SNa (M + Na)+: 391.0939, found: 391.0918.
2-((S)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-(p-tolyl)acetamide (5b): White solid,yield 38%,mp 192-193 8C,[α] D 25 -225.7 (c 1.0, MeOH); 1H NMR (600 MHz,CD3OD):δ 2.31 (3H,s,CH3),3.59-3.63 (m,2H,H-5,H-5'),3.67 (dd,1H,J = 12.0 Hz,4.2 Hz,H-5'),3.76 (d, 1H,J = 15.6 Hz,H-5),3.96 (dd,1H,J = 9.0 Hz,4.8 Hz,H-2'),4.00 (t, 1H,J = 4.2 Hz,H-3'),4.16 (dd,1H,J = 8.4 Hz,4.2 Hz,H-1'),4.37 (d, 1H,J = 16.8 Hz,CH),4.51 (d,1H,J = 14.2 Hz,CH),5.06 (dd,1H, J = 7.8 Hz,1.2 Hz,H-2),7.14 (d,2H,J = 7.8 Hz,2CH),7.43 (d,2H, J = 8.4 Hz,2CH); 13C NMR (150 MHz,CD3OD):δ 17.4,19.5,30.7, 61.7,62.6,77.5,79.1,85.5,87.6,120.0,128.9,133.7,135.5,166.7; HRESIMS: Calcd. for C17H22N2O6SNa (M + Na)+: 405.1096,found: 405.1104.
2-((R)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-(p-tolyl)acetamide (6b): White solid,yield 12%,mp 214-216 8C,[α] D 25 261.4 (c 1.0, MeOH); 1HNMR(600 MHz,CD3OD):δ 2.31 (s,3H,CH3),3.47 (d,1H, J = 15.6 Hz,H-5),3.61 (dd,1H,J = 12.0 Hz,4.8 Hz,H-5'),3.76-3.82 (m,2H,H-4',H-5'),3.94 (s,1H,H-5),4.02-4.08 (m,3H,H-2',H-3' , H-1',4.18 (d,1H,J = 8.4 Hz,CH),4.48 (d,1H,J = 16.8 Hz,CH),5.04 (s,1H,H-2),7.14 (d,2H,J = 7.8 Hz,2CH),7.44 (d,2H,J = 8.4 Hz, 2CH); 13C NMR (150 MHz,CD3OD):δ 19.5,31.6,45.9,61.5,64.6, 76.2,77.6,80.9,83.8,119.9,128.9,133.8,135.4,166.3,173.6; HRESIMS: Calcd. for C17H22N2O6SNa (M + Na)+: 405.1096,found: 405.1108.
2-((S)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-(2-oxo-2-(phenylamino) ethyl)acetamide (7a): Yellow solid,yield 33%,mp 214- 216 8C,[α] D 25 -436.4 (c 1.0,MeOH); 1H NMR (600 MHz,CD3OD):δ 3.57-3.64 (m,2H,H-5,H-5'),3.70-3.77 (m,2H,H-5,H-5'),3.98- 4.01 (m,2H,H-4',H-2'),4.06-4.09 (m,3H,H-3' ,H-1',CH),4.18 (dd, 1H,J = 7.2 Hz,4.2 Hz,CH),4.30 (d,1H,J = 15.0 Hz,CH),4.41 (d,1H, J = 14.4 Hz,CH),5.04 (d,1H,J = 7.8 Hz,H-2),7.11 (t,1H,J = 7.2 Hz, CH),7.32 (t,2H,J = 7.8 Hz,2CH),7.59 (d,2H,J = 7.8 Hz,2CH); 13C NMR (150 MHz,CD3OD):δ 30.7,42.7,61.7,62.8,77.5,78.9,85.0, 86.9,120.1,124.0,128.4,138.0,168.2,169.6,173.5; HRESIMS: Calcd. for C18H24N3O7S (M+H)+: 426.1335,found: 426.1352.
2-((R)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-(2-oxo-2-(phenylamino) ethyl)acetamide (8a): Yellow solid,yield 5%,mp 154-156 8C, [α] D 25 475.9 (c 1.0,MeOH); 1H NMR (600 MHz,CD3OD:CDCl3 = 3:1):δ 3.44 (d,1H,J = 9.0 Hz,H-5),3.59 (dd,1H,J = 12.0 Hz,5.4 Hz,H-5'), 3.76-3.81 (m,2H,H-5,H-5'),3.95-3.98 (m,1H,H-4'),4.03-4.06 (m,3H,H-3' ,H-1',H-2'),4.08-4.13 (m,2H,2CH),4.19-4.23 (m,2H, 2CH),4.98 (s,1H,H-2),7.10 (t,1H,J = 7.2 Hz,CH),7.30 (t,2H, J = 7.8 Hz,2CH),7.58 (d,2H,J = 7.8 Hz,2CH); 13C NMR (150 MHz, CD3OD:CDCl3 = 3:1):δ 31.9,42.9,46.7,47.6,47.7,47.9,61.7,65.0, 76.2,80.7,83.9,120.1,124.2,128.5,137.8,168.0,169.0,173.76; HRESIMS: Calcd. for C18H24N3O7S (M+H)+: 448.1154,found: 448.1159.
2-((S)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-(2-oxo-2-(p-tolylamino) ethyl)acetamide (7b): White solid,yield 32%,mp 130-132 8C, [α] D 25 -252.7 (c 1.0,MeOH); 1HNMR(600 MHz,CD3OD):δ 2.31(s,3H, CH3),3.56-3.64(m,2H,H-5,H-5'),3.70-3.77 (m,2H,H-4',H-3'),3.99 (s,2H,2CH),4.04 (s,2H,2CH),4.08 (s,1H,H-2'),4.18 (dd,1H, J = 7.8 Hz,4.8 Hz,H-1'),4.30 (d,1H,J = 16.8 Hz,H-5'),4.40 (d,1H, J = 16.2 Hz,H-5),5.03 (d,1H,J = 7.8 Hz,H-2),7.13 (d,2H,J = 7.8 Hz, 2CH),7.46 (d,2H,J = 8.4 Hz,2CH); 13C NMR (150 MHz,CD3OD):δ 19.5,30.7,42.7,61.7,62.8,77.5,79.0,85.0,86.9,120.2,128.9,133.8, 135.3,168.1,169.6(2C),173.6; HRESIMS: Calcd. for C19H25N3O7SNa (M + Na)+: 462.1310,found: 462.1296.
2-((R)-2-((2S,3S,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran- 2-yl)-4-oxothiazolidin-3-yl)-N-(2-oxo-2-(p-tolylamino) ethyl)acetamide (8b): White solid,yield 10%,mp 210- 212 8C,[α] D 25 1622.6 (c 1.0,MeOH); 1H NMR (600 MHz,CD3OD):δ 2.31 (s,3H,CH3),3.47 (d,1H,J = 15.6 Hz,H-5),3.60 (dd,1H, J = 12.6 Hz,6.0 Hz,H-5'),3.76-3.81 (m,2H,H-5,H-4'),3.95-3.96 (m,1H,H-5'),4.02-4.11 (m,5H,H-2',H-3' ,H-1' ,2CH),4.22 (d,1H, J = 7.2 Hz,CH),4.31 (d,1H,J = 16.8 Hz,CH),5.03 (s,1H,H-2),7.13 (d,2H,J = 7.8 Hz,2CH),7.47 (d,2H,J = 7.8 Hz,2CH); 13C NMR (150 MHz,CD3OD):δ 19.5,31.6,42.7,46.3,61.6,64.9,76.2,77.6, 80.6,83.9,120.1,128.9,133.8,135.4,168.0,169.2,173.8; HRESIMS: Calcd for C19H26N3O7S (M+H)+ : 440.1491,found: 462.1497.
2.2. Immunological activities assayhe spleens from BALb/c mice were taken out in sterile conditions and soaked in non-serum RPMI-1640 cell culture medium. The spleens were grinded using a wire mesh. Filter the cell suspension with a 200-mesh nylon net. The filtrate of the splenocytes was centrifuged at 2000×g for 10 min and then the supernatant was removed. Dissolving the precipitation in 5 mL of pH 7.2 Tris-NH4Cl solution and incubating the cells at 37 ℃for 6- 10 min in order to lyse the red cells. Then the cells were centrifuged at 2000 g for 7 min and the cell pellets were dissolved in RPMI- 1640 culture medium with 10% newborn calf serum and 20 mmol/L Con A. Counting cell and adjusting the concentration of cells solution to 5×106/mL. Add 4.5×105 cells into each well of 96 well plates. Subsequently adding different concentration of each compound into each well,and incubating at 37 8C,5% CO2 for 72 h. The supernatant was collected and centrifuged at 2000×g for 5 min. The supernatant was collected and stored at -20 ℃until for assay.
plencytes from BALb/C mice were aseptically removed and minced,and cell suspensions were incubated at 4.5×105 cell/well, 90 mL/well in 96-well microtiter plates using an RPMI 1640 mediumwith 10% FCS. Spleen cells were cultured with 20 mmol/L of Con A for 72 h at 37 ℃in5% CO2 in the presence or absence of the tested compounds. Wells containing Con A without tested compounds were used as blanks. All the tests were performed at least three times in quadruplicate (P < 0.01). Cell proliferation was measured using the MTT assay,testing OD (A) at wavelength 570 nm.
| $cell proliferation\left( \% \right) = {{{A_{treated}} - {A_{control}}} \over {{A_{control}}}} \times 100\% $ |
Following our reported procedure [19],the target C-pseudonucleosides 5-8 were conveniently synthesized by the one-pot threecomponent condensation from the unprotected sugar aldehyde 1 [20],fatty amine 2 or 3 (a-b),and mercaptoacetic acid 4 at room temperature in the presence of the condensation reagent N, N’-dicyclohexyl-carbodiimide (DCC) and the promoter 4-dimethylamino- pryidine (DMAP). The one-pot synthesis afforded the diastereomeric products 5 (7,less polar) and 6 (8,more polar) in the overall yields of 38%-50% (Table 1). Although the total yields were nearly medium,however,the result was satisfactory for the three-component reaction was performed easily using the unprotected sugar aldehyde and amino acid as starting materials without complicated operation. After simple working up and repeated purification by reverse silica (C18) gel column chromatography, the pure products were obtained,respectively. It could be found that the reaction proceeded with certain stereoselectivity and gave 5 (or 7) with S configuration on C-2 as major product, possibly due to the synergistic hindrance effects of the cis hydroxyl and hydroxymethyl groups on 2' and 5' positions,respectively, which made a dominant backside attack of the sulfur atom to the Schiff base formed by aldimine condensation.
|
|
Table 1 The synthesis of C-pseudonucleosides 5-8 and their NMR signals of H-2. |
he structures of C-pseudonucleosides 5 and 6 (or 7 and 8) were assigned based on the analyses of their spectral data of NMR and HRMS. Each diastereoisomers of 5 and 6 (or 7 and 8) exhibited the similar NMR signals in H-2 as shown in Table 1,that is,H-2 signals of the less polar 5 (7) are double or double-double peaks,while H-2 signals of the more polar 6 (8) show single peaks. By comparing the 1 H NMR signals of H-2 in 5 and 6 (or 7 and 8) with those of the known compound C (Fig. 1) [14],the configurations of the new generated chiral carbon (C-2) in 5 and 6 (or 7 and 8) could be tentatively determined to be of (S) and (R),respectively.
he effects of C-pseuodonucleosides 5-8 on the concanvalin A (Con A)-induced proliferation of the mice splenocytes were assessed by the MTT method. Our initial studies revealed that the treatment of the mice splenocytes with Con A (20 mmol/L) enhanced cell proliferation by 46% (P < 0.001) compared to the untreated cells,and all subsequent experiments were carried out using the same concentration of Con A. The assays were conducted 72 h at 37 8C,5% CO2 after the administration of 5-8 at five different concentrations (5,10,25,50,100 mmol/L),using the Con A-treated splenocytes as the experimental control and compound C (S) (Fig. 1) as positive control.
s shown in Table 2,compounds 5a,6a,7a,8a,7b,and 8b could significantly affect on Con A-induced T cell proliferation. It was interesting that they showed different actions on T cell proliferation. Among them,compounds 7a and 8a (25,50,and 100 mmol/L) promoted the T cell proliferation in the range of 34%-130% compared to the Con A-treated sample,lower than those of the parent compound C (S). At 100 mmol/L,both of them showed most pronounced effects with the proliferation rate of 125% and 130%, respectively. Inversely,compounds 5a,6a,7b,and 8b decreased Con A-induced T cell proliferation in certain degree at five concentrations. In their midst,compounds 5a,6a,and 8b induced the most negative proliferation of T cell at concentrations of 5,100, and 100 mmol/L,respectively. By comparison,compound 5a has the most potential as immunosuppressant due to its’ outstanding inhibition on T cell proliferation at low concentration of 5 mmol/L. The other compounds 5b and 6b nearly did not exhibit any effects on T cell proliferation at the tested concentrations. From the view of the structure-activity relationship (SAR),the immunomodulatory activities of such C-pseudonucleosideswere seemed to depend upon both the number of the acetamide bonds and the 4-substituent (like methyl) on the phenyl,although it was difficult to formulate a rigorous structure-activity relationship based on the observed activities. These initial biological results also suggested that subtle structural changes to this artificial base part of C-pseudonucleosides could have a significant effect on T cell proliferation bias. It was a common phenomenon and a chance in the studies on developing the potential immunomodulators,like loxoribine and immucillin-G, perhaps due to the unknown complicated immune system. Future work will involve further SAR studies in order to better understand how these C-pseudonucleosides interact with the T cell.
|
|
Table 2 The effects of compounds 5-8 on Con A-induced splenocyte proliferation. |
4. Conclusion
Some novel C-pseudonucleosides containing thiazolidin-4-one and phenyl connected by acetamide bond were designed and synthesized from the unprotected sugar aldehyde,alphatic amine, and mercaptoacetic acid at room temperature. Some compounds 5a,6a,7a,8a,7b,and 8b could significantly affect on Con Ainduced T cell proliferation. The immunomodulatory efficacy of these C-pseudonucleosides was seemed to depend upon both the number of the acetamide bonds and the 4-substituent (like methyl) on the phenyl,which could have a significant effect on T cell proliferation bias.
| [1] | R. Saxena, A. Sharma, M. Bharti, M. Rathore. Immunomodulator A new horizon: an overview. J. Pharma. Res. 5 (2012) 2306–2310. |
| [2] | A. Failli, T.J. Caggiano. Small molecule immunomodulators. Curr. Opin. Ther. Pat. 2 (1992) 882–892. |
| [3] | G.H. Werner, P. Jollès. Immunostimulating agents: What next? A review of their present and potential medical application. Eur. J. Biochem. 242 (1996) 1–19. |
| [4] | J.W. Hadden. Immunostimulants. Trends Pharmacol. Sci. 14 (1993) 169–173. |
| [5] | A.C. Allison. Immunosuppressive drugs: the first, 50 years and a glance forward. Immunopharmacology 47 (2000) 63–83. |
| [6] | K.C. Meyer, J. Bierach. Immunosuppressive therapy for autoimmune lung diseases. Immnol. Allergy. Clin. 32 (2012) 633–669. |
| [7] | D. Wu, M. Fujio, C.H. Wong. Glycolipids as immunostimulating agents. Bioorg. Med. Chem. 16 (2008) 1073–1083. |
| [8] | V.P. Vyavahare, C. Chakraborty, B. Maity, et al. , Synthesis of, 1-deoxy-1-hydroxymethyl-and 1-deoxy-1-epi-hydroxy methyl castanospermine as new potential immunomodulating agents. J. Med. Chem. 50 (2007) 5519–5523. |
| [9] | G.L. Zhang, C.S. Chen, Y.L. Xiong, et al. , Synthesis of N-substituted iminosugar derivatives and their immunosuppressive activities. Carbohydra. Res. 345 (2010) 780–786. |
| [10] | P. Huxley, D.H. Sutton, P. Debnam, et al. , High-affinity small molecule of T cell costimulation: compounds for cmmunotherapy. Chem. Biol. 11 (2004) 1651–1685. |
| [11] | J. Lee, T.H. Chuang, V. Redeck, et al. , Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: Activation of toll-like receptor, 7,. Proc. Natl. Acad. Sci. U.S.A., 100 (2003) 6646–6651. |
| [12] | A.B. Reitz, M.G. Goodman, B.L. Pope, et al. Small-molecule immunostimulants. Synthesis and activity of, 7,8-disubstituted guanosines and structurally related compounds. J. Med. Chem. 37 (1994) 3561–3578. |
| [13] | K. Clinch, G.B. Evans, R.F.G. Fröhlich, et al. , Third-generation immucillins: syntheses and bioactivities of acyclic immucillin inhibitors of human purine nucleoside phosphorylase. J. Med. Chem. 52 (2009) 1126–1143. |
| [14] | H. Chen, Q.M. Yin, C.X. Li, et al. , Synthesis of C-pseudonucleosides bearing thiazolidin-4-one as novel potential immunostimulating agents. ACS Med. Chem. Lett. 2 (2011) 845–848. |
| [15] | H. Chen, F. Gao, C.X. Li, et al. , Synthesis and immunostimulating activity of C-pseudonucleosides containing N-phenyl thiazolidin-4-one. Med. Chem. Res. 22 (2013) 5723–5729. |
| [16] | A. Banchet-Cadeddu, E. Hénon, M. Dauchez, et al. , The stimulating adventure of KRN, 7000,. Org. Biomol. Chem. 9 (2011) 3080–3104. |
| [17] | L. Zhang, X.S. Ye. Structure modifications based on KRN7000 and their SARs in activating NKT cells. J. Chin. Pharmaceut. Sci. 17 (2008) 263–271. |
| [18] | E.F. DiMauro, J. Newcomb, J.J. Nunes, et al. , Structure-guided design of aminopyrimidine amides as potent, selective inhibitors of lymphocyte specific kinase: synthesis, structure-activity relationships, and inhibition of in vivo T cell activation. J. Med. Chem. 51 (2008) 1681–1694. |
| [19] | H. Chen, L.L. Jiao, Z.H. Guo, et al. , Synthesis and biological activity of novel thiazolidin-4-ones with a carbohydrate moiety. Carbohydr. Res. 343 (2008) 3015–3020. |
| [20] | Adel A.H. Abdel-Rahman, S.H. El Ashry, R.R. Schmidt. Synthesis of C-(D-glycopyranosyl) ethylamines and C-(D-glycofuranosyl) methylamines as potential glycosidase inhibitors. Carbohydr. Res. 315 (1999) 106–116. |
 2016, Vol. 27
2016, Vol. 27