b National Centre of Mass Spectrometry in Changchun & Jilin Province Key Laboratory of Chinese Medicine Chemistry and Mass Spectrometry, Changchun Institute of Applied Chemistry, Chinese Academy of Science, Changchun 130022, China ;
c Guizhou Province and Chinese Academy of Sciences, Key Laboratory of Chemistry for Natural Products, Guiyang 550002, China ;
d College of Chinese Medicine Material, Jilin Agricultural University, Changchun 130118, China ;
e Department of Orthopedics, The Fourth People's Hospital of Guiyang, Guiyang 550000, China
Gancaofuzi decoction is composed of Radix Aconiti Lateralis Preparata (Heishunpian),Atractylodes macrocephala Koidz (Baizhu), Cinnamomi Ramulus (Guizhi) and Glycyrrhizae Radix et Rhizoma preparata (prepared Gancao),and has been widely used for the treatment of wind dampness over a long period of time in China [1]. The study found that various chemical ingredients in Heishunpian,Baizhu,Guizhi and Gancao cooperate with each other,i.e.,the chemical ingredients together play an important role in the efficacy of Gancaofuzi decoction [2]. Gancaofuzi decoction has beneficial effects of anti-inflammatory and analgesia,relating to Gancaofuzi decoction’s anti-histamine effect or its regulation of serotonin and prostaglandins. The main chemical ingredients of Heishunpian are alkaloids [3],which has the effect as an antiinflammatory, analgesia,immunomodulatory,antitumor,and so on [4]. Published studies show that oral Heishunpian decoction obviously decreased the foot swelling of the rat induced by formaldehyde or egg white [5, 6],thus some researchers think that aconitine,hypaconitine and mesaconitine in Heishunpian decoction are the active ingredients for its anti-inflammatory effect,and its anti-inflammatory mechanism may be related to the excitatory effect of these three alkaloids on the hypothalamic-pituitary- adrenal system [2, 7]. The main chemical ingredients of Gancao are flavonoids,which have the antagonizing effects on arrhythmia caused by alkaloids [8],and that the pharmacodynamic basis is that Gancao has a two-way adjustment function of NO and TNF-α [9].
Long-term practice shows that the pesticide effect of Gancaofuzi decoction is better than that of any single herbal decoction alone,and the ratio of the four Chinese herbs contained in Gancaofuzi decoction is the best proportion for effectiveness. At present,research on the interaction of four herbs in Gancaofuzi decoction and its chemical ingredients mostly concentrated on the decoction process [2, 10],and less on the interaction related to the absorption process. However,the interaction of drugs in the absorption process plays an important role in drug efficacy and therefore,modern pharmacy research,including theory and technology on the interaction mechanism of the four Chinese herbal medicines in Gancaofuzi decoction is the core content and key link of the research related to Gancaofuzi decoction.
Oral administration is the main route of administration of Chinese herbal compounds,and absorption is the key to exerting the effects of any oral medication in the body. The main route of drug absorption in the body is the small intestine [11]. Therefore, study of the absorption mechanism of drugs in the small intestine is a general trend at present. The Caco-2 cell monolayer model is one of the commonly used cell models to study the absorption mechanism of drugs in vivo as one of the effective tools to study drug absorption,transport and efflux in the small intestine,and also is the most widely used in vitro model in recent years in the world [12]. The Caco-2 cell line was originally proposed by Borchardt and WorkeM in 1989,and was first isolated from human colon cancer cells in the 1970s [13, 14]. Cultured mature Caco-2 is able to form the same dense cell monolayer and cell polarity as small intestine epithelial cells,in this case its morphology and function are similar to those of human intestinal epithelial cells [15]. Therefore,the study of compatibility of traditional Chinese medicine and the interactions of components in the medicine with Caco-2 cells is beneficial to clarify bodily absorption of complex components,to understand the action mechanism of active constituents,and to prove the rationality of the compatibility of traditional Chinese medicine. The study of traditional Chinese medicine with Caco-2 cells plays an important role in the development and modernization of Chinese traditional medicine [16].
2. Experimental 2.1. MaterialsThe Caco-2 human colon carcinoma cell line was taken from the Shanghai Cell Bank of Chinese Academy of Sciences. Heishunpian was purchased from the Sichuan Jiangyou Zhongba Aconiti Technology Development Co.,Ltd. Baizhu,Guizhi and Gancao were purchased from Changchun Tongrentang Chinese Medicine- Since. Dulbecco’s modified Eagle’s medium (DMEM) with high glucose medium,heat-inactivated fetal bovine serum (FBS),HEPES were obtained from Sigma (USA). Penicillin,streptomycin,trypsin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Dingguo Corporation (Beijing,China). DMSO was provided by Xilong Chemical Corporation (Guangdong, China). Acetate,methyl alcohol and acetonitrile were obtained from Fisher Scientific (USA). All other chemicals were of analytical grade or better. The water used in the study was from the Milli-Q water purification system (Millipore Inc.,USA). Transwell transport board (1.12 cm2 surface,0.4 mm pore size,12 mm diameter) was provided by the Corning Costar Corporation (USA); 96-well culture plates were obtained from Thermo (USA).
2.2. Sample preparationExtraction of water-soluble portion of herbs: Crude powders of Heishunpian,Gancao,Baizhu and Guizhi,each 15 g,were added to 10 times total weight of distilled water to soak for 0.5 h and then heated to reflux for 1 h. The solutions (the first extracts) were filtered,and the residue was heated to reflux for 40 min in 8 times total weight of distilled water (the second extracts). The two extracts were individually filtered,and centrifuged,and then the supernatants were mixed as well as concentrated to 0.5 g crude drug/mL. After that,the supernatant was precipitated by 95% alcohol to remove the polysaccharide. At 24 h. later,the solutions were centrifuged and the supernatant was freeze-dried into powders. Crude powders of Gancao,Guizhi and Baizhu were mixed respectively with crude powders of Heishunpian in the ratio of 1:2, 1:1 and 1:2. Crude powders of Heishunpian,Gancao,Guizhi and Baizhu were mixed in the ratio of 2:1:2:1. Then,the mixtures were extracted and freeze-dried by the same method mentioned above.
2.2.1. Preparation of sample solution for the study of MTTIn the study of MTT,dried extracts of Heishunpian were dissolved in DMEM,and further diluted with DMEM to 10 different concentrations (the concentrations of Heishunpian extracts were 0.5,0.6,0.7,0.8,0.9,1.0,1.1,1.2,1.3 and 1.4 mg/mL,respectively), the solutions so generated were used as the working standard solutions in the study.
2.2.2. Preparation of sample solution for the study of transportIn the study on transport of alkaloids in Heishunpian,the single Heishunpian extract was dissolved in HBSS to obtaining the working solutions. The final concentrations of Heishunpian extracts in the samples for the transport study were 0.4 mg/mL, 0.6 mg/mL,0.8 mg/mL and 1.0 mg/mL,respectively. To observe the mechanisms of absorption and transport of alkaloids,Baizhu, Guizhi and Gancao were each respectively mixed with Heishunpian base on the ratios,1:2,1:1 and 1:2. The mixtures were also individually dissolved in HBSS. And the concentration for each mixture was 1.0 mg/mL. The extracts of decoction herbs were further processed using the same method mentioned above.
2.3. Cell cultureThe Caco-2 cells were cultured in DMEM high-glucose medium containing 100 IU/mL penicillin,100 mg/mL streptomycin and 10% fetal bovine serum. The cells were grown in a CO2 incubator at 37 °C temperature,containing 5% CO2 and 90% relative humidity. When the cells covered 80% of the bottle bottom,0.25% trypsin was added,10-15 min. later,0.5 mL fetal bovine serum was added in order to stop the digest. After that the cells were centrifuged, complete medium was added to suspend the cells,and then the cells were counted with cell counting plate.
2.3.1. MTT cytotoxicity assayThe Caco-2 cells in logarithmic growth phase were seeded in 96-well culture plates at a density of 2 × 104 cells per well and incubated for 48 h in a CO2 incubator. After the supernatants were discarded,different concentrations of the test liquids were added to 96-well culture plates,in which,each concentration was set to five parallel holes and the negative control group was set which was only added DMEM. The cells were incubated in CO2 incubator for 2.5 h,after that,the supernatants were discarded,and then 100 mL of MTT (1 mg/mL) was added. Continually,these cells were incubated in CO2 incubator for 4 h,the supernatants were discarded,and then 100 mL of DMSO was added to dissolve the formazan crystals. The absorbance of the 96-well culture plates were measured at 490 nm by a microplate reader.
2.3.2. TransportThe Caco-2 cells in logarithmic growth phase were seeded in Transwell polycarbonate insert filters at a density of 1 × 105 cells/cm2. To the apical side (side A) 0.5 mL cell suspension was added and to the basolateral side (side B) 1.5 mL HBSS was added; in the first 14 days,the medium was replaced every 2 days, and in the next seven days,the medium was replaced every day. At 21 days later,the cells were differentiated into a dense monolayer that can be used for the cell transfer experiments.
The integrity of Caco-2 cell monolayer needed to be verified before initiating the transport experiment. The morphology of cells was observed by optical inverted microscope or electron microscope; the trans-epithelial electrical resistance (TEER) of the Caco-2 cell monolayer was detected; the permeability of Caco-2 cell monolayer was measured using propranolol (100 mmol/L).
The monolayers were rinsed three times with HBSS,but incubated with HBSS in CO2 incubator for 30 min at 37 °C for the third time. In the transport study of side A- B,to the side A 0.5 mL sample was added and to the side B 1.5 mL HBSS was added,and 100 mL solution was removed from side B at times of 20,40,60,90 and 120 min,respectively,and then 100 mL HBSS was added again to side B. In the transport study of side B-A,to the side B 0.5mL sample was added and to the side A 1.5mL HBSS was added,in this case,the procedures were opposite as mentioned above. All the samples were freeze-dried and stored at -80 °C in a refrigerator.
Before detection by UPLC-MS/MS,the samples described above were dissolved in methanol/acetonitrile (1:1,v/v),and reserpine was added to each sample as an internal standard (IS).
2.4. UPLC-MS/MS methodThe conditions of UPLC for analysis were as follows: chromatographic column: Waters (USA) Acquity UPLC BEH Cl8 (50 mm × 2.1 mm,1.7 mm,with a Van Qard BEH C18 used as the precolumn); mobile phase: (A): methanol-acetonitrile (1:1); (B): ammonium acetate solution (5 mmol/L,pH 10.5); flow rate: 0.3 mL/min; detection wavelength: 235 nm; column temperature 35 °C ± 5 °C; injection volume: 10 mL. The gradient elution program is shown in Table S1 in Supporting information.
The MS/MS conditions for the alkaloids in this study were as follows: positive ionization mode; multiple reaction monitoring (MRM); capillary voltage: 2.5 kV; source temperature: 120 °C; desolvation temperature: 350 °C; cone gas flow: 50 L/h,desolvation gas (nitrogen) flow: 700 L/h; collision gas (argon) flow: 0.15 mL/min.
2.5. Data analysisIn this study,the apparent permeability coefficient (Papp) and efflux rate (Er) were used to evaluate the intestinal absorption of alkaloids.
| $Papp=\frac{V}{SC}\frac{dC}{dt}$ | (1) |
where,V is the volume of receiving chamber in L; S is the surface area of the cell monolayers (1.12 cm2 ); C is the initial concentration of the test solution in moL/L; dC/dt is the amount of drug transporter in the unit time.
| $Er=Papp(BL\to AP)/Papp(AP\to BL)$ | (2) |
The efflux ratio (Er) is obtained by dividing the BL-AP permeability by AP-BL permeability as described in the Eq. (1).
3. Results and discussion 3.1. The verification result of Caco-2 cell monolayer modelThe cells were observed by optical inverted microscope,as shown in Fig. S1[1TD$DIF] in Supporting information,the growth of cells was uniform,the boundaries were clear,cells were intact and the connections between the cells were tight. Measuring the TEER of cells with conductivity meter,the TEER of the Caco-2 cell monolayer was in the range of 400-700 V cm2. The concentrations of propranolol before and after transport were measured using HPLC method,according to Eq. (1) to calculate the Papp value of propranolol,the result was that the Papp value of propranolol was greater than 1 × 10-6 cm/s,proving that the state of absorption and transport of Caco-2 cell monolayer were normal.
The above results showed that cells have formed a complete cell monolayer,and its tightness,integrity and permeability were good,and that these cells can be used for the transport experiment.
3.2. The result of MTT cytotoxicity assayAs shown in Table S2 in Supporting information,when the concentration of Heishunpian extract reached 1.1 mg/mL,the extract showed obvious toxic effect on cells with P values <0.01. When the concentration of the Heishunpian extract was less than 1.1 mg/mL,no effect was observed on the activity of the cells and therefore the cells could be used as the concentration of the experiment.
3.3. UPLC-MS/MS method established for the separation and identification of five alkaloids in Gancaofuzi decoctionIn this research,hypaconitine (HA),mesaconitine (MA), benzoylaconitine (BAC),benzoylhypaconitine (BHA) and benzoylmesaconitine (BMA) were taken as the main alkaloid constituents in Gancaofuzi decoction for the quantitative study by UPLC-MS/ MS and multiple reaction monitoring (MRM). At the same time, reserpine was added in the formula as an internal standard. MRM conditions and retention time of five alkaloids are presented in Table S3 in Supporting information.
Under the selected chromatographic and mass spectrometric conditions,five alkaloids were quantitatively analyzed. When the concentration of Heishunpian extract was 100-1000 ng/mL,the linear relationship was good,and the R2 value was more than 0.996,as shown in Table S4 in Supporting information.
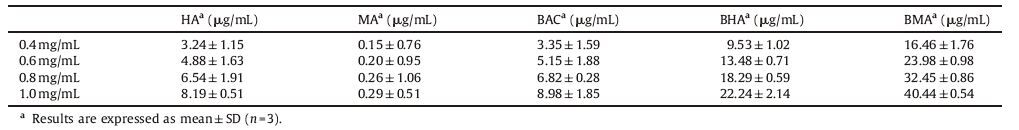
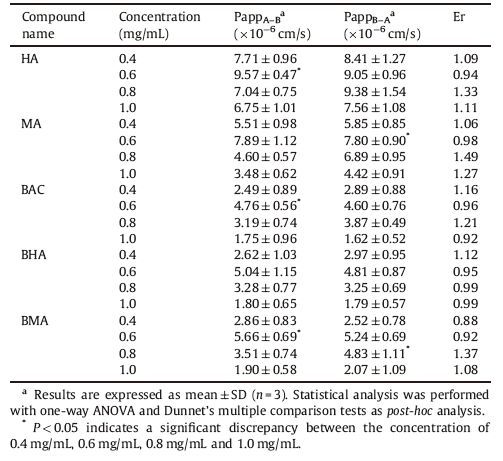
3.4. Effect of different concentrations on the transport of five alkaloids from Heishunpian extractsTo study the effect of concentration on the uptake of the five alkaloids from Heishunpian extract,the transports of AP-BL and BL-AP of five alkaloids at four different concentrations were observed over a period of 2 h. Effect of concentration on contents of the five alkaloids extract and the efflux ratios (PappB-A/ PappA-B) of five alkaloids from Heishunpian extract are shown in Tables 1 and 2.
|
|
Table 1 Polymerization of isoprene at different reaction conditionsa. |
In the Caco-2 cell model,the apparent permeability coefficient (Papp) was associated with the oral absorption of the drug,and the value of Papp can directly reflect the absorption intensities of oral drugs. If the value of Papp is greater than 1 × 10-6 cm/s,it represents a very good absorption capacity of the drug from the gastrointestinal tract [17].
Table 1 shows that there was no obvious change of alkaloids in the extracts of different concentrations from Heishunpian,but the individual quantities of the five kind alkaloids contained in that extracts changed. As can be seen from Table 2,the Papp values of the five major alkaloids from Heishunpian extract were more than 1 × 10-6 cm/s,confirming that these five alkaloids have a good intestinal absorption capacity. When the drug concentration was between 0.4 mg/mL and 1.0 mg/mL,there was no significant difference between the Papp values (P values >0.05),suggested that the Papp values of these alkaloids were not affected by the initial concentration,and the values of Er were between 0.8 and 1.5 [18]. In this case,i.e.,0.4-1.0 mg/mL for the drug concentration,the absorption of Heishunpian in the small intestine was mainly based on passive transport [19].
|
|
Table 2 Polymerization of isoprene at different reaction conditionsa. |
In comparison with monoester-diterpenoid alkaloids and diester-diterpenoid alkaloids in the Papp value of the absorption, it can be seen that the Papp value of diester-diterpenoid alkaloids was significantly higher than that of monoester-diterpenoid alkaloids. The reason may be that monoester-diterpenoid alkaloids is the hydrolysis product of diester-diterpenoid alkaloids,so diester-diterpenoid alkaloids has more basic esters than monoester- diterpenoid alkaloids and its lipid solubility and oil/water distribution coefficient is higher. Therefore,the absorption of diester-diterpenoid alkaloids in the small intestine is better than monoester-diterpenoid alkaloids [20].
3.5. Transport of herbs compatibility in Gancaofuzi decoctionTransports of the five alkaloids,hypaconitine (HA),mesaconitine (MA),benzoylaconitine (BAC),benzoylhypaconitine (BHA) and benzoylmesaconitine (BMA),in Heishunpian individually with Baizhu,Guizhi and Gancao were observed to better understand whether the five alkaloids are influenced by the above three herbs in Gancaofuzi decoction in the human intestine. For this,the investigations were divided into two cases: the first is that one extract of the three herbs was mixed initially with Heishunpian extract,and then observed; the second is that one of the three herbs was mixed initially with Heishunpian,and then decocted,as well as observed. The results show that five alkaloids in different extracts presented different varying rules (see below).
3.5.1. Transport of alkaloids in different herbs’ water extracts combinationAs described above,transports of the five alkaloids in the different herb water extract combinations were observed to determine whether these alkaloids was influenced by the decoction of Baizhu,or Guizhi,or Gancao in human intestines.
As shown in Fig. S2 in Supporting information,it is found that the absorption transport of five alkaloids from apical to basolateral direction in the Caco-2 cell monolayer increased gradually with time,this result indicated that drug through the oral administration, requires longer time,and faster the absorption of alkaloids in intestines. Compared with Heishunpian extract alone,the addition of extracts of Baizhu,or Guizhi,or Gancao can increase the transport rate of BAC,but also decreases the transport rate of HA, and this result indicates that Baizhu,Guizhi and Gancao can delay the absorption of the toxic ingredient HA,but accelerates the absorption of BAC to beneficially permit its pharmacological effects in the body.
As shown in Fig. S3 in Supporting information,it is found that Gancao single decoction can reduce the efflux transport rate of the five alkaloids. This may be attributed to P-glycoprotein mediated efflux transporter in glycyrrhetinic acid of Gancao in Caco-2 cell model [21, 22],since glycyrrhetinic acid competitively combines with P-glycoprotein,therefore the efflux transport rate of the five alkaloids was reduced. Compared with Heishunpian single decoction,the addition of Baizhu single decoction and Guizhi single decoction could reduce the efflux transport rate of diesterditerpenoid alkaloids. Thus,the addition of Baizhu,Guizhi and Gancao after decocting into Heishunpian had the effect of delaying the efflux of alkaloids.
The efflux ratio (Er) can intuitively reflect the actual absorption through the Caco-2 cells in the presence of the excretion. From Table S5 in Supporting information we can see that the Er values of HA in Heishunpian single decoction and the different herb water extract combinations were all greater than 1,which shows that the efflux of HA was greater than that of absorption in the small intestine. This is to say,HA has an active transport mechanism in the small intestine. The combinations of four herb single decoctions can partially reduce the absorption intensities of alkaloids in the small intestine,indicating that these herbs can play a role in reducing the toxicity of alkaloids.
3.5.2. Transport of alkaloids in the mixed herb water extractsTransports of five alkaloids in the mixed herb water extracts were determined to ascertain whether the uptake of the five alkaloids was influenced by the mixed herb decoction in human intestines.
The initial concentration of five alkaloids in the mixed herb water extracts are presented in Table S6 in Supporting information.As shown in Table S6,it is found that Guizhi and Heishunpian decocting together and Gancaofuzi decoction can significantly reduce the dissolution of five alkaloids in Heishunpian (because the initial reduced concentration of five alkaloids). When Baizhu and Gancao decocting together with Heishunpian,it can partially selectively reduce the dissolution of certain alkaloids,the reason may be when Gancao decocting together with Heishunpian,the acidic substance in Gancao may interact with ester alkaloids in Heishunpian to produce precipitation [23],which caused the reduced dissolution of diester-diterpenoid alkaloids in Heishunpian- Gancao water extracts.
As shown in Fig. S4 in Supporting information,when Heishunpian is decocted together with the other three herbs in Gancaofuzi decoction,the absorption transport rates of all five alkaloids were decreased,but to varying different degrees dependent on the each alkaloid. Baizhu,Guizhi and Gancao significantly reduced the absorption transport rate of HA,such as, comparing with Heishunpian single decoction at 120 min,the transport of HA was decreased more than 10%. The reduction of the transport rates of the five alkaloids in “Heishunpian-Guizhi” were the highest (all the five alkaloids were decreased more than 14%). These results show that Baizhu,Guizhi and Gancao when added before decocting into Heishunpian resulted in slowing the release and effect of the five alkaloids,but the effects were better than those herbs when added after decocting into Heishunpian.
As shown in Fig. S5 in Supporting information,Baizhu,Guizhi and Gancao could reduce the efflux transport rates of the five alkaloids, butnot significantly.As shown in Table S7 in Supporting information, Heishunpian decocting together with other three herbs in the Gancaofuzi decoction could reduce the intestinal absorption intensities of all the five alkaloids,especially HA.When Heishunpian is decocted together with Gancao,the intestinal absorption intensities of HA,BAC,BHA and BMA are obviously decreased. In the process of decoction,glycyrrhizic acid or glycyrrhetinic acid in Gancaomaybe interact with the alkaloids in Heishunpian,sothat the molecular weight or polarity of the combined product will increase, to cause the reduction of the intestinal absorption of the alkaloids [24]. From what has been discussed above,the mixed herb water extracts could reduce the toxicityof alkaloidsbyboth“[9TD$DIF]reducing toxic components dissolution” and “reducing the intestinal absorption of toxic components”.
4. ConclusionIn this study,the Caco-2 cell model was used to clarify the rationality and principle of the compatibility of four herbs in Gancaofuzi decoction from the view of the interaction between medicinal molecules and biomolecules utilizing UPLC-MS/MS method in the analyses of samples. The results suggest that the five alkaloids have good absorption in the small intestine,and the absorption of diester-diterpenoid alkaloids in the small intestine is better than monoester-diterpenoid alkaloids. The flux of alkaloids in Heishunpian were time-dependent,i.e.,the longer the exposure time,the greater the absorption of alkaloids in the intestines. The intestinal absorption mechanism of the five alkaloids was mainly based on passive transport,and the absorption mechanism of HA in the small intestine is both active transport and passive transport. The compatibility of Heishunpian,Baizhu,Guizhi and Gancao could reduce the intestinal absorption of alkaloids,especially the most toxic hypaconitine,and the attenuated effect of the mixed herb water extracts was better than that of certain individual herb water extract combinations. The results support the rational basis for compatibility of the four herbs in Gancaofuzi decoction,and should directly influence the reasonable application of Gancaofuzi decoction in pharmacodynamic study and clinical treatment.
AcknowledgmentThis research was supported by the National Natural Science Foundation of China (No. 81173507) and the Science Foundation of Jilin province (No. 20150414040GH).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.03.001.
| [1] | J.F. Li, Y.L. Wu. Clinical application of licorice aconite decoction. J. Yunnan Univ. Tradit. Chin. Med. 34 (2011) 47. |
| [2] | Q.T. Gao. Study on the Therapeutic Basis of Traditional Chinese Medicinal Preparation Gancaofuzi Decoction, Shenyang Pharmaceutical University, Shenyang. (2005) . |
| [3] | Y.P. Kao, M.J. Liu, Q.Z. Yuan. Chemical constituents and pharmacological activities of aconite. Shaanxi J. Tradit. Chin. Med. 31 (2010) 1658–1660. |
| [4] | M. Hazawa, K. Wada, K. Takahashi, et al. , Suppressive effects of novel derivatives prepared from Aconitum alkaloids on tumor growth. Investig. New Drugs 27 (2009) 111–119. |
| [5] | S.Z. Chen. Effect of Wu Tou Decoction on Rheumatoid Arthritis and its Possible Mechanism, Guangzhou University of Traditional Chinese Medicine, Guangzhou. (2006) . |
| [6] | P.J. Wang, X.G. Shi, Z. Ge, X.D. Peng, Z.S. Huang. Effects of aconite decoction on peripheral blood T lymphocytes of adjuvant arthritis rat. Pharmacol. Clin. Chin. Mater. Med. 23 (2007) 9–10. |
| [7] | W.D. Xu, X. Wang, C. Wang, et al. , Study on Rougui and Fuzi's role in tonifying kidney yang by Shenqiwan from hypothalamic-pituitary-adrenal axis. J. Zhejiang Chin. Med. Univ. 38 (2014) 831–836. |
| [8] | X.Y. Hu, G.P. Peng, R.Y. Chen. Antagonism of liquorice toward arrhythmia induced by prepared aconite root. J. Nanjing Univ. Tradit. Chin. Med. 12 (1996) 64. |
| [9] | H. Chen. Outlines of pharmacological action of glycyrrhizae. Strait Pharm. J. 17 (2005) 37–41. |
| [10] | X.J. Yao. Analyses of the Chemical Composition of Traditional Chinese Medicinal Preparation Gancaofuzi decoction, Jilin University, Changchun. (2013) . |
| [11] | P. Stenberg, C.A.S. Bergström, K. Luthman, P. Artursson. Theoretical predictions of drug absorption in drug discovery and development. Clin. Pharmacokinet. 41 (2002) 877–899. |
| [12] | Z.L. Lu, Y. Feng, D.S. Xu, X. Lin, Y. Yang. Application of Caco-2 cell model in peroral absorption of Chinese materia medica and its mechanism. Chin. Tradit. Herb. Drugs 37 (2006) 616–619. |
| [13] | M. Markowska, R. Oberle, S. Juzwin, et al. , Optimizing Caco-2 cell monolayers to increase throughput in drug intestinal absorption analysis. J. Pharmacol. Toxicol. Methods 46 (2001) 51–55. |
| [14] | P. Augustijns, R. Mols. HPLC with programmed wavelength fluorescence detection for the simultaneous determination of marker compounds of integrity and P-gp functionality in the Caco-2 intestinal absorption model. J. Pharm. Biomed. Anal. 34 (2004) 971–978. |
| [15] | A.R. Hilgers, R.A. Conradi, P.S. Burton. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm. Res. 7 (1990) 902–910. |
| [16] | B. Waltenberger, B. Avula, M. Ganzera, et al. , Transport of sennosides and sennidines from Cassia angustifolia and Cassia senna across Caco-2 monolayersan in vitro model for intestinal absorption. Phytomedicine 15 (2008) 373–377. |
| [17] | N. Li. In Vitro Study on Absorption and Mechanism of Diester-type Alkaloids in Aconite Extract. Jilin University, Changchun, (2009) . |
| [18] | J. Zhao, A.H. Liang. Caco-2 cell model and its application in the absorption and transportation research of Chinese materia medica. Chin. J. Exp. Tradit. Med. Form. 15 (2009) 79–83. |
| [19] | X. Li. In Vitro Studies on Absorptive Transportion of Alkaloids in Radix Aconiti Kusnezoffii After Processing and Combination. Jilin University, Changchun, (2012) . |
| [20] | J.W. Ma. In Vitro Study on Mechanism of Attenuation in Aconitum by Processing and Compatibility. Jilin University, Changchun, (2014) . |
| [21] | Z. Wang, Y. Kurosaki, T. Nakayama, T. Kimura. Mechanism of gastrointestinal absorption of glycyrrhizin in rats. Biol. Pharm. Bull. 17 (1994) 1399–1403. |
| [22] | T.J. Han, F.R. Song, Z.Y. Liu, et al. , Studies of the intestinal transport of the diterpenoid alkaloids in the Aconitum carmichaeli combined with different medicinal herbs in a Caco-2 cell culture system with UPLC/MS. Acta Chim. Sin. 69 (2011) 1795–1802. |
| [23] | M. Yang, X.B. Liu, Q.D. Huang. The increasing effect and reducing ill effect principle of compatibility of radix Aconiti Lateralis preparata and radix Glycyrrhizae. Lishizhen Med. Mater. Med. Res. 14 (2003) 197–198. |
| [24] | C.X. Chen, S.J. Xu. Research progress on the material basis and function of the compatibility between Licorice. Ginger and Aconite. Tradit. Chin. Drug Res. Clin. Pharmacol. 17 (2006) 472–476. |
 2016, Vol. 27
2016, Vol. 27