Isoprene is an important industrial monomer used for manufacturing synthetic rubbers called polyisoprene (PIP) and its copolymers. With a global market of over 1 million tonnes per annum,PIP has been used in a wide range of applications such as toys,adhesives,paints,automotive parts,and medical equipment [1, 2]. Industrially,PIP is synthesized by alkyllithium-based anionic polymerization [3],rare-earth metal catalyzed polymerization [4, 5] or Ziegler-Natta coordination polymerization [6, 7]. These approaches,however,are sensitive to air,humidity,and impurity, and thus require stringent reaction conditions such as rigorous drying of solvents,deep purification of monomers,and extra-low reaction temperatures. The resulting high production cost of PIP limits its range of applications.
Atom transfer radical polymerization (ATRP),catalyzed by transition-metal complexes [8-11],has been explored as one of the most successful controlled/“living” radical polymerization techniques due to its mild reaction conditions,excellent tolerance to functional groups and impurities,and precise control on molecular weight and architecture of macromolecules. Though ATRP has been applied to a large variety of monomers including styrenes, (meth)acrylates,(meth)acrylamides,acrylonitrile,and vinyl acetate [12-15],ATRP of isoprene is still troublesome. There have been some reports on polymerizations of isoprene via other controlled/ “living” radical polymerization approaches such as nitroxidemediated polymerization (NMP) [16, 17] and reversible additionfragmentation chain transfer (RAFT) polymerization [18, 19],whereas the investigation on ATRP of isoprene in open literature is very limited. For example,Matyjaszewski described ATRP of isoprene initiated by 1-phenylethyl chloride in a United States patent,but there were no detailed polymerization kinetics and characterization data provided in the patent and the obtained PIP had a broad molecular weight distribution (Mw/Mn = 2.0) [20]. Wootthikanokkhan et al. tried to use CuBr/N,N,N’,N " ,N"-pentamethyldiethylenetriamine (CuBr/PMDETA) and CuBr/tris[2- (dimethylamino)ethyl]amine (CuBr/Me6TREN) as catalysts to polymerize isoprene. Unfortunately little to no PIP was obtained due to poor solubility of the catalysts in isoprene and low chain propagation rate constant of isoprene monomer [21]. Therefore, ATRP was once regarded as incapable of exerting sufficient control over the polymerization of isoprene.
We herein report the ATRP of isoprene and the block copolymerization of isoprene with styrene for the synthesis of polyisoprene-b-polystyrene block copolymer (PS-b-PIP) usingCuBr/ 2,20-bipyridine (CuBr/Bpy) as a catalyst and methyl 2-bromopropionate (EBP) as an initiator. The “living” characteristics of the polymerization,the reaction kinetics,as well as the structural characterization of PIP homopolymer and PS-b-PIP block copolymer are presented and discussed in this work.
2. Experimental 2.1. MaterialsIsoprene (IP,99%,Aldrich) and styrene (St,99%,Aldrich) were washed with 5% (w/w) NaOH solution,dried over molecular sieve, vacuum distilled,and stored in a refrigerator. Copper (I) bromide (CuBr,98%,Aldrich) and copper (Ⅱ) chloride (CuCl,98%,Aldrich) were purified according to the literature [22]. 2,2'-Bipyridine (Bpy, 99%,Aldrich) was recrystallized from petroleum ether. N,N,N0,N " ,N"-Pentamethyldiethylenetriamine (PMDETA,98%, Shanghai Chemical Reagent Co.,) and N,N,N0,N0-tetramethylethylenediamine (TMEDA,98%,Shanghai Chemical Reagent Co.,) were used as received. Tris[2-(dimethylamino)ethyl]amine (Me6TREN) and N,N,N0,N0-tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN), 4,4'-dinonyl-2,2'-bipyridine (dNbpy,99%),ethyl 2-bromoisobutyrate (EBiB,98%),ethyl 2-bromopropionate (EBP,98%),ethyl 2- bromophenylacetate (EBPA,99%),and bromoacetonitrile (BrAN, 99%) were purchased from Sigma-Aldrich and used without any further treatment.
2.2. Characterization1H NMR(500 MHz) spectra were recorded on a Bruker Avance ⅡI 500 MHz spectrometer at 20 °C in CDCl3 with tetramethylsilane (TMS) (d = 0 ppm) as an internal standard. The monomer conversion of isoprene was determined by a gravimetric method [23]. The number-average molecular weight (Mn),weight-average molecular weight M2 and molecular weight distribution (Mw/Mn) of PIP and PS-b-PIP block copolymer were measured on a Shimadzu Prominence gel permeation chromatograph (GPC) system equipped with a Shimadzu RID-10A refractive index detector and a Waters Styragel HR-4E solvent-saving column (4.6 × 300mm,molecular weight range: 500-100,000 Da). Tetrahydrofuran (THF) was used as an eluent at a flow rate of 0.3 mL/min and the column temperature was 35 °C. A series of polystyrene standards (American Polymer Standards Corporation,molecular weights ranging from 1030 to 570,000) were employed to produce calibration curves for the calculation of molecular weights of PIP and PS-b-PIP.
2.3. ATRP of isopreneIn a typical experiment,a 48 mL Ace glass pressure tube equipped with #15 Ace-Thred,PTFE bushing,and plunger valve was charged with a magnetic stirring bar and a predetermined amount of copper halide (e.g.,CuBr and CuCl) and nitrogenous ligand (e.g. Bpy and dNbpy). The tube was degassed through the plunger valve by applying high vacuum and refilling the tube with high purity nitrogen (five cycles). A certain amount of degassed isoprene,THF,and initiator (e.g.,EBiB,EBP,or BrAN) were then injected into the tube through the small hole on the plunger valve using gastight syringes under the protection of nitrogen. The plunger valve was closed and the tube was afterward put into an oil bath and equilibrated to desired temperatures (e.g.,150 °C). After appropriate reaction time,the tube was removed from the oil bath and cooled down to room temperature. The reaction sample was collected,placed in a hermetic vial,and stored in a freezer for GPC analysis and gravimetric measurements. The kinetic data was accumulated from individual experiments since sampling of the viscous reaction solution through the plunger hole via a long needle was practically infeasible. The left polymer product in the reaction tube was dissolved in THF and precipitated in excess of methanol to remove the copper catalyst. The precipitates were collected,dried under vacuum at 35 °C,and used for NMR measurements.
2.4. Synthesis of the PS-b-PIP copolymer via ATRPThe PS-b-PIP block copolymer was synthesized by ATRP of isoprene using a PS macroinitiator (PS-Br) having narrow molecular weight distribution (Mn = 7850,PDI = 1.28). The macroinitiator was prepared by ATRP of St using CuBr/HMTETA as a catalyst and EBiB as an initiator with a feeding molar ratio of St/ EBiB/CuBr/HMTETA = 100:1:1:1 based on a reported method [15]. Briefly,PS-Br (0.395 g,0.05 mmol),CuBr (28.7 mg,0.20 mmol),and Bpy (62.4 mg,0.40 mmol) were added into a Schlenk flask. The flask was tightly sealed with a rubber septum and then degassed by five cycles of vacuum and nitrogen filling. Degassed isoprene (2.5 mL,25.0 mmol) and THF (0.5 mL) were added using nitrogenpurged gastight syringes and the mixture was heated at 130 °C in an oil bath. The polymerization was stopped 48 h later and the solution was precipitated in methanol. The precipitate was collected,vacuum dried at 30 °C,and used for NMR and GPC analysis.
3. Results and discussion 3.1. ATRP of isopreneCopper halides ligated with nitrogenous ligands are most widely used ATRP catalysts. A series of CuBr/tertiary amine or CuCl/ tertiary amine complexes were first tested for ATRP of isoprene,as shown in Table 1. Although CuBr/Me6TREN is a highly active catalyst with an activation rate constant (kact) of 4.5 × 102 [24] and has been reported to polymerize styrene,butyl acrylate,and methyl methacrylate at catalyst concentrations as low as tens of ppm level in a ICAR or ARGET process [25, 26],it failed to catalyze the polymerization of isoprene (Table 1,entry 1). Similarly,no polymerization occurred in ATRP of isoprene catalyzed by another highly active catalyst CuBr/TPEN (Table 1,entry 2). This result indicated these highly active copper catalysts were not suitable for isoprene because the chain propagation rate constant of isoprene was very low while the activation rate constants of the two catalysts were very high,which caused a high radical concentration at early stage of the polymerization and consequently led to significant irreversible radical terminations and rapid accumulations of copper (Ⅱ) complexes due to the “persistent radical effect” [27, 28]. The high concentration of copper (Ⅱ) complex acted as a deactivator to the polymerization reaction and subsequently prohibited the polymerization of isoprene.
|
|
Table 1 Polymerization of isoprene at different reaction conditionsa. |
Compared with CuBr/Me6TREN,CuBr/TEDTA is a much less active catalyst with a kact at least four orders of magnitude lower than that of CuBr/Me6TREN [24]. However,CuBr/TMEDA also showed no activity to the polymerization of isoprene (Table 1, entry 3). Only a trace amount of polyisoprene was obtained in the polymerization catalyzed by CuBr/PMEDTA (Table 1,entry 4), which was consistent with Wootthikanokkhan’s results. Fortunately, another typical ATRP catalyst CuBr/Bpy with a moderate kact of ~0.1 was found to slowly polymerize isoprene to 27.8% conversion in 72 h at 100 °C with EBiB as an initiator (Table 1,entry 5). To improve the monomer conversion,EBiB was replaced with two highly active initiators,EBPA and BrAN,but these two initiators only produced negligible polyisoprenes (Table 1,entries 6 and 7),indicating that initiators that are too active might react too fast with copper catalyst and thus generate high concentrations of free radicals at the early initiation stage of the polymerization,which inevitably led to a high accumulation of copper (Ⅱ) species and subsequent inhibition of the polymerization. A high monomer conversion was achieved when a less active initiator EBP was used to replace EBiB. The EBP-initiated polymerization reached 53.3% conversion in 72 h at 130 °C, producing PIP with a high molecular weight and relatively narrow distribution (Table 1,entry 8). With same initiator,the replacement of CuBr with CuCl or Bpy with dNbpy resulted in significant lower molecular weights and broader molecular weight distributions of PIP (Table 1,entries 9 and 10). Therefore,CuBr/Bpy combined with EBP seemed to be the best catalyst-initiator combination for ATRP of isoprene at current experimental conditions.
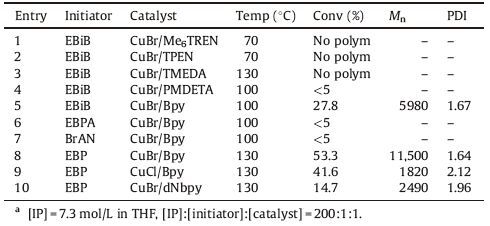
The kinetic plots of the polymerization of isoprene at different reaction temperatures with EBP as an initiator and CuBr/Bpy as a catalyst are shown in Fig. 1. The polymerizations at 100 °C,130 °C, and 150 °C proceeded smoothly and reached 48.1%,53.3%,and 71.0% conversions respectively in 72 h. The polymerization at 150 °C was significantly faster than the other two polymerizations. All ln([M]0/[M]) ~t plots were linear,indicating that the concentrations of growing radicals remained essentially constant during the polymerization and the ATRP of isoprene followed a first-order kinetics with respect to monomer concentration.
|
Download:
|
| Figure 1. Monomer conversion and ln[M]0/[M] versus time plots for ATRP of IP at 100 °C (●,○), 130 °C (◆,◇) and 150 °C (■,〼). Conditions: [IP] = 7.3 mol/L, [IP]:[EBP]:[CuBr]:[Bpy] = 200:1:1:2. | |
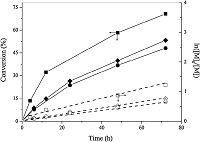
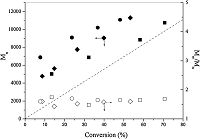
As shown in Fig. 2,the molecular weights of the produced PIP increased linearly with the monomer conversion,suggesting that CuBr/Bpy mediated a controlled/“living” radical polymerization of isoprene. The PIP prepared at 100 °C had molecular weights significantly higher than theoretical values while the molecular weights of PIP synthesized at 150 °C remained close to theoretical values (dashed line). The molecular weight distribution of obtained PIP was relatively narrow at low conversions (e.g.,Mw/Mn = 1.48, 32.2% conversion,150 °C) and broadened at high conversion (e.g.,Mw/Mn = 1.68,71.0% conversion,150 °C). This polydispersity index was,however,lower than that of PIP prepared by Matyjaszewski (Mw/Mn = 2.0) [20] and the monomer conversion was markedly higher than that reported by Wootthikanokkhan (~5%) [21]. It is possible to lower the polydispersity if a less active catalyst with a high deactivation rate constant (kdeact) is used,but the polymerization may become slower in this situation and only limited monomer conversion could be achieved. The PIP from the polymerization carried out at 150 °C showed monomodal and symmetric GPC curves with normal distributions,and the GPC curve gradually and smoothly shifted from lower molecular weight region to higher molecular weight region as isoprene conversion increased (Fig. 3),which further indicated the living character of the polymerization.
|
Download:
|
| Figure 2. The molecular weights (Mn) and polydispersity index (Mw/Mn) of PIP as a function of monomer conversion for ATRP of IP at 100 °C (●,○), 130 °C (◆,◇) and 150 °C (■,〼). See Fig. 1 for conditions. | |
|
Download:
|
| Figure 3. GPC elution curves of the PIP prepared at 150 °C. See Fig. 1 for conditions. | |
3.2. Structural analysis of PIP
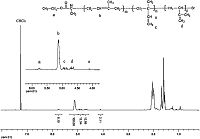
Generally,PIP has three type microstructures,namely 1,4- polyisoprene,1,2-polyisoprene,and 3,4-polyisoprene due to the different addition mode of isoprene monomer [29]. The 1H NMR spectrumof the obtainedPIP is showninFig. 4.According to reported literature [17, 30],the major resonance at 5.1 ppm corresponds to the 1,4-addition structure. The multiple peaks at 5.7-5.8 ppm and 4.8-5.0 ppmwere caused by 1,2-addition reactionwhile the peak at 4.6-4.7 ppm was due to the 3,4-addition structure. The minor peak at 4.2 ppm was assigned to the ester methylene group from the initiator EBP. Based on the integration ratio of peak b,c,and d,the calculated content of 1,4-polyisoprene was 88.8%,indicating the PIP prepared from CuBr/Bpy was primarily 1,4-polyisoprene. According to the integration ratio of peak b,c,d,and e,the molecular weight of PIP was calculated to be 7480,which is lower than 10,500 as measured by GPC (Fig. 2). This discrepancy most likely results from the GPC analysis which used polystyrene for calibration. Fig. 5 demonstrates the 13C NMR of the obtained PIP. According to literatures [6, 31],the peak at 125.0 ppm was due to cis-1,4 configuration of the backbone unit of PIP while the peak at 124.2 ppm is from trans-1,4 structure. Based on the peak integrations,the trans-structure content was determined to be 63.9%.
|
Download:
|
| Figure 4. 1H NMR spectrum of PIP prepared at 150 °C with CuBr/Bpy as a catalyst and EBP as an initiator. | |

|
Download:
|
| Figure 5. 13C NMR spectrum of PIP prepared at 150 °C with CuBr/Bpy as a catalyst and EBP as an initiator. | |
3.3. Synthesis and characterization of PS-b-PIP block copolymer
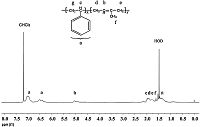
PS-b-PIP block copolymers were generally prepared by anionic or coordination polymerization. A facile synthesis of PS-b-PIP by direct initiation of isoprene with polystyrene macroinitiator was achieved using CuBr/Bpy as a catalyst. The macroinitiator (Mn = 7850,PDI = 1.28) initiated the polymerization of isoprene in the presence of CuBr/Bpy,producing PS-b-PIP (Mn = 14900,PDI = 1.68) with relatively higher polydispersity,as shown in Fig. 6. The elution peak of PS-b-PIP clearly shifts to a high molecular weight region without unreacted PS macroinitiator left in low molecular weight region,indicating a nearly complete formation of PS-b-PIP. The actual molecular weight of prepared PS-b-PIP block copolymer was determined by 1H NMR spectrum (Fig. 7). Typical signals from polystyrene segments (a) and 1,4-polyisoprene segments (b) were clearly observed. According to the integration of peaks a and b,the actual molecular weight of polyisoprene block was calculated to be 6130 and the total molecular weight of PS-b-PIP block copolymer was 13,980,which is consistent with its GPC result as illustrated in Fig. 6.

|
Download:
|
| Figure 6. GPC curves of block copolymer PS-b-PIP and the corresponding PS-Br macroinitiator. Experimental conditions: 130 °C, [PS-Br] = 0.016 mol/L, [IP] = 8.1 mol/L, [CuBr] = 0.065 mol/L, [Bpy] = 0.13 mol/L. | |
|
Download:
|
| Figure 7. 1H NMR spectrum of PS-b-PIP block copolymer. | |
4. Conclusion
In summary,ATRP of isoprene has been achieved with CuBr/Bpy as a catalyst and EBP as an initiator. The polymerizations conducted at 100 °C,130 °C,and 150 °C proceeded smoothly and reached 48.1%,53.3%,and 71.0% conversions,respectively,in 72 h. The produced PIP had 88.8% 1,4-addition microstructure and 63.9% of the backbone units were in trans-configuration. The molecular weights of PIP increased linearly with the monomer conversion and the molecular weight distributions remained relatively narrow,indicating the ATRP of isoprene mediated by CuBr/Bpy was a controlled/“living” radical polymerization process. The living characters of the polymerization were further confirmed by the block copolymerization of isoprene to synthesize PS-b-PIP block copolymer using a polystyrene macroinitiator.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21174133) and Zhejiang Science Foundation for Distinguished Young Scholars (No. LR12B04002).
| [1] | A.P. Leber. Overview of isoprene monomer and polyisoprene production processes. Chem.: Biol. Interact 135-136 (2001) 169–173. |
| [2] | R.D.F.M. Taalmann, Isoprene: background and issues. Toxicology 113 (1996) 242–246. |
| [3] | C.W. Kamienski. Lithium catalysis in industrial polymerization. Ind. Eng. Chem. 57 (1965) 38–55. |
| [4] | W. Gao, D.M. Cui. Highly cis-1,4 selective polymerization of dienes with homogeneous Ziegler-Natta catalysts based on NCN-Pincer rare earth metal dichloride precursors. J. Am. Chem. Soc. 130 (2008) 4984–4991. |
| [5] | M. Nishiura, Z.M. Hou. Novel polymerization catalysts and hydride clusters from rare-earth metal dialkyls. Nat. Chem. 2 (2010) 257–268. |
| [6] | D.T. Liu, D.M. Cui, W. Gao. Highly stereospecific polymerization of isoprene with homogeneous binary Ziegler-Natta catalysts based on NCN-pincer neodymium precursor. Sci. China Chem. 53 (2010) 1641–1645. |
| [7] | C.K.W. Tse, K.R. Kumar, M.J. Drewitt, M. C. Baird. Isobutene polymerization and copolymerization with isoprene Initiated by [Cp*TiMe2]+ in the presence of a novel type of weakly coordinating counteranion. Macromol. Chem. Phys. 205 (2004) 1439–1444. |
| [8] | M. Kato, M. Kamigaito, M. Sawamoto, T. Higashimura. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris(triphenylphosphine)ruthenium( II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules 28 (1995) 1721–1723. |
| [9] | J.S. Wang, K. Matyjaszewski, Controlled/"living" radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 117 (1995) 5614–5615. |
| [10] | K. Matyjaszewski, J.H. Xia. Atom transfer radical polymerization. Chem. Rev. 101 (2001) 2921–2990. |
| [11] | M. Kamigaito, T. Ando, M. Sawamoto. Metal-catalyzed living radical polymerization. Chem. Rev. 101 (2001) 3689–3745. |
| [12] | J.H. Xia, K .. Matyjaszewski, Controlled/"living" radical polymerization. Atom transfer radical polymerization catalyzed by copper(I) and picolylamine complexes. Macromolecules 32 (1999) 2434–2437. |
| [13] | X.S. Wang, N. Luo, S.K. Ying. Synthesis of EPDM-g-PMMA through atom transfer radical polymerization. Polymer 40 (1999) 4515–4520. |
| [14] | S.G. Gaynor, S. Edelman, K. Matyjaszewski. Synthesis of branched and hyperbranched polystyrenes. Macromolecules 29 (1996) 1079–1081. |
| [15] | H.D. Tang, M. Radosz, Y.Q. Shen. Atom transfer radical polymerization and copolymerization of vinyl acetate catalyzed by copper halide/terpyridine. AIChE J 55 (2009) 737–746. |
| [16] | D. Benoit, E. Harth, P. Fox, R.M. Waymouth, C.J. Hawker. Accurate structural control and block formation in the living polymerization of, 1,3-dienes by nitroxide-mediated procedures. Macromolecules 33 (2000) 363–370. |
| [17] | P.N. Mogaddam, A. Entezami. Synthesis of triblock copolymers of styrene and isoprene by a nitroxide-mediated living free radical polymerization. Chin. J. Polym. Sci. 22 (2004) 55–61. |
| [18] | V. Jitchum, S. Perrier. Living Radical polymerization of isoprene via the RAFT process. Macromolecules 40 (2007) 1408–1412. |
| [19] | G. Bar-Nes, R. Hall, V. Sharma, et al. , Controlled/living radical polymerization of isoprene and butadiene in emulsion. Eur. Polym. J. 45 (2009) 3149–3163. |
| [20] | K.Matyjaszewski, J.S.Wang, (Co)polymers and a novel polymerization process based on atom (or group) transfer radical polymerization, US Patents 5763548, 1998. |
| [21] | J.Wootthikanokkhan, M. Peesan, P. Phinyocheep. Atom transfer radical polymerizations of (meth)acrylicmonomers and isoprene. Eur. Polym. J. 37 (2001) 2063–2071. |
| [22] | J. Queffelec, S.G. Gaynor, K. Matyjaszewski. Optimization of atom transfer radical polymerization using Cu(I)/tris(2-(dimethylamino)ethyl)amine as a catalyst. Macromolecules 33 (2000) 8629–8639. |
| [23] | X. Huang, M.J. Wirth. Surface initiation of living radical polymerization for growth of tethered chains of low polydispersity. Macromolecules 32 (1999) 1694–1696. |
| [24] | K. Matyjaszewski. Atom transfer radical polymerization (ATRP): current status and future perspectives. Macromolecules 45 (2012) 4015–4039. |
| [25] | W. Jakubowski, K. Min, K. Matyjaszewski. Activators regenerated by electron transfer for atom transfer radical polymerization of styrene. Macromolecules 39 (2006) 39–45. |
| [26] | K. Matyjaszewski, W. Jakubowski, K. Min, et al. , Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents. Proc. Natl. Acad. Sci. USA 103 (2006) 15309–15314. |
| [27] | H. Fischer. The persistent radical effect in controlled radical polymerizations. J. Polym. Sci. A: Polym. Chem. 37 (1999) 1885–1901. |
| [28] | H. Fischer. The persistent radical effect in "living" radical polymerization. Macromolecules 30 (1997) 5666–5672. |
| [29] | J.N. Li, J. El Harfi, S.M. Howdle, K. Carmichaelc, D .J. Irvine. Controlled oligomerisation of isoprene-towards the synthesis of squalene analogues. Polym. Chem. 3 (2012) 1495–1501. |
| [30] | B.L. Wang, D.M. Cui, K. Lv. Highly 3,4-selective living polymerization of isoprene with rare earth metal fluorenyl N-heterocyclic carbene precursors. Macromolecules 41 (2008) 1983–1988. |
| [31] | G. Ricci, A. Sommazzi, F. Masi, et al. , Well-defined transition metal complexes with phosphorus and nitrogen ligands for, 1,3-dienes polymerization. Coord. Chem. Rev. 254 (2010) 661–676. |
 2016, Vol. 27
2016, Vol. 27