b Anhui Key Laboratory of Controllable Chemical Reaction & Material Chemical Engineering, Hefei 230009, China ;
c Anhui Provincial Laboratory of Heterocyclic Chemistry, Maanshan 243110, China
Cyclopentanone is an important fine chemical intermediate for the preparation of new spices,a variety of anti-inflammatory,anti-cancer drugs,pesticides and herbicides [1]. Currently,the main method of preparation of cyclopentanone in industrialized production is by intramolecular decarboxylation of adipic acid at high temperature with barium hydroxide as catalyst. But this method has disadvantages due to the shortage and high cost of raw materials,and is not in conformity with green chemistry principles. With the rapid development of the petrochemical industry,cyclopentene is abundant and the method to synthesize cyclopentanone via cyclopentene has received wide attention.
The early literature reported that cyclopentene was oxidized by N2O with high yield and the absence of a catalyst. But there were shortcomings,for example,the reaction pressure and temperature were high,N2O damaged the equipment and caused environmental pollution [2]. Takehira and others used homogeneous catalyst PdCl2-CuCl2,PdCl2-FeCl3 to catalyze the oxygenization of cyclopentene to generate cyclopentanone,however,this method had a low conversion rate and the reusability of catalyst was unsatisfactory [3, 4].
According to the mechanism of oxygenization of cyclopentene to cyclopentanone,a solid catalyst consisting of metal would be useful for this reaction. Due to good mechanical strength and thermal stability,γ-Al2O3 has been widely applied as the carrier in industry.According to literature,Pd is active to the fracture of C=C and shows good selectivity in the catalytic oxygenization of cyclopentene,but has relatively low catalytic efficiency. Nickel and copper are nonnoble metals with great catalytic ability. In the study of theWacker method to synthesize cyclopentanone,PdCl2/CuCl2 performed good catalytic activity [3, 4],consequently we speculated that Cu may be beneficial in the synthesis of C=O. Mao used Pd-Cu bimetallic catalysts for the oxygenization of styrene,showed good catalytic ability and selectivity [5]. Therefore,in our study,Pd-Cu/γ-Al2O3 was chosen as the catalyst to oxidize cyclopentene.
The aim of this paper is to report the preparation,characterization and catalytic performance of Pd-Cu/γ-Al2O3 (with difference mole ratios of Pd:Cu). Moreover,the parameters influencing the reaction of cyclopentene to cyclopentanone are intensely studied.
2. ExperimentalPreparation of Pd-Cu/γ-Al2O3 catalysts: The Pd-Cu/γ-Al2O3 catalysts were prepared by the impregnation method [6-10]. In a typical Pd-Cu (5:1)/γ-Al2O3 catalyst synthesis process,PdCl2(131.9 mg,0.75 mmol) and CuCl2▪2H2O (25.1 mg,0.15 mmol) were dissolved in 10 mL acetic acid solutions (0.0225 mol/L) with constant agitation (500 rpm/min) at r.t. To this clear solution,then was slowly added γ-Al2O3 (1.5 g) and uniformly stirred at 300 rpm/ min for 5 h. After impregnated,the solution was heated at 100 °C for removal of the redundant H2O,forming a sticky substance which was dried in vacuum oven at 80 °C for 12 h. Subsequently,the resulting catalytic forerunners were ground to powders and calcined in nitrogen (0.3 L/min) heating from r.t. to 500 °C at a rate of 5 °C/min,then kept at 500 °C for 4 h. Subsequently,the sample precursors were reduced by hydrogen (0.15 L/min) in the nitrogen at 500 °C for 2 h. Finally,the Pd-Cu (5:1)/γ-Al2O3 catalyst was obtained. In order to research the effect of the loading of metals,different catalysts of Pd/Cu mole ratios were synthesized for use in the oxygenization reaction.
Characterization: The XRD of different samples were recorded on a Rigaku D/MAX 2500 (V) Diffractometer with Cu Ka radiation and scanned at a rate of 108/min over the range 20°-90°. The XPS analysis was confirmed on Thermo Fisher Scientific ESCALAB 250 using a monochromatic Al Ka gun with photonic energy of 1486.6 eV as the X-ray source under reduced vacuum conditions.The binding energies (B.E.) acquired in the XPS analysis were compensated by using the adventitious C 1s 284.6 eV signal for any charging effects. The TEM images of catalysts were obtained on a JEOL JEM-2100F Electron Microscope equipped with EDS at an accelerating voltage of 120 kV. Samples were prepared by ultrasonic dispersion in ethanol and deposited on a copper grid for analysis.
Catalytic reaction: In a typical procedure,15mL cyclopentene (0.17 mol),60 mL ethyl alcohol (1.03 mol) and Pd-Cu (5:1)/γ-Al2O3 catalyst (0.24 g,2.0 wt% of cyclopentene) were added into 0.1 L closed autoclave. The mixture was stirred (400 rpm/min) under 1.0 MPa oxygen pressure at 100 °C for 7 h. Specific parameters are provided in Supporting information.
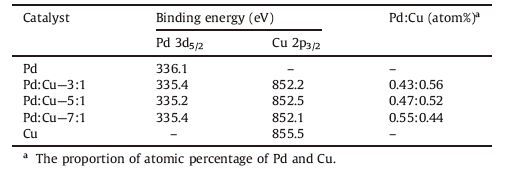
3. Results and discussion 3.1. Characterization of catalysts 3.1.1. X-ray diffractionThe XRD patterns of Pd/γ-Al2O3,Cu/γ-Al2O3 and diverse Pd-Cu/ γ-Al2O3 catalysts are exhibited in Fig. 1. The results show a low intensity level for all characteristic diffraction planes,except for the planes associated with the γ-Al2O3 support [10]. For γ-Al2O3,four apparent signals (Fig. 1A) at 2u = 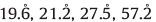

|
Download:
|
| Figure 1. XRD patterns of (A) γ-Al2O3 (JCPDS 50-1496), (B) Cu/γ-Al2O3, (C) Pd/γ-Al2O3,(D) Pd:Cu = 3:1, (E) Pd:Cu = 5:1, (F) Pd:Cu = 7:1. | |
3.1.2. X-ray photoelectron spectroscopy
The Pd-Cu/γ-Al2O3 XPS spectra of different Pd-Cu molar ratios are shown in Fig. 2. The results show that there were two oxygenization states in XPS Cu 2p spectra. The binding energies in Fig. 2 centered at 953.1 eV (2p1/2) and 932.7 eV (2p3/2) were attributed to Cu0[1TD$DIF] and CuI [13]. The differences of Cu0 and CuI were not obvious at the 2p signals because the binding energy only Exhibit 0.1 eV differences [14]. The Cu0 and CuI species,usually appear below 933 eV and CuⅡ typically appears with binding energies higher than 933 eV. The XPS spectra of Cu/γ-Al2O3 showed that oxidized CuⅡ existed,which was indicated by both second sets of spin-orbit split peaks shifted by 1.3 ± 0.2 eV to higher binding energy. It could be observed that the satellite peak at 942 eV for CuⅡ which became weaker with the addition of Pd and might indicate that CuⅡ transforms to CuI/Cu from the XPS spectra of the Pd-Cu/γ-Al2O3 [12].

|
Download:
|
| Figure 2. XPS spectra of all catalysts. (A) Pd:Cu = 3:1, (B) Pd:Cu = 5:1, (C) Pd:Cu = 7:1,(D) Cu. | |
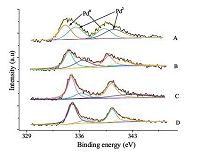
In Fig. 3,the Pd 3d5/2 appeared at 335 ± 0.1 and 336.1 ± 0.1 eV in all the catalysts,which correspond to Pd0 and PdⅡ,respectively [6, 15].The Pd primary state in the Pd/γ-Al2O3 catalyst was Pd0 and PdⅡ,while Pd0 was the main valence state in diverse Pd-Cu/γ-Al2O3samples. In Fig. 3,the peaks of PdⅡ were shifted to weaker B.E. with added Cu,which might indicate that PdⅡ transformed into Pd0 [6, 16].
|
Download:
|
| Figure 3. (A) Pd 3d peaks of Pd, (B) Pd:Cu = 3:1, (C) Pd:Cu = 5:1, (D) Pd:Cu = 7:1. | |
Furthermore,the surface atom percentages of Pd and Cu shown in Table 1 did not correspond to the preparation. According to the XPS spectra,the metals were introduced into the core of γ-Al2O3,or covered by the visible metal. The above results illustrated that appropriate ratio (1:1) of the surface atom percentages of Pd and Cu led to the simultaneous distribution of the two metals on the surface of the catalysts [8, 17].
|
|
Table 1 XPS data of catalysts. |
3.1.3. Transmission electron microscopy
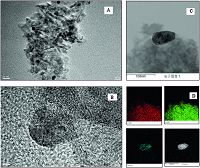
The microstructure of Pd-Cu (5:1)/γ-Al2O3 catalyst was observed by TEM. Fig. 4A and B exhibited that similar spherical particles are well-dispersed on support and clearly show that the metal particles average size was about 10 nm. Electron mapping image analysis (Fig. 4C and D) showed the distribution of oxygen (O),aluminum (Al),copper (Cu) and palladium (Pd) atoms indicated with red,green,blue and white colors,respectively,further confirmed the homogeneous distribution of Pd-Cu bimetallic nanoparticles on the surface of the γ-Al2O3 support.
|
Download:
|
| Figure 4. (A) and (B) TEM images of Pd–Cu/γ-Al2O3 = 5:1, (C) and (D) electron mapping images. | |
3.2. Activity measurements
The catalytic performance of catalysts was measured for selective oxygenization of cyclopentene under different conditions (Table 2). The results showed that all catalysts had the ability of catalytic oxygenization except for Cu/γ-Al2O3,which illustrated palladium is an essential composition of catalysts. Meanwhile,Pd- Cu(5:1)/γ-Al2O3 catalyst exhibited the best catalytic ability during the reaction. Compared with Pd-Cu(5:1)/γ-Al2O3,the catalytic activity of Pd-Cu(3:1)/γ-Al2O3 and Pd-Cu(7:1)/γ-Al2O3 was slightly lower. It was apparent that appropriate proportion of Pd and Cu of Pd-Cu/γ-Al2O3 played an important role in oxygenization of cyclopentene. According to the XPS data of catalysts in Tables 1 and 2,the Pd-Cu/γ-Al2O3 (5:1) catalyst showed the highest activity with 88% conversion of cyclopentene and it could be speculated that the surface atom percentage of Pd and Cu was close to 1:1. The inferior catalytic activity of the Pd/γ-Al2O3 sample was probably due to that plentiful PdO (according to XPS measurement) dispersed on the surface of catalyst. In addition,the catalytic activity of mixed-(Pd/γ-Al2O3 + Cu/γ-Al2O3) was not as good as the Pd-Cu/γ-Al2O3 catalyst,which illustrated that the Pd- Cu bimetal was the crucial factor in the oxygenization of cyclopentene. Simultaneously,the XPS and TEM measurements for Pd-Cu(5:1)/γ-Al2O3 catalyst demonstrated the homogeneous distribution of Pd-Cu bimetallic nanoparticles on the sample surface. We suggest that the above reasons led to Pd-Cu(5:1)/γ- Al2O3 showing the best catalytic performance. The temperature,pressure,speed (rate) and amounts of catalyst were all factors in the selective oxygenization reaction. With the changing of these factors,catalytic results were also transformed. When the values of those factors were neither too high nor too low,an optimal condition was produced. Subsequently,the impact of solvents such as 1,2-dichloroethane,ethanol,and methanol) were also studied for the reaction andwe ascertained ethanol as the solvent,and 100 °C,1 MPa oxygen pressure,400 rpm/min and Pd-Cu (5:1)/γ-Al2O3 catalyst (2.0 wt% of cyclopentene)were the optimal reaction conditions.
|
|
Table 2 The oxygenization of cyclopentene. |
4. Conclusion
In conclusion,we developed the γ-Al2O3 supported mono- (Cu and Pd),mixed- (Pd + Cu) and bi-metallic Pd-Cu catalysts for the oxygenization reaction of cyclopentene to cyclopentanone. The best results showed that the selective conversion of cyclopentene to cyclopentanone could be simultaneously up to more than 85% by employing Pd-Cu(5:1)/γ-Al2O3 catalyst under mild reaction conditions. The excellent performance of Pd-Cu(5:1)/γ-Al2O3 catalyst was attributed to the homogeneous distribution of Pd- Cu bimetallic nanoparticles on the surface of support. Our protocol provides a potential method of producing cyclopentanone in commercial production.
AcknowledgmentWe are grateful to the financial assistance from the National Natural Science Foundation of China (Nos. 21272050,21371044,21472033 and 21571047).
| [1] | C. Sui, G. Lu, X.Y. Li, et al. , Selective oxidation of cyclopentene catalyzed by Pd(CH3COO)2-NPMoV under oxygen a atmosphere. React. Kinet. Catal. Lett. 94 (2008) 191–198. |
| [2] | C. Sui, X.Y. Li, Z.P. Qu. Research progress of cyclopentanone synthesis. Chem. Ind. Eng. Prog. 27 (2008) 809–813. |
| [3] | K. Takehira, T. Hayakawa, H. Orita, M. Shimizu. Mono-oxygenation of cyclopentene by molecular oxygen catalyzed by PdCl2-CuCl2 in ethanol. J. Mol. Catal. 53 (1989) 15–21. |
| [4] | K. Takehira, H. Orita, I.H. Oh, et al. , Palladium(Ⅱ)-catalyzed oxidation of cyclopentene in the presence of copper (Ⅱ) chloride and molecular oxygen. J. Mol. Catal. 42 (1987) 247–255. |
| [5] | J.J. Mao, Y.X. Liu, Z. Chen, D.S. Wang, Y.D. Li, Bimetallic Pd-Cu nanocrystals and their tunable catalytic properties. Bimetallic Pd-Cu nanocrystals and their tunable catalytic properties. Chem. Commun. 50 (2014) 4588–4591. |
| [6] | F. Cárdenas-Lizana, S. Gómez-Quero, C. Amorim, M.A. Keane. Gas phase hydrogenation of p-chloronitrobenzene over Pd-Ni/Al2O3, Appl. Catal. A: Gen. 473 (2014) 41–50. |
| [7] | H. Yang, D. Shi, S.F. Ji, D.N. Zhang, X.F. Liu. Nanosized Pd assembled on superparamagnetic core-shell microspheres: synthesis, characterization and recyclable catalytic properties for the Heck reaction. Chin. Chem. Lett. 25 (2014) 1265–1270. |
| [8] | N.S. Babu, N. Lingaiah, P.S.S. Prasad. Characterization and reactivity of Al2O3 supported Pd-Ni bimetallic catalysts for hydrodechlorination of chlorobenzene. Appl. Catal. B: Environ. 111-112 (2012) 309–316. |
| [9] | S.J.S. Basha, P. Vijayan, C. Suresh, D. Santhanaraj, K. Shanthi. Effect of order of impregnation of Mo and Ni on the hydrodenitrogenation activity of NiO-MoO3/AlMCM-41 catalyst. Ind. Eng. Chem. Res. 48 (2009) 2774–2780. |
| [10] | J.A. Bergwerff, T. Visser, B.R.G. Leliveld, et al. , Envisaging the physicochemical processes during the preparation of supported catalysts: Raman microscopy on the impregnation of Mo onto Al2O3 extrudates. J. Am. Chem. Soc. 126 (2004) 14548–14556. |
| [11] | Y. Qiu, L. Xin, W.Z. Li. Electrocatalytic oxygen evolution over supported small amorphous Ni-Fe nanoparticles in alkaline electrolyte. Langmuir 30 (2014) 7893–7901. |
| [12] | Y.S. Feng, J. Hao, W.W. Liu, et al. , Characterization and reactivity of γ-Al2O3 supported Pd-Ni bimetallic nanocatalysts for selective hydrogenation of cyclopentadiene. Chin. Chem. Lett. 26 (2015) 709–713. |
| [13] | Y.S. Feng, C. Liu, Y.M. Kang, et al. , Selective hydrogenolysis of glycerol to, 1,2-propanediol catalyzed by supported bimetallic PdCu-KF/γ-Al2O3. Chem. Eng. J. 281 (2015) 96–101. |
| [14] | J.X. Zhou, L.Y. Guo, X.W. Guo, J.B. Mao, S.G. Zhang. Selective hydrogenolysis of glycerol to propanediol on supported Cu-containing bimetallic catalysis. Green Chem. 12 (2010) 1835–1843. |
| [15] | R. Morrish, A.J. Muscat. Nanoporous silver with controllable optical properties formed by chemical dealloying in supercritical CO2. Chem. Mater. 21 (2009) 3865–3870. |
| [16] | X.Q. Pan, Y.B. Zhang, Z.Z. Miao, X.G. Yang. A novel PdNi/Al2O3 catalyst prepared by galvanic deposition for low temperature methane combustion. J. Energy Chem. 22 (2013) 610–616. |
| [17] | X.Q. Pan, Y.B. Zhang, B. Zhang, et al. , Influence of electronic effect on methane catalytic combustion over PdNi/Al2O3. Chem. Res. Chin. Univ. 29 (2013) 952–955. |
 2016, Vol. 27
2016, Vol. 27