b Department of Chemistry, Youngstown State University, One University Plaza, Youngstown, OH 44555-3663, USA ;
c Graduate School of Natural Science, Nagoya City University, Yamanohata, Mizuho-cho, Mizuho-ku, Nagoya 467-8501, Japan ;
d CFisUC, Department of Physics, University of Coimbra, 3004-516 Coimbra, Portugal
Transition-metal complexes have been proven to be useful for the design of molecular ferromagnets,as catalysts for many organic reactions,as models for the active sites in metalloenzymes,as optical and luminescent materials,or as DNA cleavage reagents [1-5]. Furthermore,the complexes containing transition metals may be formed by bridging ligands that can mediate magnetic interactions between paramagnetic metal ions [6]. Among these materials,copper(Ⅱ) complexes have been found to have the potential for treatment of cancers and many other disease [7]. They draw great attentions due to their high nucleolytic efficiency [8, 9]. They can break the DNA chains in the presence of H2O2 and reducing agents,and are used broadly as foot printing agents for proteins and DNAs [10],probes of the dimensions of the minor groove of duplex structures [11],and identifiers of transcription starting sites [12]. Copper(Ⅱ) complexes have been probed electrochemically [13, 14] magnetically [15-19] and by EPR [20, 21] for their electronic situation. The Cu(Ⅱ) complexes have different molecular geometries,such as tetrahedral,square planar,square pyramidal and octahedral [22].
Recently,it has shown that it is possible to synthesize coordination compounds,with fascinating structures and interesting physical properties,by combining transition metal ions with pyrimidine type ligands and several pseudohalide auxiliary spacers [23]. Here a new member of this family the 4-amino-6- methoxypyrimidine cuprate(Ⅱ) sulfate trihydrate is presented,which has been obtained during our studies of the preparation of new coordination compounds with pyrimidine derivatives as ligands [24].
On other hand,in the past few years,inorganic-organic hybrid crystalline materials with interesting electrical,magnetic,and nonlinear optical (NLO) properties have attracted the attention of the material science community [25, 26]. In hybrid materials,the most usual provider of the magnetic properties is the inorganic part,whereas significant second-order NLO responses are more frequently originated from the organic counterpart. For this purpose,the essential requirement is a large molecular first-order hyperpolarizability β of the chromophore,typically obtained in planar conjugated π-electron systems,end capped with electrondonor and -acceptor moieties [27-33].
In addition to the molecular properties,it is also essential that the supramolecular organization of the chromophores maximizes the bulk electro-optic response,first of all crystallizing in noncentrosymmetric space groups and then optimizing the phase matching [34, 35].
In this paper,we present the synthesis,crystal structures,IR characterization,magnetic properties and second harmonic generation quantitative measurements of triaqua(4-amino-6- methoxypyrimidine) cuprate(Ⅱ) sulfate,which is a chiral organic- inorganic network constructed by hydrogen bonds.
2. ExperimentalChemical preparation: A solution of CuSO4-5H2O (22 mg,0.1 μmol) in water was added dropwise to a solution of 4- amino-6-methoxypyrimidine (25 mg,0.2 μmol) in ethanol (10 mL). After stirring for 30 min,the mixture was filtered. Crystals suitable for X-ray analysis were obtained after five days by evaporating the filtrate at room temperature (yield = 81%).
X-ray single crystal structural analysis: Single crystals were carefully selected under a microscope and mounted on a Mitegen micromesh mount with the help of a trace of mineral oil. X-ray diffraction data were collected at 100 K on a Bruker AXS D8 Quest CMOS diffractometer. Data were collected,the unit cell determined,and the data integrated and corrected for absorption and other systematic errors using the Apex2 suite of programs [36]. SHELXS-97 [37] was used to solve the structure using direct methods and SHELTXL6.14 [38],SHELXL-2014 [39] and SHELXLE [40] were used for refinement. The crystal was found to be racemically twinned with a twin domain ratio of 0.872(8)- 0.188(8). The drawings were made with Diamond [41]. Crystal data and experimental parameters used for the intensity data collection are summarized in Table 1.
|
|
Table 1 Experimental details |
Infrared spectroscopy: The IR spectra were recorded in the range 4000-400 cm-1 with a ‘‘Perkin-Elmer FTIR’’ spectrophotometer1000 using samples dispersed in spectroscopically pure KBr pressed into a pellet.
Magnetic properties: Magnetic measurements were carried out for a microcrystalline sample with random orientation on a SQUID (Quantum Design MPMS XL7) magnetometer down to 2 K. Temperature dependence of the dc susceptibility was measured under 500 Oe. The molar unit of the paramagnetic susceptibility xp was chosen as the quantity per one mole of [Cu(C5H7N3O)(- H2O)3]SO4. The experimental raw data were corrected for the diamagnetic contribution, -0.000173 emu mol-1,which was estimated by the Pascal diamagnetism.
Kurtz-Perry powder method: The Second-Harmonic Generation (SHG) efficiency of the compound triaqua(4-amino-6-methoxypyrimidine) cuprate(Ⅱ) sulfate was measured using the Kurtz and Perry powder method [42]. The measurements were performed at a wavelength of 1064 nm produced by a Nd:YAG laser operating at 10 Hz and producing 10 ns pulses with a pulse energy of 11 mJ. The sample preparation procedure was as follows: the material was mulled to a fine powder and compacted in a mount and then installed in the sample holder. Sample grain sizes were not standardized. Signals between individual measurements were seen to vary in some cases by as much as ±10%. For a proper comparison with the KDP reference material the measurements were averaged over several laser thermal cycles.
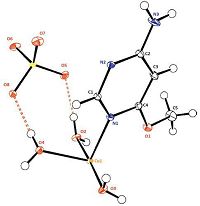
3. Results and discussion 3.1. X-ray diffraction studyThe asymmetric unit of [Cu(C5H7N3)(H2O)3]SO4 consists of one Cu(Ⅱ) cation,one 4-amino-6-methylpyrimidine (L) ligand,three water molecules and one SO42- anion (Fig. 1). The Cu(Ⅱ) ion is pentacoordinated by the three water oxygen atoms and two nitrogen atoms (N1 and N2) of the monodentate L ligand to give a distorted square pyramidal coordination geometry. The geometry of penta-coordinated metal complex my conveniently be measured by the Addison parameter [43] (trigonality index,τ = (β - α)/60,where α and β are the two largest L-M-L angles of the coordination sphere),that is zero for an ideal squarepyramidal complex and one for a trigonal bi-pyramidal complex. In the title complex,the calculated τ values of the title compound is τ(Cu) = 0.42,where β and α values are 176.55(6) and 151.17(5)°,respectively (Table S1 in Supporting information),indicating a distortion from the regular polyhedron nearly halfway between square pyramidal and trigonal bi-pyramidal. Two monodentate 4- amino-6-methoxypyrimidine ligands coordinate through N1 and N2 at equatorial positions with nearly identical Cu-N bond distances of 2.031(1) and 2.038(1)Å . The two remaining equatorial sites are occupied by the O2 and O3 water oxygen atoms with again very similar bond distances of 1.956(1) and 1.944(1)Å to the copper ion. The O4 water oxygen atom occupies the axial position and features a significantly longer Cu-O bond of 2.165(1)Å as is commonly observed for many Cu(Ⅱ) complexes. The two 4-amino- 6-methoxypyrimidine moieties are directed trans to each other at an angle of 151.17(5)°,representing the principal distortion from an ideal square pyramidal geometry,while there is only a small departure from linearity for the other trans angle O2-Cu-O3 [176.55(6)°]. In the atomic arrangement,the L ligands and the 5-connected Cu centers link each other to give a 1-D corrugated hybrid chain running along the b-axis direction (Fig. 2). These chains,located at z = ¼,are interconnected by the SO4 2× anions via O-H…O,O-H…S,C-H…O and N-H…O hydrogen bonds to form layers spreading parallel to the (011) plane (Fig. 2,Table S2 in Supporting information). These layers are located at x = n/2 and interconnected through O-H…O hydrogen bonds to form a three dimensional network (Fig. 3). Within the organic ligand 4-amino- 6-methoxypyrimidine,an examination of the C-N bond distance of the NH2 group (Table S3 in Supporting information),shows that C2-N3 [1.335(2)Å] is short for C-N single bond,but still not quite as contracted as one would expect for a fully established C55N double bond. This bond length feature is consistent with an imino resonance form as it is commonly found for C-N single bond involving sp2 hybridized C and N atoms [44, 45]. Furthermore,the distance C5-O1 [1.439(2)Å],clearly indicates a single bond,while the distance C4-O1 [1.333(2)Å] is shorter which is probably due to the donor mesomeric effect of the methoxy group [46].
|
Download:
|
| Figure 1. A view of the asymmetric unit in the crystal structure of [Cu(C5H7N3)(H2O)3]SO4 showing the atom-numbering scheme and displacement ellipsoids drawn at the 50% probability level. The dotted lines indicate hydrogen bonds. | |
|
Download:
|
| Figure 2. View along the [011] direction of an inorganic-organic layer [Cu(C5H7N3)(H2O)3]SO4. The dotted lines indicate hydrogen bonds | |

|
Download:
|
| Figure 3. Projection along the c-axis of the crystal packing of the title compound. The dotted lines indicate hydrogen bonds | |
3.2. IR spectroscopy
FTIR spectroscopy was used to verify the functional groups present in the crystal and to investigate their vibrational behavior in the solid state. The characteristic vibrational modes of this compound can be compared to those of similar materials [47-49]. The very large band in the high-frequency region,spreading between 3600 cm-1 and 2600 cm-1,correspond to the stretching vibrations of the N-H,C-H and O-H groups interconnected by a system of hydrogen bonds in the crystal [50]. The band at 1655 cm-1 is assigned to the O-H and N-H bending modes. The bands observed in the 1600 cm-1 and 1050 cm-1 region can be assigned to the valence vibrations of the C55C,C55N,C-C and C-N bonds [51]. The observed bands in the range 1000-600 cm-1 can be assigned to the CH3 and NH2 groups and to the skeleton of amino-pyrimidine rings symmetric,asymmetric,stretching and deformation modes [52, 53].
3.3. Magnetic propertiesThis material will have a one-dimensional coordination polymer structure and will be regarded as an S = 1/2 onedimensional magnetic system if superexchange interactions via the organic ligands work between copper ions. In order to investigate intensity and sign of the magnetic interactions in this material,magnetic studies were carried out. This material exhibited a paramagnetic behavior. Fig. 4a shows temperature dependence of the paramagnetic susceptibility of this material. The xp value showed a gradual increase,decreasing with temperature,and made a broad maximum at ca. 22 K. This magnetic behavior may be originated from the short-range magnetic ordering,which is characteristic of low dimensional magneticmaterials. Below 5 K,the xp value again increases,decreasing with temperature. This behavior is due to Curie term from lattice defects or magnetic impurities. Fig. 4b shows temperature dependence of the product of the paramagnetic susceptibility and temperature. The xpT value at 300 K is 0.423 emu K mol-1,and decreased,with decreasing in temperature monotonically. This fact suggests dominance of intermolecular antiferromagnetic interactions between copper atoms.

|
Download:
|
| Figure 4. Temperature dependence of the molar magnetic susceptibility xp (a) and xpT (b) of [Cu(C5H7N3)(H2O)3]SO4 for an external field of 500 Oe. The solid line corresponds to the best theoretical fit. | |
The magnetic behavior of this material can be interpreted in terms of the antiferromagnetic regular chain model using the Hamiltonian in Eq. (1),where J is the intrachain magnetic coupling constant between neighboring magnetic molecules i and j,
| $H=-2J\sum{{{s}_{i}}\cdot {{s}_{j}}}$ | (1) |
The analytical expression for the paramagnetic susceptibility x1D is given as
| ${{\chi }_{p}}=\frac{C}{T}\frac{4\left( 0.25+0.14995y+0.30094{{y}^{2}} \right)}{1+1.9862y+0.68854{{y}^{2}}+6.0626{{y}^{3}}}+\frac{{{C}_{imp}}}{T}$ | (2) |
where
This material showed no magnetic ordering transition down to 2 K in spite of the magnetic coupling constant of -36 K. This indicates that this material has a one-dimensional magnetic character [55] because the coordination chains are well separated and magnetically isolated by water molecules or the anions between the neighbor chains. This material has a bridged structure of copper atoms via the pyrimidine ligands. Ishida et al. reported that detailed magnetic properties of a di-nuclearmetal complex DPPM- Cu bridged via a pyrimidine ligand between two copper ions [56]. Their magnetic parameters were g = 2.29 and 2J/kB = -46 K. The magnetic coupling constant is inconsistent with our value.
3.4. Nonlinear optical responseTo determine the NLO response of this new coordination compound we measured the SHG efficiency with the Kurtz-Perry method [40] using a polycrystalline sample. A good SHG efficiency of 5.2 times the KDP standard was observed. We plan,in the near future,to characterize further the second order nonlinear susceptibility using the Marker fringe technique.
4. ConclusionWe have developed new nonlinear optical triaqua(4-amino-6- methoxypyrimidine) cuprate(Ⅱ) sulfate crystals,which show orthorhombic space group P212121 symmetry. The coordination polyhedron around the central Cu(Ⅱ) ion is about half way between square-pyramidal and trigonal-bipyramidal geometry. The organic ligands and the 5-connected Cu centers link each other to give a 1-D corrugated hybrid chain running along the b-axis. These chains are interconnected by the SO42- anions via a set of hydrogen bonds to form layers spreading parallel to (011) plane. The vibrational absorption bands were identified by infrared spectroscopy. Intermolecular antiferromagnetic interactions between copper atoms are expected to predominate in the compound. The crystals exhibit a good Second Harmonic Generation (SHG) efficiency of 5.2 times the potassium dihydrogen phosphate (KDP) standard.
Appendix A. Supplementary data
Supplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.02.024.
| [1] | H. Miyasaka, A. Saito, S. Abe. Magnetic assemblies based on Mn(Ⅲ) salen analogues. Coord. Chem. Rev. 251 (2007) 2622–2664. |
| [2] | K.C. Gupta, A.K. Sutar. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 252 (2008) 1420–1450. |
| [3] | D.E. Fenton. Metallobiosites and their synthetic analogues-a belief in synergism, 1997-1998 Tilden lecture. Chem. Soc. Rev. 28 (1999) 159–168. |
| [4] | G. Consiglio, S. Failla, I.P. Oliveri, R. Purrello, S. Di Bella. Controlling the molecular aggregation. An amphiphilic Schiff-base zinc(Ⅱ) complex as supramolecular fluorescent probe. Dalton Trans. 47 (2009) 10426–10428. |
| [5] | B. Dede, I. Ozmen, F. Karipcin. Synthesis, characterization, catalase functions and DNA cleavage studies of new homo and heteronuclear Schiff base copper(Ⅱ) complexes. Polyhedron 28 (2009) 3967–3974. |
| [6] | S. Alvarez, A.A. Palacios, G. Aullón. Ligand orientation effects on metal-metal, ligand-ligand and metal-ligand interactions. Coord. Chem. Rev. 185-186 (1999) 431–450. |
| [7] | C.H. Ng, K.C. Kong, S.T. Von, et al. Synthesis, characterization. DNA-binding study and anticancer properties of ternary metal(Ⅱ) complexes of edda and an intercalating ligand. Dalton Trans. (2008) 447–454. |
| [8] | B. Macías, M.V. Villa, F. Sanz, et al. Castineiras. Cu(Ⅱ) complexes with a sulfonamide derived from benzoguanamine. Oxidative cleavage of DNA in the presence of H2O2 and ascorbate. J. Inorg. Biochem. 99 (2005) 1441–1448. |
| [9] | Y.P. Li, Y.B. Wu, J. Zhao, P. Yang. DNA-binding and cleavage studies of novel binuclear copper(Ⅱ) complex with, 1,1'-dimethyl-2,2'-biimidazole ligand. J. Inorg. Biochem. 101 (2007) 283–290. |
| [10] | B.C. Bales, M. Pitié, B. Meuneir, M.M. Greenberg. A minor groove binding copperphenanthroline conjugate produces direct strand breaks via b-Elimination of, 2-deoxyribonolactone. J. Am. Chem. Soc. 124 (2002) 9062–9063. |
| [11] | J.A. Cowan. Chemical nucleases. Curr. Opin. Chem. Biol. 5 (2001) 634–642. |
| [12] | T. Thederahn, A. Spassky, M.D.Kuwabara, D.S. Sigman. Chemical nuclease activity of, 5-phenyl-1, 1'-phenanthroline-copper ion detects intermediates in transcription initiation by E. coli RNA polymerase. Biochem. Biophys. Res. Commun. 168 (1990) 756–762. |
| [13] | S. Fritzsche, P. Lönnecke, T. Höcher, E. Hey-Hawkins. Soluble monometallic salen complexes derived from O-functionalized salicylaldehydes as metalloligands for the synthesis of heterobimetallic complexes. Z. Anorg. Allg. Chem. 632 (2006) 2256–2267. |
| [14] | S. Zolezzi, E. Spodine, A. Decinti. Electrochemical studies of copper(Ⅱ) complexes with Schiff-base ligands. Polyhedron 21 (2002) 55–59. |
| [15] | C.T. Zeyrek, A. Elmali, Y. Elerman. Super-exchange interaction in a chair-piperazine bridged dicopper (Ⅱ/Ⅱ) complex: synthesis, crystal structure, magnetic properties and molecular orbital calculations. Z. Naturforsch. B 61 (2006) 237–242. |
| [16] | Z. Heren, C. Keser, C.C. Ersanli, O .Z. Yesilel, N. Ocak, Synthesis, spectrothermal studies and crystal structure of cis-bis(4-methylimidazole)bis(picolinato)copper( Ⅱ), Z. Naturforsch. B 61 (2006) 1217–1221. |
| [17] | C.T. Zeyrek, A. Elmali, Y. Elerman, I. Svoboda. Crystal structure and magnetic exchange interaction in a binuclear Copper(Ⅱ) Schiff base complex with a bridging M-phenylenediamine ligand. Z. Naturforsch. B 60 (2005) 143–148. |
| [18] | W. Kaim, C. Titze, T. Schurr, et al. Reactivity of copper(Ⅰ) complexes with tripodal ligands towards O2: structures of a precursor [L3CuI(NCCH3)](BF4). L3 = Tris(3-isopropyl-4,5-trimethylenepyrazolyl)methane and of its Oxidation Product[L3CuⅡ(m-OH)2CuⅡL3](BF4)2 with strong antiferromagnetic spin-spin coupling. Z. Anorg. Allg. Chem 631 (2005) 2568–2574. |
| [19] | J. Teichgräber, G. Leibeling, S. Dechert, et al. Thioether functionalized pyrazolate ligands and their dicopper(Ⅱ) complexes-structures and magnetic properties. Z. Anorg. Allg. Chem 631 (2005) 2613–2618. |
| [20] | J. Griebel, F. Leistner, H. Krautscheid, R. Kirmse. Tetrakis(1-adamantylcarboxylato) dikupfer(Ⅱ) Cu2(1-Ad)4-synthese. Struktur und X-/Q-Band-EPR-untersuchungen. Z. Anorg. Allg. Chem. 632 (2006) 866–870. |
| [21] | J. Moncol, M. Korabik, P. Segl'a, et al. Preparation, structure, spectral, and magnetic properties of Copper(Ⅱ) halogenonicotinates: crystal and molecular structure of tetrakis(m-2-chloronicotinato-O,O')-diaquadicopper(Ⅱ), Z. Anorg. Allg. Chem., 2007, 633: 298-305. Z. Anorg. Allg. Chem 633 (2007) 298–305. |
| [22] | P.S. Subramanian, D. Srinivas. Synthesis, spectral characterization and magnetic properties of dicarboxylato-bridged dinuclear copper(Ⅱ) complexes with n-pyridylsalicylidenaminato ligand. Polyhedron 15 (1996) 985–989. |
| [23] | A.B. Caballero, A. Rodríguez-Diéguez, E. Barea, M. Quirós, J.M. Salas. Influence of pseudohalide ligands on the structural versatility and properties of novel ternary metal complexes with, 1, 2,4-triazolo[1,5-a]pyrimidine. CrystEngComm 12 (2010) 3038–3045. |
| [24] | K. Kaabi, M. Zeller, P.S. Pereira Silva, C. Ben Nasr. Synthesis, characterization and supramolecular structure of three new Cu(Ⅱ) and Ni(Ⅱ) complexes with the potentially bidentate ligand 2-amino-6-methylpyrimidin-4(1H)-one (AMPO). norg. Chim. Acta 388 (2012) 52–59. |
| [25] | J.L. Knutson, J.D. Martin, D.B. Mitzi. Tuning the band gap in hybrid tin iodide perovskite semiconductors using structural templating. Inorg. Chem. 44 (2005) 4699–4705. |
| [26] | P. Rabu, M. Drillon. Layered organic-inorganic materials: a way towards controllable magnetism. Adv. Eng. Mater. 5 (2003) 189–210. |
| [27] | T. Verbiest, S. Houbrechts, M. Kauranen, K. Clays, A. Persoons. Second-order nonlinear optical materials: recent advances in chromophore design. J. Mater. Chem. 7 (1997) 2175–2189. |
| [28] | L.R. Dalton, W.H. Steier, B.H. Robinson, et al. From molecules to opto-chips: organic electro-optic materials. J. Mater. Chem. 9 (1999) 1905–1920. |
| [29] | I. Ledoux, I.D.W. Samuel, J. Zyss, et al. Third-order microscopic nonlinearities of very long chain polyenes: saturation phenomena and conformational effects. Chem. Phys. 245 (1999) 1–16. |
| [30] | G.R. Meredith, in: J.D. Williams (Ed.), Nonlinear Optical Properties of Organic and Polymeric Materials, ACS Symposium Series, American Chemical Society, Washington, D C. 1983. . |
| [31] | D.S. Chemla, J. Zyss, Nonlinear Optical Properties of Organic Molecules and Crystals, Academic Press, Orlando, F L. 1987. . |
| [32] | S.R. Marder, J.E. Sohn, G.D. Stucky. Materials for Nonlinear Optics: Chemical Perspectives, ACS Symposium Series, 455, American Chemical Society, Washington, DC. . |
| [33] | P.N. Prasad, D.J. Williams. Introduction to Nonlinear Optical Effects in Molecules and Polymers, John Wiley, New York. (1991) . |
| [34] | J. Zyss, J.L. Oudar. Relations between microscopic and macroscopic lowest-order optical nonlinearities of molecular crystals with one-or two-dimensional units. Phys. Rev. A 26 (1982) 2028–2048. |
| [35] | D.S. Chemla, J. Zyss, Nonlinear Optical Properties of Organic Molecules and Crystals, Academic Press Inc. Orlando, FL. (1987) . |
| [36] | Apex2, Bruker Advanced X-ray Solutions, Bruker AXS Inc. Madison, Wisconsin, USA. (2009) . |
| [37] | G.M. Sheldrick. A short history of SHELX. Acta Crystallogr. Sect. A 64 (2008) 112–122. |
| [38] | SHELXTL (Version 6.14), Bruker Advanced X-ray Solutions, Bruker AXS Inc. Madison, Wisconsin, USA. (2003) . |
| [39] | G.M. Sheldrick, SHELXL2013. University of Gö ttingen, Germany. (2013) . |
| [40] | C.B. Hü bschle, G.M. Sheldrick, B. Dittrich. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr 77 (2001) 1281–1284. |
| [41] | K. Brandenburg, Diamond Version, 2. 0 Impact GbR, Bonn, Germany. (1998) . |
| [42] | S.K. Kurtz, T.T. Perry. A powder technique for the evaluation of nonlinear optical materials. J. Appl. Phys. 39 (1968) 3798–3813. |
| [43] | A.W. Addison, T.N. Rao, J. Reedijk, J. van Rijn, G.C. Verschoor. Synthesis, structure, and spectroscopic properties of copper(Ⅱ) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(Nmethylbenzimidazol-2'-yl)-2,6-dithiaheptane]copper(Ⅱ) perchlorate. J. Chem. Soc. Dalton Trans (1984) 1349–1356. |
| [44] | R.N. Yang, D.M. Wang, Y.M. Hou, et al. Thioether functionalized pyrazolate ligands and their dicopper(Ⅱ) complexes-structures and magnetic properties. Z. Anorg. Allg. Chem. 631 (2005) 2613–2618. |
| [45] | R. Grobelny, T. Glowiak, J. Mrozinski, W. Baran, P. Tomasik. Crystal structure, spectroscopic and magnetic studies of diacetato-bis(2-aminopyridine) copper(Ⅱ) complex. Pol. J. Chem. 69 (1995) 559–565. |
| [46] | R. Kefi, S. Abid, C. Ben Nasr, M. Rzaigui. Synthesis and characterization of a new monophosphate (5-Cl-2,4-(OCH3)2C6H2NH3) H2PO4. Mater. Res. Bull. 42 (2007) 404–412. |
| [47] | N.L. Calve, F. Romain, M.H. Limage, A. Novak. Etude par spectroscopic Raman et infrarouge des verres de pseudo-spin Rb0.65(NH4)0.35H2PO4. J. Mol. Struct 200 (1989) 131–147. |
| [48] | H. Ratajczak. Structural studies of some hydrogen-bonded ferroelectrics using polarized IR radiation. J. Mol. Struct. 3 (1969) 27–41. |
| [49] | A. Navak. Vibrational studies of structural phase transitions in partially ordered solids. J. Mol. Struct. 217 (1990) 35–49. |
| [50] | W. Smirani, C. Ben Nasr, M. Rzaigui. Synthesis and crystal structure of a new oethylphenylammonium triphosphate. Mater. Res. Bull. 39 (2004) 1103–1111. |
| [51] | K. Kamel, A. Rayes, C. Ben Nasr, M. Rzaigui, F. Lefebvre. Synthesis and crystal structure of a new dihydrogenomonophosphate (4-C2H5C6H4NH3)H2PO4. Mater. Res. Bull. 38 (2003) 741–747. |
| [52] | A.C. Chapman, L.E. Thirlwell. Spectra of phosphorus compounds-I the infra-red spectra of orthophosphates. Spectrochim. Acta 20 (1964) 937–947. |
| [53] | F. Scheinmann, L.E. Thirtwel. An Introduction to Spectroscopic Methods for the Identification of Organic Compounds[M]. New York: Pergamon Press, 1970 . |
| [54] | J.C. Bonner, M.E. Fisher. Linear magnetic chains with anisotropic coupling. Phys. Rev. 135 (1964) A640–A658. |
| [55] | L.R. Carlin. Magnetochemistry, Springer-Verlag[M]. Heidelberg: Berlin, 1986 . |
| [56] | T. Ishida, T. Kawakami, S.I. Mitsubori, et al. Antiferromagnetic coupling of transition metal spins across pyrimidine and pyrazine bridges in dinuclear manganese(Ⅱ), cobalt(Ⅱ), nickel(Ⅱ) and copper(Ⅱ), 1,1,1,5,5,5-hexafluoropentane-2,4-dionate complexes. J. Chem. Soc. Dalton Trans (2002) 3177–3186. |
 2016, Vol. 27
2016, Vol. 27 



