b Engineering Research Center of Materials-Oriented Chemical Engineering of Xinjiang Production and Construction Corps, Shihezi 832003, China ;
c Key Laboratory of Materials-Oriented Chemical Engineering of Xinjiang Uygur Autonomous Region, Shihezi 832003, China
For the last few decades,graphite has been the most used commercial anode material in lithium-ion batteries (LIBs) with a theoretical capacity of 372 mAh g-1 [1]. With the increasing demand of LIBs as power sources in electric vehicles (EV) and hybrid electric vehicles (HEV),much attention has been paid to alternative anode materials [2-5]. A major breakthrough for LIB anodes,made by Tarascon et al. in 2000,is the development of transition metal oxides as anode materials,which exhibit a much higher theoretical specific capacity than traditional graphite electrodes [6]. Iron oxide,Fe3O4,is one of the few transition metal oxide materials that possesses both a high theoretical capacity (926 mAh g-1) and a high electronic conductivity (2 × 104S m-1),in addition to the advantages of nontoxicity,natural abundance,and low cost [7, 8].
The electrochemical conversion reaction of Fe3O4 with Li+ is shown in the reaction [9, 10]:
| $\text{F}{{\text{e}}_{\text{3}}}{{\text{O}}_{\text{4}}}\text{+8L}{{\text{i}}^{\text{+}}}\text{+8}{{\text{e}}^{\text{-}}}\to 3\text{Fe+4L}{{\text{i}}_{\text{2}}}\text{O}$ |
Each formula unit of Fe3O4 can react with eight Li+ to form a composite containing Fe nanoclusters embedded in an amorphous Li2O matrix,which reversibly converts back to Fe3O4 on charging process. The quite reversible reaction ensures excellent coulombic efficiency and high reversible capacity of Fe3O4.
However,the large volume variation (>200%) that inherently accompanies the conversion reaction process and severe destruction of the electrode hamper the use of Fe3O4 as an anode material in LIBs. This holdback can partially be solved by fabricating nanostructured materials. Hollow nanomaterials of transition metal oxides (SnO2 [11-13],Co3O4 [14],Fe2O3 [15],Ni(OH)2 [16],Co(OH)2 [17],and TiO2 [18]) have been the research focus as promising high-energy electrode materials for next-generation lithium-ion batteries for a long time. The results from these previous works prove that construction of hollow nanostructures is an effective strategy to improve the cyclability,resulting from the large surface area,the sufficient contact of active material/ electrolyte,and the short diffusion length of Li+. Inspired by this,we attempt to produce hollow nanostructured Fe3O4. The synthesis strategies of hollow Fe3O4 [19-21] have been reported in many ways,however,a tiny change in experimental parameters or operations leads to the morphological transformation from hollow to solid [22],resulting in bad repeatability. Therefore,there is an urgent need for a new synthesis strategy of H-Fe3O4 microspheres which is more repeatable.
Herein,we report a facile one-step solvothermal synthesis of H-Fe3O4 microspheres based on oriented aggregation and Ostwald ripening mechanisms. The synthesis is performed in an ethylene glycol (EG)-diethylene glycol (DEG) mixed solvent using polyethylene glycol (PEG) as the stabilizer. Significantly,the approach is suitable for the high-yield mass production of HFe3O4 microspheres with nearly 100% morphological yield. Benefiting from the hollow nanostructures,when used as the anode material in lithium-ion batteries,not only is the Li+ diffusion is much easier,but also the strain associated with Li+ intercalation and the volume expansion of active materials are often better accommodated,resulting in significantly improved electrochemical performance.
2. Experimental 2.1. Preparation of H-Fe3O4 microspheresThe synthesis of H-Fe3O4 microspheres was performed in an EG-DEG mixed solvent. Typically,FeCl3·6H2O (1.35 g) and PEG 20000 (1.5 g) were dissolved in EG (20 mL) and DEG (20 mL),respectively. After mixing the two solutions,NaAc (3.6 g) was added. The mixture was stirred vigorously for 60 min at 50℃ and then sealed in a 50 mL Teflon-lined stainless steel autoclave. The autoclave was treated in an air-flow electric oven at 200℃ for 24 h. After cooling down naturally,the precipitate was collected using a centrifuge and was washed with ethanol. The washed products were dried in vacuum at 60℃ overnight. The synthesis of S-Fe3O4 microspheres is performed in an EG solvent,then followed by the same conditions and procedures applied in the synthesis of HFe3O4 microspheres.
2.2. Characterization techniquesThe morphologies of the samples were examined by scanning electron microscopy (SEM; Zeiss Supra 40 FE) and transmission electron microscopy (TEM; FEI Tecnai G20 and Hitachi H600). The crystal phases of the samples were characterized by X-ray diffraction analysis (XRD) recorded on a powder diffractometer (Bruker D8 Advanced Diffractometer System) with Cu-Ka (1.5406A˚ ) source.
2.3. Electrochemical measurementsThe electrochemical measurements were carried out on cointype half cells using a Land battery system (CT2001A). To prepare the working electrode,80 wt% of the active material,10 wt% carbon black and 10 wt% Polytetrafluoroethylene (PTFE) dissolved in N-methylpyrrolidone (NMP) were mixed to form a slurry. The slurry was pasted on the Ni foil and dried in a vacuum oven for 12 h. The loading of the working electrodes was typically in the range of 1-2 mg (6 mg cm-2 ). Li foil was used as both the counter and reference electrodes. 1 mol L×1 LiPF6 in ethylene carbonate and diethyl carbonate (EC/DEC,v/v ¼ 1:1) solution was used as the electrolyte. Galvanostatic charge and discharge measurement was carried out in the voltage range between 3.0 and 0.05 V at a current density of 100 mA g-1. The impedance spectrum measurements were carried out in the frequency range from 100 kHz to 0.01 Hz.
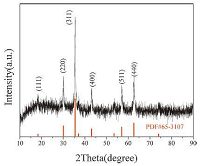
3. Results and discussionFig. 1 shows the XRD pattern of the H-Fe3O4 microspheres after solvothermal treatment for 24 h at 200 ℃. The reflections of the HFe3O4 microspheres can be mainly attributed to the face-centered cubic structural (Fd3m space group) magnetite Fe3O4 (JCPDS card No. 65-3107). Fig. 2a shows the TEM image of the monodispersed H-Fe3O4 microspheres at low magnification obtained at 200℃ for 24 h via a solvothermal route with diameters of 400-500 nm. Through the clear variation in contrast,the porous and hollow nature of the microspheres was confirmed. The TEM image of a single Fe3O4 microsphere was shown in Fig. 2b and c. Most pores were channel-like,connecting the central hollow interior and the outside space. According to our understanding,this structure represents an ideal candidate for LIBS anode material. For example,the hollow interior could act as a good container for Li+,while the interstitials between primary crystals,which enable Li+ diffusion,could work as "nanochannels" for material exchange between the interior and exterior of the spheres. To achieve more detailed crystallographic information of the constituent nanocrystals,a high resolution TEM image was taken at the conjunction of two constituent Fe3O4 nanocrystals,which was shown in Fig. 2d. Interestingly,both nanocrystals shared one common crystallographic orientation. The lattice fringe calculated from the HRTEM image was 0.25 nm,fitting well with the (3 1 1) planes of a cubic Fe3O4 structure.
|
Download:
|
| Figure 1. XRD pattern of H-Fe3O4 microspheres after solvothermal treatment for 24 h | |

|
Download:
|
| Figure 2. (a and b) Representative TEM images of microspheres of H-Fe3O4 after solvothermal treatment for 24 h; (c and d) HRTEM images of a Fe3O4 sphere | |
In order to investigate the growth mechanism of such hollow microstructures,we carried out time-dependent experiments,as shown in Fig. S1a-d in Supporting information. The first capture of the discrete nanocrystals layer was after solvothermal treatment for 12 h (Fig. S1a). With a synthesis time up to 16 h (Fig. S1b),the discrete nanocrystals layer became slightly thicker,and the partial Fe3O4 microspheres transformed from solid to hollow,which could be inferred from the slight contrast between the dark edge and the pale center. When the reaction was extended to 20 h (Fig. S1c),an obvious increase in the thickness of the discrete nanocrystal layer and a distinct contrast between the dark edges and the pale center were observed. Upon reaction for 24 h (Fig. S1d),the hollow interior became larger,and the H-Fe3O4 microspheres were finally synthesized.
Apart from the reaction time,the reaction solvent is another synthetic parameter that has a significant effect on the as-prepared H-Fe3O4,as shown in Fig. S2 in Supporting information. In agreement with previous report [22],the Fe3O4 microspheres synthesized in EG were solid (Fig. S2a and d). With the addition of DEG,the morphology of the Fe3O4 microspheres transformed from solid to hollow (Fig. S2b and e). However,the morphology transformed into irregular spheres when synthesized in pure DEG (Fig. S2c and f). We tried to give a reasonable explanation for this phenomenon. During the formation of Fe3O4 microspheres,the newly formed Fe3O4 nanoparticles in solution slowly attached on the surface of the pre-formed Fe3O4 aggregates. In an effort to reduce the overall energy of the system,the approaching Fe3O4 nanoparticles rotated so that adjacent primary Fe3O4 nanoparticles self-organized into a common crystallographic orientation,and finally fused together to form the secondary Fe3O4 microspheres. At the reaction temperature (200 ℃),the addition of DEG,which possesses a boiling point of 245 ℃,much higher than that of EG (197.3 ℃),enhanced the solvent viscosity. This ensures that the Fe3O4 primary crystals aggregate slower and self-organize more uniformly,which laid the foundation for the next Ostwald ripening stage.
On the basis of all the above observations,a simple plausible mechanism is proposed addressing the formation of H-Fe3O4 microspheres. The synthetic procedure is drawn as shown in Fig. 3. At the initial stage of the formation of H-Fe3O4 microspheres,oriented aggregation mechanism plays a dominant role in the synthetic procedure. Primary magnetite nanocrystals first nucleate in a supersaturated solution,resulting from the solvent-mediated hydrolysis of Fe3+. Afterward,the newly formed nanocrystals aggregate into round spheres. The driving force for this process comes fromthe decrease in surface energy through self-organization of adjacent particles along common crystallographic orientations and joining at the planar interface. Therefore,the formed aggregates present a single-crystal characteristic [23]. From here,the spheres develop according to the Ostwald ripening mechanism. The inner crystallites of the aggregate go through mass transfer to the outer shellbya dissolution-recrystallizationprocess at the costof thesmall crystals.This thermodynamically drivenspontaneousprocess occurs because larger particles aremore energetically favored than smaller particles [24]. Through a lengthened ripening process,outward migration of crystalswould result in continued expansion of interior space within the original aggregates,and the inner space of the spheres is further increased.

|
Download:
|
| Figure 3. Schematic illustration of the formation of H-Fe3O4 microspheres | |
Coin cells were made to test the electrochemical performance of the H-Fe3O4 microspheres. For comparison,S-Fe3O4 microspheres were tested under the same condition. Fig. 4a shows representative charge/discharge profiles of H-Fe3O4 microspheres at a current density of 100 mA g-1 between 0.05 V and 3 V (vs. Li+/Li). The gravimetric capacities referred to hereafter are based on the total mass of the composites (including graphene). The first discharge and charge capacities are 1304.1 mAh g-1 and 928.3 mAh g-1,respectively. The lithium storage capacities are much higher than the theoretical capacity of Fe3O4 owing to the formation of a solid electrolyte interface on the surfaces of the H-Fe3O4 NPs [25-28]. It should be emphasized that no obvious change in both charge and discharge profiles is observed for the H-Fe3O4 NPs microspheres in subsequent cycles,compared with S-Fe3O4 microspheres (Fig. 4b). Fig. 4c further compares the cycle performance of the H-Fe3O4 and S-Fe3O4 microspheres at a current density of 100 mA g-1 between 0.05 V and 3 V (vs. Li+/Li). For S-Fe3O4 microspheres,the specific capacity decreased significantly and retained only 61.2 mAh g-1 at 100 mA g-1 after 50 cycles. Meanwhile,the H-Fe3O4 microspheres displayed much better cyclic performance,retaining 453.3 mAh g-1 at 100 mA g-1 after 50 cycles,which is much higher than the theoretical capacity of graphite. As we had expected,the hollow nanostructure can greatly improve cyclic stability,which can shorten diffusion length of Li+,alleviating the volume expansion of active materials.

|
Download:
|
| Figure 4. (a) Discharge-charge curves of H-Fe3O4 vs. Li/Li+ at a current density of 100 mA g-1; (b) Discharge-charge curves of S-Fe3O4 vs. Li/Li+ at a current density of 100 mA g-1; (c) comparative cycle performance of different electrodes at a current density of 100 mA g-1 | |
In order to gain an in-depth understanding of the difference in electrochemical performance between the H-Fe3O4 and S-Fe3O4 microspheres,electrochemical impedance spectroscopy (EIS),a promising tool for investigating diffusion issues,was conducted at frequencies from 100 kHz to 0.01 Hz to identify the relationship between the electrochemical performance and electrode kinetics. Fig. S3 shows the Nyquist plots for the H-Fe3O4 and S-Fe3O4 microspheres after 10 cycles at 100 mA g-1,which share the common feature of a high-frequency depressed semicircle and a medium-frequency depressed semicircle followed by a linear tail in the low frequency region. Following are the common equivalent circuit descriptions of these features: the intercept on the Z' axis at the high-frequency end is the electrolyte resistance (Rs),the size of the semicircular that encompasses the medium-frequency response is an indication of the charge-transfer resistance (Rct) in the electrode reaction,and the inclined line in the low-frequency region represents the Warburg impedance (Zw) related to lithium diffusion in the solid. Evidently,the diameter of the semicircle for H-Fe3O4 electrode in the high-medium-frequency region is significantly smaller than that of S-Fe3O4 microspheres,demonstrating that H-Fe3O4 electrodes possess lower contact and chargetransfer impedances,which can lead to rapid electron transport during the electrochemical lithium insertion/extraction reaction and thus result in significant improvement on the cycle performance.
4. ConclusionIn summary,we have developed a facile one-step solvothermal synthesis of H-Fe3O4 microspheres,based on oriented aggregation and Ostwald ripening mechanisms. With the mediation of DEG and PEG,the current method is suitable for inexpensive,mass production of H-Fe3O4 microspheres. The as-prepared H-Fe3O4 microspheres exhibit superior lithium storage capacity and cycle performance when used as the anode material in lithium-ion batteries .
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.02. 003.
| [1] | J.B. Goodenough, Y. Kim. Challenges for rechargeable Li batteries. Chem. Mater. 22 (2009) 587–603. |
| [2] | M. Armand, J.-M. Tarascon. Building better batteries. Nature 451 (2008) 652–657. |
| [3] | K. Kang, Y.S. Meng, J. Bréger, C.P. Grey, G. Ceder. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 311 (2006) 977–980. |
| [4] | S.L. Yang, B.H. Zhou, M. Lei, et al. Sub-100 nm hollow SnO2@C nanoparticles as anode material for lithium ion batteries and significantly enhanced cycle performances. Chin. Chem. Lett. 26 (2015) 1293–1297. |
| [5] | W. Wei, L.X. Song, L. Guo. SnO2 hollow nanospheres assembled by single layer nanocrystals as anode material for high performance Li ion batteries. Chin. Chem. Lett. 26 (2015) 124–128. |
| [6] | P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, J. Tarascon. Nano-sized transitionmetal oxides as negative-electrode materials for lithium-ion batteries. Nature 407 (2000) 496–499. |
| [7] | J.M.D. Coey, A.E. Berkowitz, L. Balcells, F.F. Putris, F.T. Parker. Magnetoresistance of magnetite. Appl. Phys. Lett. 72 (1998) 734–736. |
| [8] | Z.M. Cui, L.Y. Jiang, W.G. Song, Y.G. Guo. High-yield gas-liquid interfacial synthesis of highly dispersed Fe3O4 nanocrystals and their application in lithium-ion batteries. Chem. Mater. 21 (2009) 1162–1166. |
| [9] | S. Ito, K. Nakaoka, M. Kawamura, et al. Lithium battery having a large capacity using Fe3O4 as a cathode material. J. Power Sources 146 (2005) 319–322. |
| [10] | T. Muraliganth, A.V. Murugan, A. Manthiram. Facile synthesis of carbondecorated single-crystalline Fe3O4 nanowires and their application as high performance anode in lithium ion batteries. Chem. Commun (2009) 7360–7362. |
| [11] | S. Han, B. Jang, T. Kim, S.M. Oh, T. Hyeon. Simple synthesis of hollow tin dioxide microspheres and their application to lithium-ion battery anodes. Adv. Funct. Mater. 15 (2005) 1845–1850. |
| [12] | Y. Wang, H.C. Zeng, J.Y. Lee. Highly reversible lithium storage in porous SnO2 nanotubes with coaxially grown carbon nanotube overlayers. Adv. Mater. 18 (2006) 645–649. |
| [13] | X.W. Lou, Y. Wang, C. Yuan, J.Y. Lee, L.A. Archer. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 18 (2006) 2325–2329. |
| [14] | W.Y. Li, L.N. Xu, J. Chen. Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 15 (2005) 851–857. |
| [15] | L.S. Zhong, J.S. Hu, H .P. Liang, et al. , Self-Assembled 3D flowerlike iron oxide nanostructures and their application in water treatment, Adv. Mater. 18 (2006) 2426–2431. |
| [16] | F.S. Cai, G.Y. Zhang, J. Chen, et al. Ni(OH)2 tubes with mesoscale dimensions as positive-electrode materials of alkaline rechargeable batteries. Angew. Chem. Int. Ed. 43 (2004) 4212–4216. |
| [17] | X.L. Huang, X. Zhao, Z.L. Wang, L.M. Wang, X.B. Zhang. Facile and controllable onepot synthesis of an ordered nanostructure of Co(OH)2 nanosheets and their modification by oxidation for high-performance lithium-ion batteries. J. Mater. Chem. 22 (2012) 3764–3769. |
| [18] | H.G. Wang, D.L. Ma, X.L. Huang, Y. Huang, X.B. Zhang. General and controllable synthesis strategy of metal oxide/TiO2 hierarchical heterostructures with improved lithium-ion battery performance. Sci. Rep. 2 (2012) 701. |
| [19] | Y. Chen, H. Xia, L. Lu, J.M. Xue. Synthesis of porous hollow Fe3O4 beads and their applications in lithium ion batteries. J. Mater. Chem. 22 (2012) 5006–5012. |
| [20] | Q.Q. Xiong, J.P. Tu, Y. Lu, et al. Synthesis of hierarchical hollow-structured singlecrystalline magnetite (Fe3O4) microspheres: the highly powerful storage versus lithium as an anode for lithium ion batteries. J. Phys. Chem. C 116 (2012) 6495–6502. |
| [21] | B.P. Jia, L. Gao. Morphological transformation of Fe3O4 spherical aggregates from solid to hollow and their self-assembly under an external magnetic field. J. Phys. Chem. C 112 (2008) 666–671. |
| [22] | H. Deng, X.L. Li, Q. Peng, et al. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 44 (2005) 2782–2785. |
| [23] | R.L. Penn, J.F. Banfield. Imperfect oriented attachment: dislocation generation in defect-free nanocrystals. Science 281 (1998) 969–971. |
| [24] | P.W. Voorhees. The theory of Ostwald ripening. J. Stat. Phys. 38 (1985) 231–252. |
| [25] | Z.L. Wang, D. Xu, H.G. Wang, Z. Wu, X.B. Zhang. In situ fabrication of porous graphene electrodes for high-performance energy storage. ACS Nano 7 (2013) 2422–2430. |
| [26] | X.L. Huang, R.Z. Wang, D. Xu, et al. Homogeneous CoO on graphene for binderfree and ultralong-life lithium ion batteries. Adv. Funct. Mater. 23 (2013) 4345–4353. |
| [27] | Y. Huang, X.L. Huang, J.S. Lian, et al. Self-assembly of ultrathin porous NiO nanosheets/graphene hierarchical structure for high-capacity and high-rate lithium storage. J. Mater. Chem. 22 (2012) 2844–2847. |
| [28] | X.L. Huang, J. Chai, T. Jiang, et al. Self-assembled large-area Co(OH)2 nanosheets/ionic liquid modified graphene heterostructures toward enhanced energy storage. J. Mater. Chem. 22 (2012) 3404–3410. |
 2016, Vol. 27
2016, Vol. 27 


