As common cations,protons play key roles in both of metabolism and cellular events,such as cell growth,calcium regulation chemotaxis,and cell adhesion [1]. Many detection methods in the determination of pH have resulted in the research of pH sensors [2-11]. Among these methods,fluorescent probes are regarded as outstanding detection systems due to their terms of high sensitivity,selectivity,and more convenient operation in many applications [12-14]. So far,strenuous efforts have been made to the development of efficient fluorescent chemosensors for the detection of pH [15-19]. Rhodamine derivatives have been widely used for detection of various metal ions due to their high photostability,high quantum yield,and special structure [20-27]. The sensing mechanism is based on fluorescence enhancement caused by spirolactam ring-opening of rhodamine derivatives [28]. The spirolactam structure is also sensitive to the pH value of the environment. Under neutral or basic conditions,the spirolactam remains closed and the rhodamine derivatives are non-fluorescent,while under acidic conditions,proton leads to spirolactam ringopening and the rhodamine derivatives exhibit strong fluorescence emission [29-31]. Thus,rhodamine derivatives are suitable to monitor the pH value in acidic environment with enhanced fluorescence signals. This type of rhodamine-based pH probes have been prepared and attained considerable effects [30-34],while pH-sensitive rhodamine derivatives as pH fluorescence probes are less common. Among these rhodamine-based probes,dual-switch pH sensors that emit fluorescence with dual emission wavelengths and enable a built in correction for the undesired environmental effects have received more and more concerns.
Perylene tetracarboxylic diimides (PDI) have also generated great interest in the fields of sensors because of their excellent photo-stability,chemical stability,and high electron-accepting ability [35-44]. Most perylenediimide fluorometric sensors were designed to sense photophysical changes produced upon complexation,including photoinduced electron transfer (PET),intramolecular charge transfer (ICT) [45],and fluorescence resonance energy transfer (FRET) [46]. In these sensors,the PET-based PDI fluorometric sensors [47, 48] have been widely utilized in the design of sensors due to the inherently higher sensitivity of PET in comparison with the normal fluorescence quenching motif. However,PET-based PDI fluorometric sensors with dual-switch sensor for proton have not been reported before.
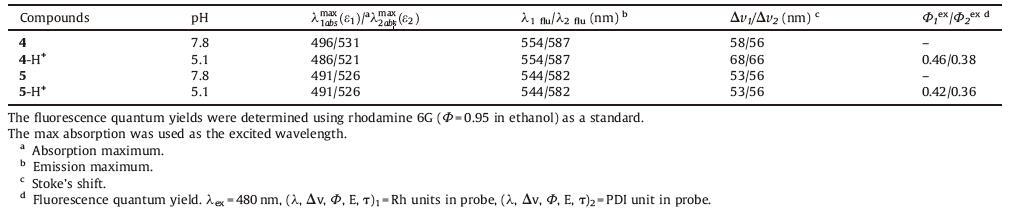
Herein,based on the unique structural transformations of rhodamine in acid media and the high electron-accepting ability of PDI,two novel dual-switch fluorescent probes 4 and 5 were designed and synthesized as shown in Fig. 1. In these probes,the perylene diimide chromophore plays as an electron acceptor; the rhodamine units serve as electron donors,and the ethylenediamine plays as spacer,which separates the two units. In order to improve the solubility of PDI-core in probes,two PDI cores have been prepared via modifying the bay region of perylene diimides with 4-tert-butylphenol. In these cases,A PET process can occur accompanying with the spirolactam ring-opening of rhodamine. The probes represent off-state when the rhodamine units are in spirocyclic,the protonation coordination of the amine fluorophore would generate the rhodamine ring-open. At the same time,the PET process should be blocked and the probes represented "switched on" state.
|
Download:
|
| Figure 1. Structure of the compounds 4 and 5 | |
2. Experimental 2.1. Materials
Commercially available rhodamine B,ethylenediamine and perylene-3,4:9,10-tetraboxylic acid bisanhydride were used without purification. The starting compounds 3 [49] and (1,7-bis(4-tert-butylphenyloxy) perylene-3,4:9,10-tetracarboxylic acid bisanhydride) 2 [50, 51]werepreparedaccording to the reportedprocedures. All solvents used in spectroscopic measurements were of analytical grade. Reactions were monitored by thin layer chromatography using Merck TLC Silica gel 60 F254. Silica gel column chromatographywas performed overMerck Silica gel 60. Dilute hydrochloric acid or sodium hydroxide was used for tuning pH values.
2.2. Methods1H NMR and 13C NMR spectra were recorded on a Bruker DMX 300 NMR spectrometer and a Bruker ADVANCE 500 NMR spectrometer in CDCl3 with tetramethylsilane (TMS) as internal standard. Mass spectra were recorded on Agilent Technologies 6530 Accurate-Mass Q-TOF LC/MS. HRMS were recorded on an Ultraflex Ⅱ MALDI-TOF mass spectrometer. UV-visible absorption spectra were determined on a Shimadu UV-3600 spectrophotometer. Fluorescence spectra were measured on a HORIBA FL-4 Max spectrometer. FT-IR spectra were recorded on a Nicolet 750 series in the region of 4000-400 cm-1 using KBr pellets. DFT calculations of compounds were performed using the Gaussian 03 program package. The calculations were optimized at the B3LYP/6- 31G (d) level of theory. The molecular orbitals were visualized using GaussView.
2.3. Synthesis of amino-functional rhodamine B (3)Rhodamine B 5.0 g (11.2 mmol) and ethylenediamine 9.0 mL (134.8 mmol) were dissolved in ethanol (50 mL) in a 250 mL flask,then the mixture was heated at 80℃ for 7 h. After ethanol was removed under vacuum,the residue was purified by column chromatography on silica gel (CH2Cl2/MeOH,10:1) to give 3 as a pale yellow powder 4.7 g,yield: 85%. M.p. 216-218℃ (the reference value 217-219 ℃),yield: 75%. 1H NMR (CDCl3,ppm): δ 7.89 (m,1H),7.42 (m,2H),7.07 (m,1H),6.40 (dd,4H,J = 8.9,2.6 Hz ),6.26 (dd,2H,J = 8.8,2.7 Hz),3.32 (m,8H),3.18 (t,2H,J = 6.6 Hz),2.40 (t,2H,J = 6.6 Hz),1.15 (t,12H,J = 6.9 Hz).
2.4. Synthesis of 1,7- bis(4-tert-butylphenyloxy)perylene-3,4:9,10- tetracarboxylic acid bisanhydride (2)(1) A mixture of 5.0 g (13 mmol) of perylene-3,4:9,10-tetraboxylic acid bisanhydride (1) and sulfuric acid (100 mL) was stirred at 50℃ for 12 h,and subsequently I2 (0.56 g) was added. The reaction mixture was heated to 80℃,and bromine 2.2 g (15 mmol) was added dropwise over a time period of 2 h. After bromine addition,the reaction mixture was heated for an additional 48 h,the excess bromine was removed by saturated aqueous solution of K2CO3,and water (100 mL) was added carefully. The precipitate was separated by filtration,washed with water (100 mL),and dried in a vacuum to give a red powder 6.0 g. Without further purification it was used to the next reaction.
(2) A mixture of compound 1,7-dibromoperylene-3,4:9,10-tetracarboxylic acid bisanhydride 2.0 g (3.6 mmol),4-tert-butylphenol 1.8 g (12.0 mmol),and K2CO3 2.4 g (6.8 mmol) in dry DMF (120 mL) was heated at the refluxing temperature for 4 h under an N2 atmosphere. The reaction mixture was poured into water (100 mL) and neutralized with aqueous 1.2 mol/L HCl solutions. The formed precipitate was collected by filtration and washed with water and methanol to give crude product 2 3.1 g,yield: 92%. As this product showed poor solubility in common organic solvents,it was used for the next reaction without further purification.
2.5. Rhodamine-perylenediimide (4) Compound 3 1.1 g (2.3 mmol),compound 2 0.42 g (0.6 mmol),and N (C3H7)3 (0.5 mL) were dissolved in DMF (25 mL),the mixture was heated at 120℃ for 12 h under nitrogen and the reaction was followed by TLC (DCM: methanol = 10:1). On completion of the reaction the solvent was removed under reduced pressure,a redblack solid got,washed with waters (100 mL),dry in 60℃,a more rigorous purification was then carried out via column chromatography (DCM/ methanol = 20:1) to give a purple solid 4 0.79 g,yield: 80%. m.p. > 300℃. FT-IR (KBr,cm-1): 3422 (vNH),2950-2863 (vCH),1696 (vasN-C=O),1665 (vsN-C=O),and 1578 (vN-C=O). 1H NMR (CDCl3,ppm): δ 8.53 (s,2H),8.33 (d,4H,J = 8.1 Hz),7.79-7.71 (m,2H),7.43-7.41 (m,8H),7.14 (s,2H),7.05 (d,2H,J = 6.0 Hz),6.96 (d,4H,J = 8.4 Hz),6.62~6.60 (m,4H),6.43~6.40 (m,4H),6.29~6.26 (m,4H),3.74 (t,4H,J = 4.5 Hz),3.52 (t,4H,J = 4.5 Hz),3.28 (m,16H),1.65 (s,18H),1.13 (t,24H,J = 6.3 Hz); 13C-NMR (CDCl3): δ 168.4 163.2,155.7,153.8,152.8,147.0,132.8,131.1,128.8,127.8,126.5,123.7,122.9,122.7,120.1,119.5,119.2,44.4,44.3,44.2,39.4,34.3,
31.6,31.4,29.6,and 12.5. MALDI-TOF-MS: m/z. Calcd.: [M + H]+ = 1621.7635,found: 1621.7639.
Compound 5 was prepared according the same method of compound 4 . A oxblood red solid 5 was got 1.1 g,yield: 84%. m.p. = 292-294 ℃. FT-IR (KBr,cm-1): 3423 (vNH),2969-2863 (vCH),1695 (vasN-C=O),1616 (vsN-C=O),1592 (vN-C=O). 1H NMR (300 MHz): δ 8.45 (m,8H,J = 8.1 Hz),7.94-7.85 (m,2H),7.45-7.36 (m,4H),7.02 (dd,2H,J = 5.1,4.2 Hz),6.5 (d,4H,J = 8.7 Hz),6.31 (d,4H,J = 2.1 Hz),6.03 (dd,4H,J = 8.7,1.8 Hz),4.25 (t,4H,J = 3.2 Hz),3.57 (t,4H,J = 3.2 Hz),3.12 (m,16H),1.04 (t,24H,J = 6.9 Hz); 13C-NMR (CDCl3): δ 168.8,162.5,153.4,148.6,135.4,135.4,134.1,132.6,132.3,132.1,131.2,130.8,131.3,128.0,127.8,126.4,124.4,123.7,123.3,122.7,
129.8,118.3,108.0,97.8,77.4,77.0,76.5,73.6,61.6,58.8,45.8,41.5,38.8,and 12.5. MALDI-TOF-MS: m/z Calcd.: [M + H]+ = 1325.5859,found: 1325.5854.
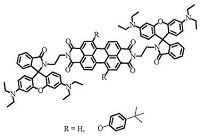
Compounds 2,4,and 5 were synthesized via simple steps using standard organic reactions,as depicted in Schemes 1 and 2. Details of the synthesis of compounds 4 and 5 have been discussed above. Compound 2 was synthesized by refluxing 1,7-dibromoperylene- 3,4:9,10-tetracarboxylic acid bisanhydride and 4-tert-butylphenol in DMF,as its poor solubility in common organic solvents,it was used for the next reaction without further purification. Compounds 4 and compound 5 were prepared by refluxing compounds 3 and 2 in imidazole in the presence of Et3 N,their yields were 80% and 82%,respectively. All of the new compounds were fully characterized by FT-IR,1H NMR,13C NMR and high-resolution mass spectrometry (HRMS-MALDI-TOF).

|
Download:
|
| Scheme 1. Synthesis of 1,7-bis(4-tert-butylphenyloxy)perylenediimide 2 | |
|
Download:
|
| Scheme 2. Synthesis of the probes 4 and 5 | |
3.2. The pH dependence of absorption and fluorescence spectra of compounds 4 and 5
Fig. 2 presents the absorption spectra of compounds 4 and 5 in DMSO/H2O at different pH values. The maximum absorption peaks of probe 4 are at 480 and 530 nm,which are corresponding to the absorption of rhodamine and PDI units in probes,respectively. As shown in Fig. 2-left the absorption spectra of probe 4 showed nearly no changes when the pH value > 7.0,which is ascribed to its spirolactam form of probe in solution; while the pH decreases to pH value < 7.0 the absorption intensity of probe 4 at 530 nm and 480 nm increases,respectively. Meanwhile,an obvious absorption blue shifts generates,indicating the formation of the ring-opened amide form of rhodamine units in probe 4. The nearly same results can be obtained in the probe 5 (Fig. 2 right). The synchronous changes of absorption spectra at 480 nm and 530 nm maybe can prove the dual-switch process happened in the probes.

|
Download:
|
| Figure 2. The pH dependence of absorption spectra of compounds 4 and 5 (c = 10 μmol/L, in DMSO/H2O, 1:9). | |
The pH dependence of fluorescence spectra of compounds 4 and 5,obtained after excitation within the spectral region of maximal absorption of the peripheral fluorophore (λex = 480 or 530 nm),show two emission bands at 550 nm and 580 nm,corresponding to the emission bands of rhodamine units and the PDI core in compounds 4 and 5 as shown in Fig. 3. The corresponding data is showed in Table 1. From the insets photons,we can see that the probe 4 is non-fluorescent when the pH value > 7.0,when the value of the pH falls to less than 7.0,the fluorescence intensity gradually increases to about 170-fold from pH 7.8 to 5.4. The quantum yield of the probe 4 is determined to be 0.46 in acidic condition (pH 5.1) and < 0.01 in neutral condition with rhodamine 6G as a standard [52]. The similar results can be obtained for compound 5,as shown in Fig. 3 right. Compared with to the fluorescent spectra of the two probes,the nearly same results can be obtained in probes 4 and 5,which can be attributed to the nearly same molecular structure of probes. As shown in Fig. 3,the synchronous enhanced fluorescence intensity of probes to the various pH values at 550 nm and 580 nm further prove that the dual-switch processes exactly happened in the probes.

|
Download:
|
| Figure 3. The pH dependence of fluorescence spectra of compounds 4 and 5 (c = 10 μmol/L, λex = 480 nm, in DMSO/H2O, 1:9). Inset: fluorescence intensity of probes at various pH values | |
|
|
Table 1 Fluorescent properties of compounds 4 and 5 in DMSO/H2O, 1/9 |
| $\log \left( \frac{{{I}_{F\max }}-{{I}_{F}}}{{{I}_{F}}-{{I}_{F\min }}} \right)=\text{pH-pKa}$ | (1) |
The abilities of the probes to recognize proton were also investigated. From Fig. 3,it can be seen that the linear response range covers the acidic pH range from 5.1 to 7.0. The acidity constants apparent pKa of probes 4 and 5 were determined by fluorimetric titration as a function of pH,taking the part of the inset graph in Fig. 3 located between pH 5.1 and 7.0,the pKa values of the light harvesting probes 4 and 5 have been calculated by Eq. (1) [53]. The calculated pKa values are 6.12 and 6.30 for probes 4 and 5,respectively. These pKa values prove that the probes are valuable for the study of acidic environment.
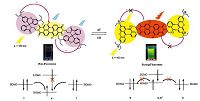
3.3. Sensing mechanism and DFT calculationsIn these probes,there are two important factors that lead to the fluorescence quenching of compounds 4 and 5. The first factor is that the PET process from the rhodamine units to the perylenediimide core in probes. The other is the closed spirolactam of the rhodamine units in the probes as shown in Fig. 4. Therefore,while the pH decreased to pH value < 7.0 the PET process in probes was pressed and the fluorescence of PDI core in probe recovered. Meanwhile,the closed spirolactam of the rhodamine units in the probes also opened,the probes show strong fluorescence with dual emission wavelength at 550 nm and 580 nm. The fluorescence response processes of probes proved that the design of the dualswitch state is favorable for the probe to work as a pH indicator. Moreover,the ring-open of rhodamine can be confirmed by 1H NMR,a new multiplet around 9.6 ppm is assigned to ring-open of probe 4 (Fig. S5 in supporting information).
|
Download:
|
| Figure 4. Photo-induced electron transfer (PET) process for the proposed sensors | |
DFT calculations were performed to understand the PET progress of 4 at the molecular level. The HOMOs and LUMOs of model compounds 4 and 4-H+ are showed in Fig. S10 in Supporting information. The LUMOs (-5.44 eV) of model compound 4 are evenly delocalized over the rhodamine units and its HOMOs (-10.9072 eV) are mainly delocalized over perylenediimide units. On the other hand,The HOMOs (-8.3504 eV) of model compound 4-H+ are mainly delocalized over rhodamine units and its LUMOs (-2.2848 eV) are mainly delocalized over perylenediimide unit. Meanwhile,in order to understand the PET process,three model compounds 6,7,and 8 (Fig. 5) are used for DFT calculations. The Optimized geometries and calculated HOMO and LUMO density maps are showed in Fig. 6. The calculated HOMOs of compounds 7 and 8 are law compared with to that of compound 6. These results reflect that the energy level of PDI units in probe 4 in acid environment changes higher than the HOMOs of PDI units in probe 4 in neutral environment. So,it can be concluded that the PET of probes can be repressed in acid environment.
|
Download:
|
| Figure 5. Structure of the model compounds 6, 7, and 8 | |

|
Download:
|
| Figure 6. Optimized geometries and calculated HOMO and LUMO density maps for model compounds according to DFT calculations at B3LYP/6–31* level | |
3.4. Interference of metal cations in the fluorescence intensity of dyes
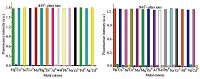
Fig. 7 shows the selectivity of probes 4 and 5 to various ions,respectively. Upon addition of Hg2+,Na+,K+,Ca2+,Mg2+,Co2+,Ni2+,Cu2+,Zn2+,Mg2+,Pd2+,Pb2+,Fe2+,Mn2+,and Al3+ ions to the 10 μmol/L probe solutions,no significant fluorescence intensity changes were observed. However,the same amount of H+ led to a remarkable enhancement in fluorescence intensity,and the fluorescence intensity changes caused by H+ are not obviously influenced by the coexisting metal ions such as Hg2+,Na+,K+,Ca2+,Mg2+,Co2+,Ni2+,Cu2+,Zn2+,Mg2+,Pd2+,Pb2+,Fe2+,Mn2+,and Al3+ ions. Meanwhile,the fluorescence spectra of probes 4 and 5 to the mixture of various metal salts were showed in Fig. 8. The results indicate that probes 4 and 5 have high selectivity to protons in the presence of other metal ions.
|
Download:
|
| Figure 7. Fluorescence responses of compounds 4 and 5 to various metal salts of metal cations, such as K+, Fe3+, Ca2+, Mg2+, Mn2+, Cr3+, Al3+, and Co2+ (4 eq.) | |

|
Download:
|
| Figure 8. Fluorescence responses of probes to the mixture of various metal salts, such as K+, Fe3+, Ca2+, Mg2+, Mn2+, Cr3+, Al3+, and Co2+ (4 eq.) | |
3.5. Reproducibility,reversibility,and response time
In order to study the response time,and reversibility of the sensors,the reversible nature of the sensors were examined by recording the ratio of fluorescence intensity at 550 nm with respect to the change of pH from acidic (pH 2.0) to alkaline (pH 7.8) range and vice versa up to 7 cycles. Fig. 9 shows the fluorescence intensity change with time upon switching from one solution to the other. The relative standard deviations from sevenmeasurements for blank solution of pH 7.0 was found to be 0.1% and the relative standard deviations in fluorescence intensities recorded from five replicates of pH 2.0 was estimated as 1.1%. The response time were 5-8 s for probes of pH 2.0,meanwhile,it was found that the recovering time was independent of the H+ concentration change. Results indicate the reversibility between the protonated and deprotonated forms of the sensors. Thus the sensors might be applicable for real time pH monitoring.

|
Download:
|
| Figure 9. The ratio of fluorescence intensity of probes at 550 nm(c = 10 μmol/L, λex = 480 nm, in DMSO/H2O, 1:9) upon consecutive addition of HCl (1 mol/L) and NaOH (1 mol/L) solution up to seven cycles | |
3.6. Long-time stability and lifetime
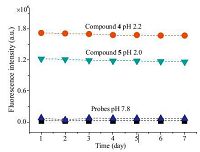
To investigate the short-term stability of the sensors,the fluorescence intensity of the sensors exposed to solution of pH 2.0 and 7.8 were tested over a period of seven days. The fluorescence emission intensities were recorded every 24 h. As shown in Fig. 10,the relative standard error of < 1% was obtained for the solution. The solution exhibits good stability and has a lifetime of at least one month. Thus,the probes have remarkably long lifetime.
|
Download:
|
| Figure 10. The time courses of fluorescence intensity of probes in buffers of various pH values (7.8, 2.2, and 2.0, respectively) λex = 480 nm, lem = 550 nm. | |
3.7. Confocal fluorescence images
HeLa cell was cultured in media (RPMI 1640 supplemented with 10% PBS,100 units/mL of penicillin and 100 units/mL of streptomycin) at 37℃ in a humidied incubator,and culture media were replaced with fresh media every day. The utilities of probes 4 and 5 in living cells were studied. The HeLa cell lines were incubated with receptor probes [1.0 mmol/L in DMSO/H2O (1:9,v/ v) buffered with HEPES,pH 7.0] in a RPMI-1640 medium for 1 h at 37℃ and washed with a phosphate-buffered saline (PBS) buffer (pH 7.2) to remove excess receptor 4 or 5. The cells were then treated with pH 5.8 in the RPMI-1640 medium,incubated again for 30 min at 37℃,and washed with a PBS buffer. After treatment with pH 5.8 in the RPMI-1640 medium,confocal fluorescence images were studied,the cells show enhanced red fluorescence emission as shown in Fig. 11-B and D. These results suggested that probes 4 and 5 are effective dual-switch fluorescent pH probes intracellular imaging.

|
Download:
|
| Figure 11. Confocal fluorescence images of Hela cells incubated with 1 mmol/L probes 4 (Fig. A and B) and 5 (Fig. C and D) for 30 min (pH 5.8, Fig. B and D: fluorescence image; pH 7.2, Fig. A and C: photos in bright field). | |
4. Conclusion
In summary,two novel pH fluorescent chemosensors based on rhodamine-perylenediimide have been designed,synthesized,and fully characterized by 1H NMR,13C NMR,and HRMS-MALDI-TOF. The dual-switches of the sensors were based on the structural transformation of rhodamine units and an intramolecular photoinduced electron transfer (PET) process that occurred with the switching effects of rhodamine units in the probes. These probes showed excellent fluorescent sensitivity for protons with enhanced emission from 550 nm to 580 nm. The fluorescence changes of probes 4 and 5 were reversible within a wide range of pH values from 2.0 to 11.0. Furthermore,the sensors exhibited short response time,long lifetime,and high selectivity toward protons in the presence of various other metal cations,such as Hg2+ ,Fe3+,Cu2+,Mg2+,Ca2+,Mn2+,Co2+,Ni2+,Zn2+,Cd2+,Ba2+,and Pb2+. The possible mechanism was investigated by the DFT calculation and 1H NMR. According to the experiments of confocal laser scanning microscopy,compounds 4 and 5 could be used to detect the acidic pH variations in living cells with an effective dualswitches fluorescent signal. Thus,we are convinced that the design strategy will help to develop a new platform for the design of new dual-switches fluorescent probes for other target analytes.
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.039.
| [1] | W.S. Zhang, B. Tang, X. Liu, et al. A highly sensitive acidic pH fluorescent probe and its application to HepG2 cells. Analyst 134 (2009) 367–371. |
| [2] | H. Deveci, A. Akcil, I. Alp. Bioleaching of complex zinc sulphides using mesophilic and thermophilic bacteria: comparative importance of pH and iron. Hydrometallurgy 73 (2004) 293–303. |
| [3] | M.K. Nielsen, N. Arneborg. S12.7 The effect of citric acid and pH on growth and metabolism of anaerobic Saccharomyces cerevisiae and Zygosaccharomyces bailii cultures. Food Microbiol 24 (2007) 101–105. |
| [4] | J. Hynes, T. O'Riordan, D. Papkovsky, Y. Will. The use of oxygen and pH-sensitive fluorescent probes for the investigation of perturbed cell metabolism. Biochim. Biophys. Acta 1777 (2008) S77. |
| [5] | M. Kroez, E.J. Kanzy, P. Gronski, G. Dickneite. Hypotension with intravenous immunoglobulin therapy: importance of pH and dimer formation. Biologicals 31 (2003) 277–286. |
| [6] | J. Hynes, T.C. O'Riordan, A.V. Zhdanov, et al. In vitro analysis of cell metabolism using a long-decay pH-sensitive lanthanide probe and extracellular acidification assay. Anal. Biochem. 390 (2009) 21–28. |
| [7] | T. Hasegawa, Y. Kondo, Y. Koizumi, et al. A highly sensitive probe detecting low pH area of HeLa cells based on rhodamine B modified b-cyclodextrins. Bioorg. Med. Chem. 17 (2009) 6015–6019. |
| [8] | S. Maskula, J. Nyman, A. Ivaska. Titration of strong and weak acids by sequential injection analysis technique. Talanta 52 (2000) 91–99. |
| [9] | T.R.L.C. Paixão, L. Kosminsky, M. Bertotti. Use of electrochemically pretreated glassy carbon electrodes as pH sensors in potentiometric titrations. Sens. Actuators B 87 (2002) 41–46. |
| [10] | M. Iga, A. Seki, Y. Kubota, K. Watanabe. Acidity measurements based on a heterocore structured fiber optic sensor. Sens. Actuators B 96 (2003) 234–238. |
| [11] | H.F. Ji, K.M. Hansen, Z. Hu, T. Thundat. Detection of pH variation using modified microcantilever sensors. Sens. Actuators B 72 (2001) 233–238. |
| [12] | Z. Li, S.Q. Wu, J.H. Han, S.F. Han. Imaging of intracellular acidic compartments with a sensitive rhodamine based fluorogenic pH sensor. Analyst 136 (2011) 3698–3706. |
| [13] | S. Kang, S. Kim, Y.K. Yang, S. Bae, J. Tae. Fluorescent and colorimetric detection of acid vapors by using solid-supported rhodamine hydrazides. Tetrahedron Lett. 50 (2009) 2010–2012. |
| [14] | Y.Q. Miao, J.R. Chen, K.M. Fang. New technology for the detection of pH. J. Biochem. Biophys. Methods 63 (2005) 1–9. |
| [15] | M.Z. Tian, X.J. Peng, F. Feng, et al. Fluorescent pH probes based on boron dipyrromethene dyes. Dyes Pigments 81 (2009) 58–62. |
| [16] | B. Tang, F.B. Yu, P. Li, et al. A near-infrared neutral pH fluorescent probe for monitoring minor pH changes: imaging in living HepG2 and HL-7702 cells. J. Am. Chem. Soc. 131 (2009) 3016–3023. |
| [17] | Z.P. Liu, C.L. Zhang, W.J. He, et al. A charge transfer type pH responsive fluorescent probe and its intracellular application. New J. Chem. 34 (2010) 656–660. |
| [18] | L.J. Liu, P. Guo, L. Chai, et al. Fluorescent and colorimetric detection of pH by a rhodamine-based probe. Sens. Actuators B 194 (2014) 498–502. |
| [19] | M.Z. Tian, X.J. Peng, J.L. Fan, J.Y. Wang, S.G. Sun. A fluorescent sensor for pH based on rhodamine fluorophore. Dyes Pigments 95 (2012) 112–115. |
| [20] | X. Zhang, Y. Shiraishi, T. Hirai, Cu (Ⅱ)-Selective Green fluorescence of a rhodamine-diacetic acid conjugate. Org. Lett 24 (2007) 5039–5042. |
| [21] | Y. Zhao, Y. Sun, X. Lv, et al. Rhodamine-based chemosensor for Hg2+ in aqueous solution with a broad pH range and its application in live cell imaging. Org. Biomol. Chem. 8 (2010) 4143–4147. |
| [22] | L. Yuan, W.Y. Lin, Y.M. Feng. A rational approach to tuning the pKa values of rhodamines for living cell fluorescence imaging. Org. Biomol. Chem. 9 (2011) 1723–1726. |
| [23] | L. Feng, H. Li, Y.J. Lv, Y.F. Guan. Colorimetric and "turn-on" fluorescent determination of Cu2+ ions based on rhodamine-quinoline derivative. Analyst 137 (2012) 5829–5833. |
| [24] | S.T. Cai, Y. Lu, S. He, et al. A highly sensitive and selective turn-on fluorescent chemosensor for palladium based on a phosphine-rhodamine conjugate. Chem. Commun. 49 (2013) 822–824. |
| [25] | N.R. Chereddy, S. Thennarasu, A.B. Mandal. Incorporation of triazole into a quinoline-rhodamine conjugate imparts iron (Ⅲ) selective complexation permitting detection at nanomolar levels. Dalton Trans. 41 (2012) 11753–11759. |
| [26] | H.L. Li, H. Guan, X.R. Duan, et al. An acid catalyzed reversible ring-opening/ringclosure reaction involving a cyano-rhodamine spirolactam. Org. Biomol. Chem. 11 (2013) 1805–1809. |
| [27] | P. Mahato, S. Saha, E. Suresh, et al. Ratiometric detection of Cr3+ and Hg2+ by a naphthalimide-rhodamine based fluorescent probe. Inorg. Chem. 51 (2012) 1769–1777. |
| [28] | H. Zheng, X.Q. Zhan, Q.N. Bian, X.J. Zhang. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem. Commun. 49 (2013) 429–447. |
| [29] | X.L. Wu, X.L. Jin, Y.X. Wang, et al. Synthesis and spectral properties of novel chlorinated pH fluorescent probes. J. Lumin. 131 (2011) 776–780. |
| [30] | N.I. Georgiev, A.M. Asiri, A.H. Qusti, K.A. Alamry, V.B. Bojinov. A pH sensitive and selective ratiometric PAMAM wavelength-shifting bichromophoric system based on PET. FRET and ICT. Dyes Pigments 102 (2014) 35–45. |
| [31] | H.S. Lv, J. Liu, J. Zhao, B.X. Zhao, J.Y. Miao. Highly selective and sensitive pHresponsive fluorescent probe in living Hela and HUVEC cells. Sens. Actuators B 177 (2013) 956–963. |
| [32] | Z.Q. Hua, M. Li, M.D. Liu, W.M. Zhuang, G.K. Li. A highly sensitive fluorescent acidic pH probe based on rhodamine B diethyl-2-aminobutenedioate conjugate and its application in living cells. Dyes Pigments 96 (2013) 71–75. |
| [33] | H.S. Lv, S.Y. Huang, B.X. Zhao, J.Y. Miao. A new rhodamine B-based lysosomal pH fluorescent indicator. Anal. Chim. Acta 788 (2013) 177–182. |
| [34] | J.L. Fan, C.Y. Lin, H.L. Li, et al. A ratiometric lysosomal pH chemosensor based on fluorescence resonance energy transfer. Dyes Pigments 99 (2013) 620–626. |
| [35] | J.Q. Feng, B.L. Liang, D.L. Wang, L. Xue, X.Y. Li. Novel fluorescent dyes with fused perylene tetracarboxlic diimide and BODIPY analogue structures. Org. Lett. 10 (2008) 4437–4440. |
| [36] | C.T. Zhao, Y.X. Zhang, R.J. Li, X.Y. Li, J.Z. Jiang. Di (alkoxy)-and di (alkylthio)-substituted perylene-3, 4;9,1'-tetracarboxy diimides with tunable electrochemical and photophysical properties. J. Org. Chem 72 (2007) 2402–2410. |
| [37] | W. Xu, H.L. Chen, Y.F. Wang, et al. Photoinduced electron and energy transfer in dyads of porphyrin dimer and perylene tetracarboxylic diimide. Chem. Phys. Chem. 9 (2008) 1409–1415. |
| [38] | B.A. Jones, M.J. Ahrens, M.H. Yoon, et al. High-Mobility air-stable n-type semiconductors with processing versatility: dicyanoperylene-3,, 4:9, 1'-bis (dicarboximides). Angew. Chem. Int. Ed. 43 (2004) 6363–6366. |
| [39] | K.L. Liu, Z.J. Xu, M.Z. Yin, et al. A multifunctional perylenediimide derivative (DTPDI) can be used as a recyclable specific Hg2+ ion sensor and an efficient DNA delivery carrier. J. Mater. Chem. B 2 (2014) 2093–2096. |
| [40] | N.I. Georgiev, A.R. Sakr, V.B. Bojinov. Design and synthesis of novel fiuorescence sensing perylene diimides based on photoinduced electron transfer. Dyes Pigments 91 (2011) 332–339. |
| [41] | L.M. Huang, S.W. Tam-Chang. 9-Piperazine substituted perylene-3, 4-dicarboximide as a fluorescent probe in ratiometric analysis. Chem. Commun 47 (2011) 2291–2293. |
| [42] | Y.X. Wu, X.B. Zhang, J.B. Li, et al. Bispyrene-fluorescein hybrid based FRET cassette: a convenient platform toward ratiometric time-resolved probe for bioanalytical applications. Anal. Chem. 86 (2014) 10389–10396. |
| [43] | S.S. You, Q. Cai, K. Müllen, W.T. Yang, M.Z. Yin. pH-sensitive unimolecular fluorescent polymeric micelles: from volume phase transition to optical response. Chem. Commun 50 (2014) 823–825. |
| [44] | F.S. Goodson, D.K. Panda, S. Ray, et al. Tunable electronic interactions between anions and perylenediimide. Org. Biomol. Chem. 11 (2011) 4797–4803. |
| [45] | U. Hahn, J.F. Nierengarten, B. Delavaux-Nicot, et al. Fullerodendrimers with a perylenediimide core. New J. Chem. 35 (2011) 2234–2244. |
| [46] | J.H. Hurenkamp, W.R. Browne, R. Augulis, et al. Intramolecular energy transfer in a tetra-coumarinperylene system: influence of solvent and bridging unit on electronic properties. Org. Biomol. Chem. 5 (2007) 3354–3362. |
| [47] | J.S. Kim, D.T. Quang. Calixarene-derived fluorescent probes. Chem. Rev. 107 (2007) 3780–3799. |
| [48] | C.T. Chen, W.P. Huang. A highly selective fluorescent chemosensor for lead ions. J. Am. Chem. Soc. 124 (2002) 6246–6247. |
| [49] | J.H. Huang, Y.F. Xu, X.H. Qian. A rhodamine-based Hg2+ sensor with high selectivity and sensitivity in aqueous solution: A NS2-containing receptor. J. Org. Chem. 74 (2009) 2167–2170. |
| [50] | M.J. Ahrens, M.J. Tauber, M .R. Wasielewski, Bis (n-octylamino) perylene-3, 4: 9, 1'-bis (dicarboximide)s and their radial cations: synthesis, electrochemistry, and ENDOR spectroscopy. J. Org. Chem 71 (2006) 2107–2114. |
| [51] | T.E. Kaiser, V. Stepanenko, F. Würthner. Fluorescent J-aggregates of core-substituted perylene bisimides: studies on structure-property relationship, nucleationelongation mechanism, and sergeants-and-soldiers principle. J. Am. Chem. Soc 131 (2009) 6719–6732. |
| [52] | G.A. Crosby, J.N. Demas. The measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 75 (1971) 991–1024. |
| [53] | D. Staneva, P. Bosch, A.M. Asiri, L.A. Taib, I. Grabchev. Studying pH dependence of the photophysical properties of a blue emitting fiuorescent PAMAM dendrimer and evaluation of its sensor potential. Dyes Pigments 105 (2014) 114–120. |
 2016, Vol. 27
2016, Vol. 27