b School of Mechanical and Materials Engineering, Washington State University, Pullman, WA 99164, United States
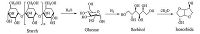
In the past decades,there have been increasing concerns about the fast consumption of fossil resources and growing environmental problems caused by petroleum-based materials [1, 2]. Utilization of abundant and renewable resources such as starch,cellulose and lignin as a platform to develop new chemicals and polymeric materials appears to be of importance both from an ecological and economical point of view [3-6]. Among the studied bio-based chemicals,isosorbide is a valuable V-shaped diol consisting of two cis-fused tetrahydrofuran rings with 120° angle and can be produced in large scale from starch through three main chemical reactions (shown in Fig. 1). First,starch is hydrolyzed to glucose and then hydrogenated to sorbitol. At last sorbitol is dehydrated to isosorbide. Due to the distinctive properties of isosorbide such as attractive price,molecular rigidity,high thermal stability,biodegradability,renewability,and non-toxicity,a lot of research have focused on exploring new isosorbide-based derivatives (monomers) to prepare high-performance materials for a wide range of applications [7-11].
|
Download:
|
| Figure 1. Scheme of the conversion of starch to isosorbide | |
Smart materials,which could respond to environmental change in automatic ways,have attracted much attention and represent a new paradigm in materials design. Self-healing polymers are one of most interesting smartmaterials [12, 13]. In thesematerials,damage can trigger autonomic healing response. Norbornene-based derivative,such as dicyclopentadiene (DCPD),was widely studied as potential self-healing agent because it could easily performed ringopening metathesis polymerization (ROMP) and form valuable materials in the presence of Grubbs' catalyst [14, 15]. Recently,our research group reported the synthesis of a new norbornenylfunctionalized vegetable oil and prepared two kinds ofROMP-based thermosets with good thermal and mechanical properties [16].
In this paper,continuing to develop novel bio-based reactive systems and monomers suitable for high performance materials,we took advantage of isosorbide as a platform and incorporated high reactive norbornene functional group into isosorbide backbone to synthesize one novel bio-based norbornenylfunctionalized isosorbide (ISN). The ROMP curing behaviors of ISN were evaluated in comparison with conventional petroleumbased DCPD and the dynamic mechanical properties and tensile strength of cured poly(ISN) thermosets were also investigated and compared with cured poly(DCPD).
2. ExperimentalHigh purity isosorbide (99.5%,1),5-norbornen-2-yl(ethyl)- chlorodimethylsilane (2),anhydrous triethylamine (TEA),4- dimethylaminopyridine (DMAP) and 2nd generation Grubbs' catalyst [1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]- dichloro(phenylmethylene)-(tricyclohexylphosphine) ruthenium were purchased from Sigma-Aldrich (Milwaukee,WI). 5-Norbornene- 2,3-dicarboxylic anhydride (3),sodium bicarbonate,anhydrous magnesium sulfate,ammonium chloride and dichloromethane (CH2Cl2) were supplied by Fisher Scientific (Fair Lawn,NJ).Dicyclopentadiene (endo-DCPD)was obtained fromAcros Organics (Belgium).CH2Cl2 was refluxed with sodiumhydride under nitrogen flow and distilled immediately prior to use. All other reagents were used as received without further purification.
The norbornenyl-functionalized isosorbide monomers were synthesized by following procedures. For ISN,in a three-neck flask equipped with a nitrogen inlet and magnetic stirrer,1 (10 mmol),TEA (33 mmol),DMAP (1.5 mmol) and CH2Cl2 (100 mL) were charged and mixed with vigorous stirring. Under ice bath,2 (21 mmol) dissolved in 10 mL CH2Cl2 was dropwise added into the solution of 1 in 1 h. Then the reaction mixture was kept stirring at room temperature for 12 h,followed by adding saturated NH4Cl solution. The organic layer was subsequently separated and evaporated under reduced pressure to obtain the desired product ISN with a high yield of 96%.
The corresponding thermosets were prepared via ringopening metathesis polymerization (ROMP) in the presence of Grubbs's catalyst. First,2nd generation Grubbs's catalyst was freeze-dried prior to dissolution in monomer. In a typical procedure,catalyst (100 mg) was dissolved in benzene (2 mL) in a small vial and then flash-frozen in a liquid nitrogen bath. The frozen solution was placed in an ice bath under vacuum for 5 h to remove the benzene. Freeze-dried 2nd generation Grubbs's catalyst (0.05 wt%) was added to ISN resin cooled in an ice bath,which was vigorously stirred to dissolve catalyst completely. Then the resin mixture was placed in Teflon molds and cured according to the following schedule: isothermal cure at 50℃ for 1 h and 110℃ for 2 h,followed by post-cure at 130℃ for 12 h. After curing the resulting thermosets were removed from oven and cool down in the air.
1H NMR spectra were recorded on a Bruker AC 400 nuclear magnetic resonance instrument with CDCl3 as solvent and tetramethylsilane as internal standard. Differential scanning calorimetry (DSC,TA instruments) was used to evaluate the curing behaviors of ISN monomer. The thermal stabilities of cured materials were studied using a thermogravimetric analyzer (Discovery TGA,TA Instruments). The dynamic mechanical properties of poly(ISN) thermosets were investigated using a strain-controlled rheometer (ARES G2,TA Instruments) in DMA mode with the torsion geometry. The specimens were tested in a temperature range from -50℃ to 220℃ at a heating rate of 3℃/ min,a strain of 0.065%,and an oscillation frequency of 1 Hz. Tensile properties were tested at room temperature by an Instron 4505 universal testing apparatus under a constant crosshead rate of 5 mm/min. Each batch included five specimens to yield an average value.
3. Results and discussionAs shown in Scheme 1,two different chemicals 5-norbornen-2- yl(ethyl)chlorodimethylsilane (2) and 5-norbornene-2,3-dicarboxylic anhydride (3) were selected as modification reagents to functionalize isosorbide (1). Through a facile one-step reaction at room temperature the corresponding two norbornenyl-functionalized isosorbide derivatives ISN and IN were synthesized,respectively. In contrast to solid IN (Tm = 47 ℃),ISN has low melting point (×15 ℃) and is low viscous liquid at room temperature,which means that ISN is more likely to wet the crack surface through capillary effects and perform ROMP to repair the potential microcracks in the materials for the future selfhealing application. In addition,non-isothermal differential scanning calorimetry (DSC) indicated that IN could not conduct ROMP in the presence of Grubbs' catalyst because no obvious exothermic peaks were observed during the whole curing process. This could be explained by the difference of molecular structures between ISN and IN. Compared to ISN,the two rigid reactive norbornene end groups in IN were too close to the center rigid twofused tetrahydrofuran rings,which made the molecular structure of IN even more crowded and then inhibited the formation of macromolecules. Meanwhile,the presence of carboxylic acid groups attached to norbornene groups also could form intramolecular hydrogen bonds in IN and further increased the molecular rigidity. Additionally,Lambeth et al. ever reported that the existence of carboxylic acid group was likely to react and form coordinated complex with transition metals of Grubbs' catalyst,which dramatically decreased the catalyst efficiency [17].

|
Download:
|
| Scheme 1. Synthetic route of norbornenyl-functionalized isosorbide ISN and IN | |
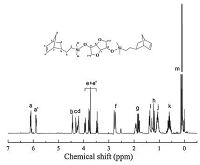
The chemical compositions of ISN were characterized and verified by 1H NMR spectroscopy (shown in Fig. 2). The resonances centered at 6.11-6.09 and 5.90-5.87 ppm were assigned to the two different protons of -CH55CH- of norbornene moiety,while the peaks located in the area ranging from 4.45 ppm to 3.44 ppm was corresponded to the eight protons of two-fused rings of isosorbide segment. The signals at 2.78-2.73 and 1.92-1.78 ppm were caused by the end-protons (CH) andmiddle-protons (CH2) of bridged -CH- CH2-CH- of norbornenemoiety. In addition,multiplets corresponding to the protons of -CH2-CH2-Si(CH3)2- chain-linker appeared around 1.11-1.01,0.67-0.50 and 0.15-0.05 ppm,respectively.
|
Download:
|
| Figure 2. 1H NMR spectrum of bio-based monomer ISN. | |
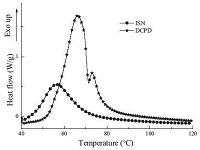
The curing behaviors of ISN in the presence of Grubbs' catalyst was investigated by non-isothermal DSC at heating rate of 5 ℃/min and compared with the conventional petroleum-based DCPD. As presented in Fig. 3,pure ISN only exhibited one exothermic peak,while there were two different exothermic peaks appeared in the DCPD curing spectrum. As we know,the cyclic carbon double bonds could easily be triggered by the Grubbs' catalysts to conduct ring-opening metathesis polymerization (ROMP) and form macromolecules. As a difunctional monomer,ISN is comprised of two identical norbornene end-groups in the isosorbide backbone. When ROMP took place,much reaction heat was released at the same time. According to Fig. 3,the heat flow of ISN reached maximum value when the temperature was 56.6℃. In the contrary,there were two different cyclic C55C double bonds in DCPD molecule. The major and minor exothermic peaks were attributed to the ROMP of cyclic C55C double bonds in norbornene and cyclopentene groups,respectively,because the reactivity of C55C double bonds in norbornene was usually regarded to be higher than that of C55C double bonds in cyclopentene group. The corresponding cure peak temperatures were 66.4℃ and 73.1℃. It was obvious that in comparison with DCPD,ISN curing peak temperature was nearly 10℃ lower than DCPD,which indicated ISN had higher reactivity and was more likely to conduct ROMP at the same conditions.
|
Download:
|
| Figure 3. DSC thermograms of ISN and DCPD at heating rate of 5℃/min | |
Fig. 4 presented the plots of dynamic storage modulus G' and loss factor tanδ as a function of temperature for the cured poly(ISN) and poly(DCPD) and the date were summarized in Table 1. It demonstrated that at low temperature (blew 0 ℃) the G' of poly(ISN) was almost the same as that of poly(DCPD),which suggested poly(ISN) was a rather stiff material at the glassy state. With the temperature increasing,the value of G' gradually decreased and leveled off at high temperature (above 150 ℃). Interestingly,in contrast to the glass state,the G' of poly(ISN) was as much as ten times higher than that of poly(DCPD) at the rubbery state. For example,the G' was as high as 110.5 MPa for poly(ISN) while the G' of poly(DCPD) was only 9.3 MPa at 175 ℃. These results suggested that compared to poly(DCPD),poly(ISN) was more suitable to be applied in the areas under harsh conditions. In addition,Fig. 4b showed that Tg of poly(ISN) determined by the arelaxation peak of tan was lower than that of poly(DCPD) because poly(ISN) had flexible and elastic Si-C long chains in macromolecular backbones,while poly(DCDP) mainly consisted of rigid fivemember rings. The presence of less rigid and elastic Si-C long chains also made the β-relaxation of poly(ISN),attributed to the vibration of minor side groups,was more obvious at the temperature ranging from -30℃ to 20℃ in comparison with poly(DCPD).

|
Download:
|
| Figure 4. DMA curves for poly(ISN) and poly(DCPD) thermosets: (a) storage modulus and (b) loss tangents | |
The thermal stability of cured poly(ISN) was evaluated by thermogravimetric analysis (TGA) under nitrogen atmosphere. As presented in Fig. 5,three-stage thermal degradation was observed in poly(ISN) samples,while poly(DCPD) only exhibited one major degradation. Compared to compact and rigid backbone of poly(DCPD),poly(ISN) was formed through cross-linking of the long and flexible O-Si-C chains. At the temperature ranging from 260℃ to 440℃,the weight loss in the first stage was attributed to the breakage of flexible O-Si-C chains. With temperature increasing,the severe weight loss occurred in the second stage,which was mainly caused by the cleavages of rigid cyclopentyl backbone originated from norbornene moieties of ISN. The last degradation started from 540℃ to 600℃ and corresponded to gradual thermolysis of more stable two-fused five-member-ring isosorbide segments.

|
Download:
|
| Figure 5. TGA curves of cured poly (ISN) and poly(DCPD) thermosets | |
The mechanical properties of poly(ISN) was analyzed and preliminarily compared with poly(DCPD). As presented in Table 1,due to the presence of flexible and elastic Si-C long-chain unit in the poly(ISN) the Young's modulus (E) and tensile strength (σ) were decreased from 700 Mpa and 63.6 Mpa to 615 Mpa and 45.1 Mpa,while the elongation at break (ε) was increased from 16.5% to 21.3%,respectively. Although the tensile strength was not as high as poly(DCPD),poly (ISN) still exhibited satisfying mechanical properties.
|
|
Table 1 DMA parameters and mechanical properties of poly (ISN) and poly (DCPD) thermosets |
4. Conclusion
We have demonstrated an easy and convenient strategy to design and synthesize novel bio-based monomer (ISN) from renewable isosorbideunder mild conditions. Due to the presence of flexible and elastic Si-C long chains and two reactive norbornene end groups,DSC curing thermograms indicated that ISN had higher reactivity andmore easily conducted ROMP at the same conditions compared to conventional petroleum-based DCPD. The DMA and tensile tests illustrated that the obtained cured poly(ISN) not only had good mechanical properties but also exhibited much higher storage modulus than poly(DCPD) at the rubbery state.
| [1] | A. Gandini. Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules 41 (2008) 9491–9504. |
| [2] | N. Hernández, R.C. Williams, E.W. Cochran. The battle for the "green" polymer. Different approaches for biopolymer synthesis: bioadvantaged vs. bioreplacement. Org. Biomol. Chem 12 (2014) 2834–2849. |
| [3] | B.T. Wang, Y. Zhang, P. Zhang, Z.P. Fang. Functionalization of polyhedral oligomericsilsesquioxanes with bis(hydroxyethyl) ester and preparation of the corresponding degradable nanohybrids. Chin. Chem. Lett. 23 (2012) 1083–1086. |
| [4] | K.J. Yao, C.B. Tang. Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 46 (2013) 1689–1712. |
| [5] | M. Rose, R. Palkovits. Cellulose-based sustainable polymers: state of the art and future trends. Macromol. Rapid Commun. 32 (2011) 1299–1311. |
| [6] | M.A.R. Meier. Metathesis with oleochemicals: new approaches for the utilization of plant oils as renewable resources in polymer science. Macromol. Chem. Phys. 210 (2009) 1073–1079. |
| [7] | M. Chrysanthos, J. Galy, J.P. Pascault. Preparation and properties of bio-based epoxy networks derived from isosorbidediglycidyl ether. Polymer 52 (2011) 3611–3620. |
| [8] | R. Vendamme, W. Eevers. Sweet solution for sticky problems: chemoreological design of self-adhesive gel materials derived from lipid biofeedstocks and adhesion tailoring via incorporation of isosorbide. Macromolecules 46 (2013) 3395–3405. |
| [9] | J. Łukaszczyk, B. Janicki, A. López, et al. Novel injectable biomaterials for bone augmentation based on isosorbidedimethacrylic monomers. Mater. Sci. Eng. 40 (2014) 76–84. |
| [10] | V. Besse, R. Auvergne, S. Carlotti, et al. Synthesis of isosorbide based polyurethanes: an isocyanate free method. React. Funct. Polym. 73 (2013) 588–594. |
| [11] | K.L. Xie, Y.H. Su, C.X. Zhang. Synthesis and optical activity of isosorbide chiral derivative containing fluorocarbon group as chiral dopant in liquid crystal materials. Chin. Chem. Lett. 22 (2011) 1447–1450. |
| [12] | J.A. Syrett, C.R. Becer, D.M. Haddleton. Self-healing and self-mendable polymers. Polym. Chem. 1 (2010) 978–987. |
| [13] | V.K. Thakur, M.R. Kessler. Self-healing polymer nanocomposite materials: a review. Polymer 69 (2015) 369–383. |
| [14] | S. Sutthasupa, M. Shiotsuki, F. Sanda. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 42 (2010) 905–915. |
| [15] | C.E. Hobbs, B.H. Lin, T. Malinski. Norbornene derivatives from a metal-free, strainpromoted cycloaddition reaction: new building blocks for ring-opening metathesis polymerization reactions. J. Polym. Sci. 53 (2015) 2357–2362. |
| [16] | R. Ding, Y. Xia, T.C. Mauldin, M.R. Kessler. BiorenewableROMP-based thermosetting copolymers from functionalized castor oil derivative with various crosslinking agents. Polymer 55 (2014) 5718–5726. |
| [17] | R.H. Lambeth, S.J. Pederson, M. Baranoski, A.M. Rawlett. Methods for removal of residual catalyst from polymers prepared by ring opening metathesis polymerization. J. Polym. Sci. 48 (2010) 5752–5757. |
 2016, Vol. 27
2016, Vol. 27 



