b School of Chemistry and Chemical Engineering, Xuzhou Institute of Technology, Xuzhou 221111, China
Hexavalent chromium (Cr(Ⅵ)) is highly toxic and highly soluble in water,and has been classified as a priority pollutant by many countries in the world [1-3]. Photocatalytic reduction has now been widely considered as a simple,effective,low cost and environmentally-friendly method for the treatment of aqueous Cr(Ⅵ) [4-8]. However,at present,the lack of efficient visiblelight- activated photocatalysts has hindered the industrial application of photocatalysis technology in treating large-scale Cr(Ⅵ) wastewater. For instance,TiO2,the most studied photocatalyst hitherto,can absorb only the UV light (only~5% of solar energy) and exhibits little photocatalytic activity in the reduction of aqueous Cr(Ⅵ) under visible-light irradiation,due to its large bandgap (Eg = 3.2 eV) [8-10].
The modification of TiO2 by smaller bandgap semiconductors has been proved to be a simple and effective way to achieve the visible-light photocatalysis [9-11]. Furthermore,composite photocatalysts can exhibit higher photocatalytic efficiency than their individual components,because of the synergistic effect of their components,such as the enhanced separation of photogenerated electrons and holes [9-12]. Bi2Ti2O7 has a bandgap of 2.58-2.74 eV [13, 14],and has been studied as a visible-light-active photocatalyst in the degradation of organics [14, 15] and production of hydrogen by splitting water [16]. However,the reports on the use of Bi2Ti2O7/TiO2 composites as photocatalysts were scarce so far. Herein,we report the synthesis of Bi2Ti2O7/TiO2 composites by modifying the method proposed to synthesize Bi2Ti2O7 [17],and the initial study of Bi2Ti2O7/TiO2 composites in the photocatalytic reduction of aqueous Cr(Ⅵ).
2. ExperimentalPreparation: 18 mL of absolute ethanol and 7 mL of glycerin were added to a 50 mL Teflon jar,and the mixture was stirred until a homogeneous solution was obtained. A certain amount (0- 1.76 mmol) of Bi(NO3)3-5H2O was added to the absolute ethanol- glycerin solution and the mixture was stirred until the dissolution of Bi(NO3)3-5H2O. 1.5 mL (4.4 mmol) of tetrabutyl titanate was added dropwise to the above solution under magnetic stirring,and after the addition of tetrabutyl titanate,the resulting mixture was further magnetically stirred for 20 min. Diethyl ether (5 mL) was added to the above mixture and the mixture was stirred for 5 min. The Teflon jar containing the reactants was placed into a stainless steel autoclave,sealed and heated at 110℃ for 12 h,then cooled to ambient temperature naturally. The resultant amorphous precipitate was centrifuged,washed with absolute ethanol and deionized water,dried in air at 100℃ for 10 h,and finally calcined at 500℃ for 3 h.
For the convenience of description,the products prepared using the Bi/Ti molar ratios of 0,10%,20%,30% and 40% were abbreviated as BT-0,BT-10,BT-20,BT-30 and BT-40 (Table 1),respectively.
|
|
Table 1 Abbreviations and compositions of the products prepared using different Bi/Ti molar ratios in the reactants. |
Characterization: The compositions,structures and optical properties of the products were characterized by X-ray diffraction (XRD,German Bruker AXS D8 ADVANCE X-ray diffractometer),field emission scanning electron microscopy (FESEM,Japan Hitachi S-4800 field emission scanning electron microscopy) and UV-vis diffuse reflectance spectra (American Varian Cary 5000 UV-vis- NIR spectrophotometer). Photocatalytic properties of the products (whose dosage was 300 mg) were tested in the reduction of Cr6+ in 300 mL of 50 mg/L K2Cr2O7 aqueous solution under visible-light (λ > 420 nm) irradiation,with the addition of 1.0 mL of 100 mg/mL citric acid aqueous solution as the sacrificing reagent [18]. The photocatalytic reaction process was monitored by measuring the Cr(Ⅵ) concentration using the standard diphenylcarbazide colorimetric method [19].
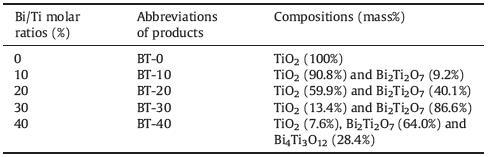
3. Results and discussionFig. 1 shows the XRD patterns of BT-0,BT-10,BT-20,BT-30 and BT-40. All the XRD peaks of BT-0 can be indexed to pure anatase TiO2 (Joint Committee on Powder Diffraction Standards (JCPDS) card number 89-4921). The XRD peaks of BT-10,BT-20 and BT-30 revealed the preparation of the composites of anatase TiO2 and cubic phase Bi2Ti2O7 (JCPDS card number 32-0118),whereas those of BT-40 suggested the formation of a mixture of anatase TiO2,cubic phase Bi2Ti2O7 and orthorhombic phase Bi4Ti3O12 (JCPDS card No. 35-0795). The phase compositions of BT-10,BT-20,BT-30 and BT-40 were estimated by the method of XRD quantitative analysis,and the obtained results are shown in Table 1. The results revealed that the molar ratio of Bi/Ti played a crucial role in the phase compositions of the resultant products. This was also consistent with the previous report [20]. The formation mechanisms of different products may be described as the following equations:
|
Download:
|
| Figure 1. XRD patterns of BT-0, BT-10, BT-20, BT-30 and BT-40 | |
| $\text{Ti}{{\left( \text{O}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}} \right)}_{\text{4}}}\text{+6ROH}\to \text{Ti}\left( \text{OH} \right)_{\text{6}}^{\text{2-}}\text{+2}{{\text{R}}^{\text{+}}}\text{+4RO}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}$ | (1) |
| $\text{B}{{\text{i}}^{\text{3+}}}\text{+6ROH}\to \text{Bi}\left( \text{OH} \right)_{\text{6}}^{\text{3-}}\text{+6}{{\text{R}}^{\text{+}}}$ | (2) |
| $\text{Ti}\left( \text{OH} \right)_{\text{6}}^{\text{2-}}\to \text{Ti}{{\text{O}}_{\text{2}}}\cdot n{{\text{H}}_{\text{2}}}\text{O+}\left( \text{3}-\text{n} \right){{\text{H}}_{\text{2}}}\text{O}$ | (3) |
| $\text{2Bi}\left( \text{OH} \right)_{\text{6}}^{\text{3-}}\to \text{B}{{\text{i}}_{\text{2}}}{{\text{O}}_{\text{3}}}\cdot m{{\text{H}}_{\text{2}}}\text{O}+\left( 3-\text{m} \right){{\text{H}}_{\text{2}}}\text{O+6O}{{\text{H}}^{\text{-}}}$ | (4) |
| $\text{B}{{\text{i}}_{\text{2}}}{{\text{O}}_{\text{3}}}\cdot m{{\text{H}}_{\text{2}}}\text{O+2Ti}{{\text{O}}_{\text{2}}}\cdot n{{\text{H}}_{\text{2}}}\text{O}\to \text{B}{{\text{i}}_{\text{2}}}\text{T}{{\text{i}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{+}\left( \text{m+2n} \right){{\text{H}}_{\text{2}}}\text{O}$ | (5) |
| $\text{2B}{{\text{i}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{ }\!\!\times\!\!\text{ m}{{\text{H}}_{\text{2}}}\text{O+3Ti}{{\text{O}}_{\text{2}}}\cdot n{{\text{H}}_{\text{2}}}\text{O}\to \text{B}{{\text{i}}_{\text{4}}}\text{T}{{\text{i}}_{\text{3}}}{{\text{O}}_{\text{12}}}\text{+}\left( \text{2m+3n} \right){{\text{H}}_{\text{2}}}\text{O}$ | (6) |
The microstructures of BT-0,BT-10,BT-20,BT-30 and BT-40 were characterized by FESEM (Fig. 2). The FESEM image of BT-0 showed that the as-prepared TiO2 was comprised of aggregates of nanoparticles. The FESEM images of BT-10,BT-20 and BT-30 showed that the prepared composites of TiO2 and Bi2Ti2O7 were comprised of nanoparticles and micrometer-sized spheres. Furthermore,the number of micrometer-sized spheres increased with the increase of Bi2Ti2O7 content from BT-10 to BT-30. Besides,the higher magnification FESEM image of BT-30 (Fig. 2,BT-30-B) revealed that the micrometer-sized spheres were constructed with nanoparticles. The FESEM image of BT-40 showed that the prepared composite of TiO2,Bi2 Ti2O7 and Bi4Ti3O12 were mainly comprised of micrometer-sized spheres decorated with nanoparticles.

|
Download:
|
| Figure 2. FESEM images of BT-0, BT-10, BT-20, BT-30 and BT-40 | |
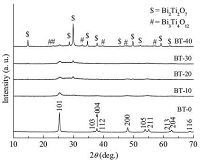
Fig. 3 shows the UV-vis diffuse reflectance spectra of BT-0,BT- 10,BT-20,BT-30 and BT-40 in the absorbance mode. It can be seen that BT-0 (TiO2) displays almost no absorption of visible-light,whereas BT-10,BT-20,BT-30 and BT-40 display distinct absorption of visible-light in the range of about 400-550 nm.
|
Download:
|
| Figure 3. UV–vis diffuse reflectance spectra of BT-0, BT-10, BT-20, BT-30 and BT-40 | |
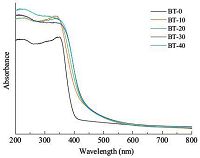
Fig. 4 shows the photocatalytic reduction of aqueous Cr(Ⅵ) over BT-0,BT-10,BT-20,BT-30 or BT-40 in the presence of citric acid as the sacrificing reagent,as well as the results obtained in the absence of a catalyst under visible-light (λ > 420 nm). In the absence of a catalyst,the Cr(Ⅵ) concentration remained unchanged,suggesting that Cr(Ⅵ) cannot be reduced directly by citric acid under visible-light (λ > 420 nm) irradiation. In the presence of BT-0 (TiO2),the Cr(Ⅵ) concentration decreased slowly with the increase of irradiation time. In contrast,the Cr(Ⅵ) concentration decreased much more quickly with the increase of irradiation time in the presence of BT-10,BT-20,BT-30 or BT-40. These results indicated that BT-10,BT-20,BT-30 and BT-40 all had higher visiblelight- driven photocatalytic activity than BT-0 in the reduction of aqueous Cr(Ⅵ). The photocatalytic activity of the samples followed an order of BT-0 < BT-10 < BT-20 < BT-40 < BT-30. In the presence of the most efficient BT-30,a complete reduction of Cr(Ⅵ) can be achieved under visible-light (λ > 420 nm) irradiation in 90 min.
|
Download:
|
| Figure 4. Photocatalytic reduction of aqueous Cr(Ⅵ) over BT-0, BT-10, BT-20, BT-30 or BT-40 in the presence of citric acid as the sacrificing reagent, as well as the results from mere irradiation (no catalyst) under visible-light (λ > 420 nm). Note: C0 and Ct are the Cr(Ⅵ) concentrations at the irradiation times of 0 and t min, respectively | |
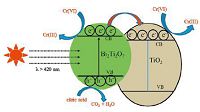
The possible mechanisms involved in the photocatalytic reduction of aqueous Cr(Ⅵ) over Bi2Ti2O7/TiO2 under visible-light (λ > 420 nm) irradiation in the presence of citric acid as the sacrificing reagent were proposed in Fig. 5. The electrons in the valence band of Bi2Ti2O7 can be excited upon visible light irradiation to its conduction band with the simultaneous generation of an equal number of holes in its valence band,but TiO2 cannot absorb the incidental visible-light (λ > 420 nm) due to its wide band gap (Eg = 3.2 eV). Because the valence band and conduction band potentials of Bi2Ti2O7 are more negative than those of TiO2 [21],the photo-generated electrons (e-1) can migrate from the conduction band of Bi2Ti2O7 to the conduction band of TiO2,whereas the photo-generated holes (h+) still remain on the valence band of Bi2Ti2O7. The e-1 can reduce Cr(Ⅵ) to Cr(Ⅲ),and at the same time,the h+ are consumed by the oxidation of citric acid [18]. The matched band structure and heterojunction interface between Bi2Ti2O7 and TiO2 could benefit the transfer and separation of e-1-Hh+ pairs,contributing to the higher photocatalytic activity of Bi2Ti2O7/TiO2 composite than TiO2 [21]. In addition,the more content of the visible-light-active Bi2Ti2O7 in the Bi2Ti2O7/TiO2 composite resulted in the higher photocatalytic activity. Thus,BT-30 with the largest content of Bi2Ti2O7 exhibited the highest photocatalytic activity. Nevertheless,the presence of Bi4Ti3O12 in the composite would decrease the photocatalytic activity (such as BT-40),because Bi4Ti3O12 cannot absorb the incidental visible-light (λ > 420 nm) either due to its larger band gap of 3.08 eV [22].
|
Download:
|
| Figure 5. Schematic diagram for photocatalytic reduction of aqueous Cr(Ⅵ) over Bi2Ti2O7/TiO2 under visible-light (λ > 420 nm) irradiation in the presence of citric acid as the sacrificing reagent | |
4. Conclusion
Bi2Ti2O7/TiO2 composites with tunable compositions were synthesized via a solvothermal-calcination two-step method,simply by changing the molar ratios of Bi(NO3)3·5H2O to tetrabutyl titanate in the reactants. The as-synthesized Bi2Ti2O7/TiO2 composites exhibited much higher photocatalytic activity than TiO2 nanoparticles in the reduction of aqueous Cr(Ⅵ) under visible-light (λ > 420 nm) irradiation.
| [1] | H. Zhang, F. Huang, D.L. Liu, P. Shi. Highly efficient removal of Cr(Ⅵ) from wastewater via adsorption with novel magnetic Fe3O4@C@MgAl-layered double-hydroxide. Chin. Chem. Lett. 26 (2015) 1137–1143. |
| [2] | M. Lu, X.H. Guan, X.H. Xu, D.Z. Wei. Characteristic and mechanism of Cr(Ⅵ) adsorption by ammonium sulfamate-bacterial cellulose in aqueous solutions. Chin. Chem. Lett. 24 (2013) 253–256. |
| [3] | Y.C. Zhang, J. Li, H.Y. Xu. One-step in situ solvothermal synthesis of SnS2/TiO2 nanocomposites with high performance in visible light-driven photocatalytic reduction of aqueous Cr(Ⅵ), Appl. Catal.. B: Environ 123-124 (2012) 18–26. |
| [4] | J. Li, M. Yang, Z.B. Jiang. One-step solvothermal synthesis of N-doped TiO2 nanoparticles with high photocatalytic activity in the reduction of aqueous Cr(Ⅵ). Chin. Chem. Lett. 25 (2014) 283–286. |
| [5] | J.M. Meichtry, C. Colbeau-Justin, G. Custo, M.I. Litter. TiO2-photocatalytic transformation of Cr(Ⅵ) in the presence of EDTA: Comparison of different commercial photocatalysts and studies by Time Resolved Microwave Conductivity, Appl. Catal.. B: Environ 144 (2014) 189–195. |
| [6] | Y.C. Zhang, L. Yao, G.S. Zhang, et al. One-step hydrothermal synthesis of highperformance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr(Ⅵ), Appl. Catal.. B: Environ 144 (2014) 730–738. |
| [7] | K. Zhang, K.C. Kemp, V. Chandra. Homogeneous anchoring of TiO2 nanoparticles on graphene sheets for waste water treatment. Mater. Lett. 81 (2012) 127–130. |
| [8] | Y.C. Zhang, M. Yang, G.S. Zhang, D.D. Dionysiou. HNO3-involved one-step low temperature solvothermal synthesis of N-doped TiO2 nanocrystals for efficient photocatalytic reduction of Cr(Ⅵ) in water, Appl. Catal.. B: Environ 142-143 (2013) 249–258. |
| [9] | M. Pelaez, N.T. Nolan, S.C. Pillai, et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications, Appl. Catal.. B: Environ 125 (2012) 331–349. |
| [10] | S. Bera, S.B. Rawal, H.J. Kim, W.I. Lee. Novel coupled structures of FeWO4/TiO2 and FeWO4/TiO2/CdS designed for highly efficient visible-light photocatalysis. ACS Appl. Mater. Interfaces 6 (2014) 9654–9663. |
| [11] | K. Sridharan, E. Jang, T.J. Park. Novel visible light active graphitic C3N4-TiO2 composite photocatalyst: synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants, Appl. Catal.. B: Environ 142-143 (2013) 718–728. |
| [12] | H.Y. Wang, Y. Yang, X. Li, L.J. Li, C. Wang. Preparation and characterization of porous TiO2/ZnO composite nanofibers via electrospinning. Chin. Chem. Lett. 21 (2010) 1119–1123. |
| [13] | A. McInnes, J.S. Sagu, K.G.U. Wijayantha. Fabrication and photoelectronchemical studies of Bi2Ti2O7 pyrochlore thin films by aerosol assisted chemical vapour deposition. Mater. Lett. 137 (2014) 214–217. |
| [14] | L.Z. Pei, H.D. Liu, N. Lin, H.Y. Yu. Bismuth titanate nanorods and their visible light photocatalytic properties. J. Alloys Compd. 622 (2015) 254–261. |
| [15] | J.G. Hou, S.Q. Jiao, H.M. Zhu, R.V. Kumar. Bismuth titanate pyrochlore microspheres: directed synthesis and their visible light photocatalytic activity. J. Solid State Chem. 184 (2011) 154–158. |
| [16] | B. Allured, S. DelaCruz, T. Darling, M.N. Huda, V. Subramanian. Enhancing the visible light absorbance of Bi2Ti2O7 through Fe-substitution and its effects on photocatalytic hydrogen evolution, Appl. Catal.. B: Environ 144 (2014) 261–268. |
| [17] | J.K. Ren, G.Q. Liu, Y.P. Wang, Q. Shi. A novel method for the preparation of Bi2Ti2O7 pyrochlore. Mater. Lett. 76 (2012) 184–186. |
| [18] | L.X. Yang, Y. Xiao, S.H. Liu, et al. Photocatalytic reduction of Cr(Ⅵ) on WO3 doped long TiO2 nanotube arrays in the presence of citric acid, Appl. Catal.. B: Environ 94 (2010) 142–149. |
| [19] | Y.C. Zhang, Q. Zhang, Q.W. Shi, Z.Y. Cai, Z.J. Yang. Acid-treated g-C3N4 with improved photocatalytic performance in the reduction of aqueous Cr(Ⅵ) under visible-light. Sep. Purif. Technol. 142 (2015) 251–257. |
| [20] | W.F. Su, Y.T. Lu. Synthesis, phase transformation and dielectric properties of solgel derived Bi2Ti2O7 ceramics, Mater. Chem. Phys 80 (2003) 632–637. |
| [21] | J.H. Yi, X.J. Yuan, H.J. Wang, H. Yu, F. Peng. Preparation of Bi2Ti2O7/TiO2 nanocomposites and their photocatalytic performance under visible light irradiation. Mater. Des. 86 (2015) 152–155. |
| [22] | K.S. Chen, R. Hu, X.R. Feng, et al. Bi4Ti3O12/TiO2 heterostructure: synthesis, characterization and enhanced photocatalytic activity. Ceram. Int. 39 (2013) 9109–9114. |
 2016, Vol. 27
2016, Vol. 27