Proteins and surfactants coexist in many biochemical and physiological systems [1-14]. Interactions between surfactants and proteins, which can modulate the functional properties of proteins [1-14], have been the subject of intensive research in the past years and various techniques have been developed for that purpose such as surface tension [5, 6], viscosimetry [7] and fluorescence probe [8-10]. Therefore, a better understanding of protein-surfactant interaction plays an important role in many applications such as foods [1, 9], cosmetics [2, 7], pharmaceuticals [11, 12] and drug delivery [13, 14].
Alkyl polyglycosides (APGs), including one or several glucose molecules in their polar moiety, constitute a relatively new class of surfactants that have been taken into consideration as feasible alternatives to conventional nonionic ethoxylated surfactants in applications such as the solubilization and purification of membrane proteins [15, 16], owing to their mildness and solubility, biodegradability and safety. Consequently, understanding how proteins affect these surfactants in the protein-surfactant systems is of great significance.
In recent years more attention is paid to the use of liquid crystals (LCs) as sensing materials in the development of simple, fast, and label-free techniques for transduction and amplification of a range of chemical and biological events such as protein binding events [17-20], DNA hybridization [21-24], and enzymatic reactions [25-29] into optical signals that could be visible to the naked eye under crossed-polarizers [17-29]. For example, Pal and Das have recently reported a simple method to study protein- Lipopolysaccharide interactions using liquid crystals as an imaging tool [20].
In this work, the interactions of APG monolayer with gelatin and BSA at the LC-aqueous interface were studied using liquid crystal as an optical probe. The optical changes of LC at the APGdecorated LC-aqueous interface from a dark to a bright state occurred in the presence of gelatin and BSA, which is triggered by the interactions of APG with gelatin and BSA. On the other hand, the effect of the different chemical structure of proteins on the rearrangement of APG monolayer at the LC-aqueous interface was also discussed.
2. Experimental 2.1. Materials4-Cyano-40-pentylbiphenyl (5CB), alkyl polyglycosides (APG, C12-C14) , gelatin and bovine serum albumin (BSA) were obtained from Sinopharm Chemical Reagent Co., Ltd. Sodium chloride (NaCl) and hydrochloric acid were supplied by Kelong Chemical Technology Co., Ltd. (Chengdu China). All chemicals were used without further purification. Glass microscopy slides and copper TEM grids (100 mesh, 18 mm thickness, 285 mm grid spacing, and 55 mm bar width) were obtained from Beijing Zhongjingkeyi Technology Co., Ltd. All aqueous solutions were prepared with deionized water.
2.2. Preparation of LC optical cellsThe LC optical cells were prepared following the procedures reported in the previous studies [30, 31]. Briefly, glass slides were cleaned by sonication in ethanol for 15 min and then dried in a 100 ℃ oven for 30 min. Next, a silicon plate support with holes of 5 mm in diameter and 0.5 mm in depth was placed onto the slide and the bubbles between the slide and the silicon plate were removed under pressure. Subsequently, the silicon plate was secured on the slide via adhesion. Meanwhile, TEM copper grids (100 mesh, 18 mm thickness, 285 mm grid spacing, and 55 mm bar width) were first cleaned in methanol, ethanol, and acetone (sonication for 10 min in each solvent), then heated overnight at 45 ℃ to evaporate the residual solvents. The copper grid was then impregnated with about 0.5 mL of 5CB using a capillary tube. Excess 5CB was removed by contacting the LCs with the other end of the capillary tube. The grids containing LCs were then put on the wells of the plate containing samples of interest. This optical cell was then ready for examination under cross-polarized lighting.
2.3. Image capture and analysisThe optical texture of the 5CB films filled in the pores of the copper grids was examined using plane-polarized light in the transmission mode on an UOP UB200i microscope with a crossed polarizer. All optical microscopic images were taken at room temperature with a digital camera (DPIXEL DP330C CCD Camera) mounted on the polarizing optical microscope. Arthroscopic examinations were performed with the source light intensity set to 50% of full illumination and the aperture set to 10% to collimate the incident light. Luminosities of the images were analyzed using an image processing software Adobe Photoshop CS6. Luminosity (L) of the optical images of 5CB upon exposure of 50 mg/L gelatin aqueous solution to the APG-decorated LC interface at different time intervals were calculated using the following Eq. (1) , where S is the raw luminance of each image of the 5CB decorated with 10 mg/L APG aqueous solution and exposed to gelatin (or BSA) aqueous solutions, Smin is the luminance of the image of the 5CB contacted with 10 mg/L APG aqueous solution and Smax is the luminance of the images of 5CB contacted with gelatin (or BSA) aqueous solutions.
In every set Smax was taken as 100%, therefore, we do not report error bar for this data point.
| $L\left( \% \right) = {{S - {S_{\min }}} \over {{S_{\max }} - {S_{\min }}}}$ | (1) |
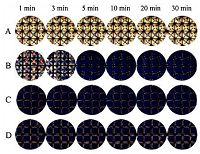
Driven by the interest in the interactions between APG and different proteins at the LC-aqueous interface, we initially investigated the optical response of LC in the presence of APG aqueous solutions, at different time intervals. It could be seen from Fig. 1 that the increasingly dark images observed with increasing time intervals were closely related to the occupation of APG molecules at the LC-aqueous interface, leading to the formation of APG-decorated LC-aqueous interface, and then inducing the orientational transition of LC from planar to the homeotropic alignment. On the other hand, it was obvious that at higher APG concentrations, the time taken to achieve optical transition of LC was shorter. Therefore no orientational transition of LC could be observed at concentrations greater than 10 mg/L APG after 1 min (Fig. 1C and D), suggesting that the interactions between LC and APG are both time-dependent and concentration-dependent. The above results are in agreement with previous reports [32, 33] that hydrophobic interactions at the interface created between LC molecules and surfactant tails play a dominant role in determining the homeotropic alignment of LCs.
|
Download:
|
| Figure 1. Optical images of LC confined with a copper grid in different time intervals after exposure to aqueous solutions of APG at increasing concentrations (mg/L): (A) 0, (B) 5, (C) 10, (D) 20. All the optical images were taken at room temperature. | |
To obtain a stable APG-decorated LC-aqueous interface to examine the interactions between APG and gelatin (or BSA), copper grids confined with LC were placed onto a 10 mg/L aqueous solution of APG for 10 min, so that the APG molecules could fully occupy the LC-aqueous interface before exposing to various proteins.
Next, we sought to determine the influence of gelatin on the orientational anchoring of the LC within the APG-decorated LCaqueous interface. It is shown in Fig. 2 that remarkable changes in the optical appearance of the LC from dark to bright occurred within 45 min (Fig. 2A), 15 min (Fig. 2B) and 5 min (Fig. 2C), respectively, after the introduction of 25 mg/L, 50 mg/L and 100 mg/L of gelatin, implying an orientational anchoring transition of LC from a homeotropic to planar/tilted alignment upon the addition of gelatin. The planar/tilted alignment, combining with previous reports of biochemical interactions in LC [19, 20], is suggestive of a physical phenomenon that gelatin absorbs and selfassemblies at the APG-decorated LC-aqueous interface [5, 34]. This observation could be due to the hydrophobic interactions between gelatin and APG molecules, which play an important role in the rearrangement of the APG monolayer, and thus, result in a transition in the orientation of LC from dark to bright [19, 20]. Moreover, the APG-decorated LC films remain stable in the dark state for days, therefore, anchoring transitions of LC from dark to bright in Fig. 2 were triggered by the presence of gelatin.

|
Download:
|
| Figure 2. Optical images of LC confined with copper grids which were decorated with 10 mg/L APG for 10 min after exposure to aqueous solutions with increasing concentrations of gelatin (A) 25, (B) 50, (C) 100 mg/L in different time intervals. All the optical images were taken at room temperature. | |
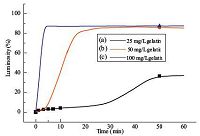
To gain insight into the rearrangement of APG monolayer triggered by gelatin at the LC-aqueous interface, the luminosities of the time-course optical images were analyzed using an image processing software Adobe Photoshop CS6 [33, 35, 36], in which the luminosities of the LC films in 16 square pore of the grid were calculated by Eq. (1) . We quantified the optical observation by plotting the luminance as a function of time after introducing gelatin (or BSA) onto the LC-aqueous interface decorated with APG, obtaining the information regarding the rearrangement of APG monolayer induced by proteins at the LC-aqueous interface. The curves shown in Fig. 3 (for example, curve a) reveal the lag-burst phase, which is a slow phase (termed as lag phase), followed by a rapid phase (termed as burst phase). The lag-phase could reflect the duration of absorption of protein at the LC-aqueous interface decorated with APG. The burst-phase is a measurement of the rearrangement of APG monolayer at the LC-aqueous interface triggered by protein. The slope of the curve of the burst-phase suggests the rearrangement rate of APG at the LC-aqueous interface. As obtained in Fig. 3 that the rearrangement rate of APG monolayer increases with an increasing gelatin concentration. The increase of gelatin concentrations could improve the availability of gelatin at the LC-aqueous interface decorated with APG, shortening the lag-time and enhancing the rearrangement rate of APG. Thus there is no lag-phase observed if the concentration of gelatin is higher than 100 mg/L (Fig. 3, curve c), indicating a time-dependent and concentration-dependent rearrangement of APG at the LC-aqueous interface.
|
Download:
|
| Figure 3. Luminosity of the optical images of 5CB films upon exposure of gelatin aqueous solutions to the APG-decorated LC interface at different time intervals. | |
In order to compare with the gelatin-APG system, our next goal was to investigate whether the interaction of BSA with APGdecorated LC-aqueous interface underwent a similar anchoring transition to that of gelatin-APG system mentioned above. Thus BSA was added into the LC-aqueous interface decorated with APG. It is obvious in Fig. 4 that a significantly continuous dark-to-bright shift in the optical response of LCs occurred in the presence of BSA, which showed a similar phenomenon to that of the gelatin-APG system (Fig. 2), reinforcing the notion that the orientational transition of LC from a homeotropic to a planar alignment was caused by the formation of protein-surfactant interaction at the LC-aqueous interface.

|
Download:
|
| Figure 4. Optical images of LC confined with copper grids which were decorated with 10 mg/L APG for 10 min after exposure to aqueous solutions with increasing concentrations of BSA (A) 5, (B) 10, (C) 50 mg/L in different time intervals. All the optical images were taken at room temperature. | |
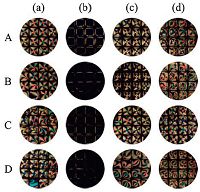
Furthermore, the influence of pH and NaCl concentration on the interactions between gelatin and APG molecules imaged by LC was investigated (Fig. 5). It can be observed from Fig. 5 that the orientation transition at different pH values and NaCl concentrations were the same with that in water, which indicates that the ordering of the LC is independent of electrostatic interactions [20]. This result also demonstrates that the interactions between gelatin (or BSA) and APG molecules are accompanied not by electrostatic but hydrophobic interactions.
|
Download:
|
| Figure 5. Optical images of LC confined within a copper grid after exposure to aqueous solutions with increasing concentrations of APG (a) 0 mg/L and (b) 10 mg/L, optical images of LC confined with copper grids which were decorated with 10 mg/L APG for 10 min after exposure to aqueous solution of (c) 100 mg/L gelatin and (d) 100 mg/L BSA. 3 mmol/LNaCl (A), 5 mmol/L NaCl (B), pH 3.7 (C) and pH 5.9 (D) aqueous solution. | |
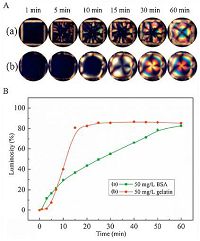
To provide further insight into the effect of the different chemical structure of proteins on the rearrangement rate of APG at the LC-aqueous interface, we conducted more experiments. Fig. 6A showed that a square pore of bright domains in LC films grow in the presence of two proteins BSA and gelatin. A detail observation should be noted that a time-dependent nucleation and growth of bright domains in LC occurred in the BSA-APG system (Fig. 6A, part a). Meanwhile, a remarkable difference in the growth behavior of bight domains without nucleation in LC could be found in the gelatin-APG system compared to BSA-APG system (Fig. 6A, part b), which might be coupled to the difference in the chemical structures. Namely, BSA is a globular protein with Trp, Tyr, and phenylalanine (Phe) residues while gelatin is a fibrous protein with Tyr and Phe residues [37-39], which therefore induced different behaviors in the growth of bright domains. Furthermore, Fig. 6B showed that in the gelatin-APG system, the time-course luminosity of the LC films reaching a maximum value was faster than that in the BSA-APG system at the same concentration, implying a higher rearrangement rate of APG in gelatin-APG system. Moreover, the molecular weights and amino acid sequence for BSA and gelatin are different, which probably contributed to the difference in the time-course luminosities.
|
Download:
|
| Figure 6. (A) The growth process of the bright domains after exposure the APGdecorated LC-aqueous interface to 50 mg/L BSA (a) and 50 mg/L gelatin (b). (B) Luminosity of the optical images of 5CB films upon exposure of the APG-decorated LC interface to 50 mg/L gelatin and 50 mg/L BSA at different time intervals. | |
4. Conclusion
In conclusion, we have studied the interactions of APG monolayer with proteins (gelatin or BSA) through the optical transition of the LC at the LC-aqueous interface. The optical changes from dark to bright are closely coupled to the concentrations of the proteins. Moreover, the comparison of the growth behaviors of the bright domains between the gelatin-APG system and the BSA-APG system also indicated that the effect of the proteins on the rearrangement of APG monolayer strongly relied on the intrinsic structures of the proteins, which therefore made the difference in the growth behavior of bight domains in LC, between the gelatin-APG system and the BSA-APG system. The results presented in this work exhibited that the LC-based interface might provide a simple, general path to study the protein- surfactant interactions, which are essential in determining their future use as excipients in membrane mimetics and pharmaceutical/ drug delivery-related compilations.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 51273220, 50903011) and the Project of Postgraduate Degree Construction, Southwest University for Nationalities (No. 2015XWD-S0703) .
| [1] | S.R. Derkach. Interfacial layers of complex-forming ionic surfactants with gelatin. Adv. Colloid Interface Sci. 222 (2015) 172–198. |
| [2] | C.M.C. Faustino, A.R.T. Calado, L. Garcia-Rio. Gemini surfactant-protein interactions: effect of pH, temperature, and surfactant stereochemistry. Biomacromolecules 10 (2009) 2508–2514. |
| [3] | K.T. Naidu, N.P. Prabhu. Protein-surfactant interaction: sodium dodecyl sulfateinducedunfolding of ribonuclease A. J. Phys. Chem. B 115 (2011) 14760–14767. |
| [4] | S. Mehan, V.K. Aswal, J. Kohlbrecher. Cationic versus anionic surfactant in tuning the structure and interaction of nanoparticle, protein, and surfactant complexes. Langmuir 30 (2014) 9941–9950. |
| [5] | D. Wu, G.Y. Xu, Y.J. Feng, Y.M. Li. Aggregation behaviors of gelatin with cationic gemini surfactant at air/water interface. Int. J. Biol. Macromol. 40 (2007) 345–350. |
| [6] | S.F. Santos, D. Zanette, H. Fischer, R. Itri. A systematic study of bovine serum albumin (BSA)and sodium dodecyl sulfate (SDS) interactions by surface tension and small angle X-ray scattering. J. Colloid Interface Sci. 262 (2003) 400–408. |
| [7] | V. Sovilj, J. Milanović, L. Petrović. Viscosimetric and tensiometric investigations of interactions between gelatin and surface active molecules of various structures. Food Hydrocolloid 32 (2013) 20–27. |
| [8] | S. De, A. Girigoswami, S. Das. Fluorescence probing of albumin-surfactant interaction. J. Colloid Interface Sci. 285 (2005) 562–573. |
| [9] | M.A. Branco, L. Pinheiro, C. Faustino. Amino acid-based cationic gemini surfactant-protein interactions. Colloids Surf. A: Physicochem. Eng. Aspects 480 (2015) 105–112. |
| [10] | S.X. Tai, X.G. Liu, W.Y. Chen, Z.N. Gao, F. Niu. Spectroscopic studies on the interactions of bovine serum albumin with alkyl sulfate gemini surfactants. Colloids Surf. A: Physicochem. Eng. Aspects 441 (2014) 532–538. |
| [11] | B. An, M. Zhang, R.W. Johnson, J. Qu. Surfactant-aided precipitation/on-pelletdigestion (SOD) procedure provides robust and rapid sample preparation for reproducible, accurate and sensitive LC/MS quantification of therapeutic protein in plasma and tissues. Anal. Chem. 87 (2015) 4023–4029. |
| [12] | A. Marin, D.P. DeCollibus, A.K. Andrianov. Protein stabilization in aqueous solutions of polyphosphazenepoly electrolyte and non-ionic surfactants. Biomacromolecules 11 (2010) 2268–2273. |
| [13] | A.W. Du, M.H. Stenzel. Drug carriers for the delivery of therapeutic peptides. Biomacromolecules 15 (2014) 1097–1114. |
| [14] | C.G. Zhou, Y.C. Mao, Y. Sugimoto, et al. , SPANosomes as delivery vehicles for small interfering RNA (siRNA). Mol. Pharm. 9 (2012) 201–210. |
| [15] | Y.M. Liu, J.L. Tao, J. Sun, W.Y. Chen. Removing polysaccharides-and saccharidesrelated coloring impurities in alkyl polyglycosides by bleaching with the H2O2/TAED/NaHCO3 system. Carbohydr. Polym. 112 (2014) 416–421. |
| [16] | H. Messinger, W. Aulmann, M. Kleber, W. Koehl. Investigations on the effects of alkyl polyglucosides on development and fertility. Food Chem. Toxicol. 45 (2007) 1375–1382. |
| [17] | J.M. Seo, W. Khan, S.Y. Park. Protein detection using aqueous/LC interfaces decorated with a novel polyacrylic acid block liquid crystalline polymer. Soft Matter 8 (2012) 198–203. |
| [18] | D. Hartono, C.Y. Xue, K.L. Yang, L.Y.L. Yung. Decorating liquid crystal surfaces with proteins for real-time detection of specific protein-protein binding. Adv. Funct. Mater. 19 (2009) 3574–3579. |
| [19] | L.N. Tan, V.J. Orler, N.L. Abbott. Ordering transitions triggered by specific binding of vesicles to protein-decorated interfaces of thermotropic liquid crystals. Langmuir 28 (2012) 6364–6376. |
| [20] | D. Das, S. Sidiq, S.K. Pal. A simple quantitative method to study protein-lipopolysaccharide interactions by using liquid crystals. ChemPhysChem 16 (2015) 753–760. |
| [21] | H. Tan, X. Li, S.Z. Liao, R.Q. Yu, Z.Y. Wu. Highly-sensitive liquid crystal biosensor based on DNA dendrimers-mediated optical reorientation. Biosens. Bioelectron. 62 (2014) 84–89. |
| [22] | P.S. Noonan, P. Mohan, A.P. Goodwin, D.K. Schwartz. DNA hybridization-mediated liposome fusion at the aqueous liquid crystal interface. Adv. Funct. Mater. 24 (2014) 3206–3212. |
| [23] | A.D. Price, D.K. Schwartz. DNA hybridization-induced reorientation of liquid crystal anchoring at the nematic liquid crystal/aqueous interface. J. Am. Chem. Soc. 130 (2008) 8188–8194. |
| [24] | A.C. McUmber, P.S. Noonan, D.K. Schwartz. Surfactant-DNA interactions at the liquid crystal-aqueous interface. Soft Matter 8 (2012) 4335–4342. |
| [25] | M.M. Zhang, C.H. Jang. Sensitive detection of trypsin using liquid-crystal droplet patterns modulated by interactions between poly-l-lysine and a phospholipid monolayer. ChemPhysChem 15 (2014) 2569–2574. |
| [26] | X.Y. Bi, D. Hartono, K.L. Yang. Real-time liquid crystal pH sensor for monitoring enzymatic activities of penicillinase. Adv. Funct. Mater. 19 (2009) 3760–3765. |
| [27] | Q.Z. Hu, C.H. Jang. A simple strategy to monitor lipase activity using liquid crystalbased sensors. Talanta 99 (2012) 36–39. |
| [28] | D. Hartono, X.Y. Bi, K.L. Yang, L.Y.L. Yung. An air-supported liquid crystal system for real-time and label-free characterization of phospholipases and their inhibitors. Adv. Funct. Mater. 18 (2008) 2938–2945. |
| [29] | J.S. Park, N.L. Abbott. Ordering transitions in thermotropic liquid crystals induced by the interfacial assembly and enzymatic processing of oligopeptide amphiphiles. Adv. Mater. 20 (2008) 1185–1190. |
| [30] | Z.J. Liao, Z.L. Qin, S.N. Du, et al. , Orientational transitions of liquid crystal driven by hydrogen-bond interaction at the liquid crystal-aqueous interface. Acta Phys. Chim. Sin. 31 (2015) 1733–1740. |
| [31] | M.I. Kinsinger, B. Sun, N.L. Abbott, D.M. Lynn. Reversible control of ordering transitions at aqueous/liquid crystal interfaces using functional amphiphilic polymers. Adv. Mater. 19 (2007) 4208–4212. |
| [32] | F. Zuo, Z.J. Liao, C.X. Zhao, et al. , An air-supported liquid crystal system for realtime reporting of host-guest inclusion events. Chem. Commun. 50 (2014) 1857–1860. |
| [33] | Z.J. Liao, S. Du, Z.L. Qin, et al. , Use of liquid crystals for imaging different inclusion abilities of a-cyclodextrin and b-cyclodextrin toward cetyltrimethyl ammonium bromide. Chem. Phys. Lett. 637 (2015) 189–194. |
| [34] | A.M. Howe, A.R. Pitt. Rheology and stability of oil-in-water nanoemulsions stabilised by anionic surfactant and gelatin, 2) addition of homologous series of sugar-based co-surfactants. Adv. Colloid Interface Sci. 144 (2008) 30–37. |
| [35] | S.R. Kim, N.L. Abbott. Rubbed films of functionalized bovine serum albumin as substrates for the imaging of protein-receptor interactions using liquid crystals. Adv. Mater. 13 (2001) 1445–1449. |
| [36] | R. Nandi, S.K. Singh, H.K. Singh, B. Singh, R.K. Singh. Fabrication of liquid crystal based sensor for detection of hydrazine vapours. Chem. Phys. Lett. 614 (2014) 62–66. |
| [37] | H.Y. Ren, X. Xin, L. Wang, et al. , A direct comparison of the interaction of bovine serum albumin and gelatin with sodium deoxycholate in aqueous solutions. J. Mol. Liq. 207 (2015) 164–170. |
| [38] | C. Onesippe, S. Lagerge. Study of the complex formation between sodium dodecyl sulphate and gelatin. Colloids Surf. A: Physicochem. Eng. Aspects 337 (2009) 61–66. |
| [39] | G. Rath, T. Hussain, G. Chauhan, T. Gary, A.K. Goyal. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater. Sci. Eng. C 58 (2016) 242–253. |
 2016, Vol. 27
2016, Vol. 27 


