Recently, there has been growing interest in transition metalcatalyzed C-N and C-C bond forming processes [1]. In particular, the copper-catalyzed Ullmann-type N-arylations have received enormous attention and have been widely used to prepare quinazolinones derivatives [2], whose subunits are extensively used in medicinal chemistry as a pharmacophore [3], with applications such as hypnotic, sedative, analgesic, antibacterial, and antitumor agents [4], or to modify known bioactive molecules and to potentiate their biological activities [5].
Generally, copper salts have been used as homogeneous catalyst systems to catalyze synthesis of quinazolinones derivatives from ortho-amino- or -nitrobenzoic acid derivatives, aryl halides [6]. Although homogeneous copper catalysts have many advantages, such as high turnover number, good activity, and good selectivity [7], they are difficult to separate from reaction mixtures and reuse. Furthermore, the residual copper metal along with the final products may cause serious problems in the synthesis of pharmaceuticals [8]. In light of these problems, various catalysts supports have been explored, such as polymers [9], zeolite [10] and mesoporous particles [11] that can be efficiently reused while keeping the inherent activity of the catalytic center. In recent years, magnetic nanoparticles supported on various materials for recovery have provided new ways to develop recyclable catalyst [12].
It is well known that graphene oxide (GO), one of the most important derivatives of graphene, has a unique planar structure[13], huge surface area (2630 m2m2 g-1)) [14], and a variety of oxygen-containing functional groups [15], which renders it a good candidate for supporting other functional materials. What’s more, the acidic and oxidative nature of these oxygen functionalities allow it to function as a solid acid catalyst or green oxidant [16].
In this study, we prepared a magnetically recyclable heterogeneous catalyst GO/Fe3O4-CuI for the synthesis of quinazolinones by the condensation of halide benzamide with amino acid, which is rarely reported [17]. The catalyst gave the desired products in good yields and could be reused several times without any loss of its catalytic activity, which showed its applicability as a reusable and promising catalyst for quinazolinones synthesis.
2. Experimental 2.1. Sample preparation proceduresGO was synthesized by natural graphite powder oxidation according to the Hummers method [18]. GO (200 mg) was dispersed in 60 mL of 1-methyl-2-pyrrolidone with ultrasonication for 1 h [19], and the mixture was heated to 190 ℃ under nitrogen atmosphere (Scheme 1). Fe(acac)3 (1.413 g) was dissolved in 40 mL of 1-methyl-2-pyrrolidone and then dropped into the GO solution under stirring for 2 h. After the reaction finished and cooled to room temperature, the precipitate was separated by magnet and dispersed in ethanol. Finally, the resulting black powder was washed several times with acetone and vacuum dried at 45 ℃ overnight.

|
Download:
|
| Scheme. 1. Preparation of GO/Fe3O4–CuI catalyst and magnetic separation of GO/Fe3O4–CuI catalyst from water. | |
The obtained GO/Fe3O4 (0.50 g) and CuI (0.25 g) were dispersed in 50 mL ethanol under nitrogen atmosphere (Scheme 1). Then the mixture was stirred at 80 ℃ overnight.The GO/Fe3O4-CuI powder was retrieved by an external permanent magnet, and washed with deionized water and ethanol.Finally, the GO/Fe3O4-CuI powder was dried under vacuum at 45 ℃ overnight for further use. The prepared GO/Fe3O4-CuI was characterized by FTIR, powder XRD, and SEM. The doped concentration of CuI was detected by ICP-AES.
2.2. Typical procedure for the condensation synthesis of quinazolinones2-Iodobenzamide 1a (1.0 equiv.), 2-aminoisovaleric acid 2a (3.0 equiv.), Cs2CO3 (3.0 equiv.) and GO/Fe3O4-CuI (Cu+0.03equiv.) were added to DMSO/ethylene glycol (60:1, v/v) in a round bottom flask. The reaction was monitored by TLC. The product was purified by column chromatography with petroleum ether/ethyl acetate (3:1, v/v) to give pure white solid 3a. Other products were synthesized through the same procedure and characterized by 1H NMR and 13C NMR. Their molecular masses were determined by HRMS.
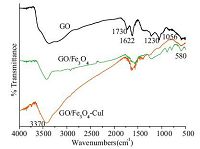
3. Results and discussionFourier-transform infrared spectroscopy (FTIR) spectra of GO, GO/Fe3O4, and GO/Fe3O4-CuI are presented in Fig. 1. Characteristic peaks of GO/Fe3O4-CuI are basically the same with GO and GO/ Fe3O4. The FTIR pattern of GO shows the presence of the oxygencontaining functional groups. The peaks at 3370 cm-1, 1730 cm-1 are the results of the stretching of O-H, C55O in COOH. The band at 1622 cm-1, 1230 cm-1, and 1056 cm-1 can be assigned to aromatic C55C, carboxy C-O, and alkoxy C-O stretches [20], respectively. The FTIR spectra of GO/Fe3O4-CuI and GO/Fe3O4 differ from that of GO as evidenced by the new band at 580 cm-1 [21]. The peak at 1730 cm-1 disappeared in the FTIR pattern of GO/ Fe3O4-CuI and the new peaks that emerged from 800 cm-1 to 1000 cm-1 are attributed to the formation of -COO- after the complex reaction with CuI.
|
Download:
|
| Figure 1. IR spectra of GO, GO/Fe3O4 and GO/Fe3O4–CuI. | |
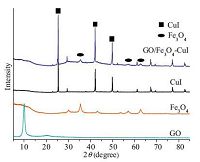
Fig. 2 shows the XRD pattern of GO/Fe3O4-CuI. The main peaks at 25.4°, 41.6°, and 49.2°, marked by their indices (1 1 1) , (2 2 0) , and (3 1 1) [22], show the characteristics of CuI on GO/Fe3O4. Besides these, no significantly different peaks are observed among the diffraction patterns of GO, GO/Fe3O4 and GO/Fe3O4-CuI, which indicates that the CuI was finely attached on GO/Fe3O4.
|
Download:
|
| Figure 2. XRD patterns of GO, Fe3O4, CuI and GO/Fe3O4–CuI. | |
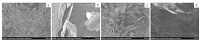
SEM observation was also undertaken to characterize the morphologies of the GO/Fe3O4 and GO/Fe3O4-CuI composites. As is shown in Fig. 3a, GO/Fe3O4 sheets show the sheet-like structure with smooth surfaces and wrinkled edges. GO/Fe3O4-CuI sheets present similar morphology with the parent GO/Fe3O4. CuI composites are uniformly distributed on the surface of GO/ Fe3O4-CuI sheets. ICP-AES detected the CuI doped concentration is up to 10.1 wt% which implies CuI successfully doped on the GO/ Fe3O4 sheets.
|
Download:
|
| Figure 3. SEM images of GO/Fe3O4–CuI ((a) and (b)), GO/Fe3O4 (c) and GO (d). | |
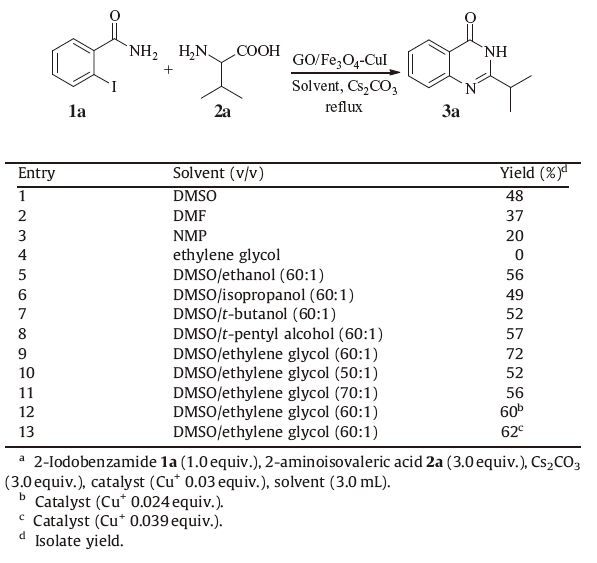
To demonstrate its activity for the synthesis of quinazolinones derivatives, the reaction of 2-iodobenzamide 1a and 2-aminoisovaleric acid 1b was used as a model reaction catalyzed by magnetic GO/Fe3O4-CuI. Clearly, the results in Table 1 show that the reaction could be catalyzed by several copper salts.While with the amount of copper was 0.03 equivalent immobilized on GO/Fe3O4, the reaction went smoothly with a respective high productivity (compare entries 2-8, Table 1). To further obtain the optimal conditions, different copper content, various bases and solvent were screened. The results revealed that the yield was up to 72% with the moderate addition of DMSO/ethylene glycol (60:1, v/v) and Cs2CO3 (3.0 equiv.) as base at 120 ℃ (Table 2, entry 9) .
|
|
Table 1 Effect of different catalyst and base on the synthesis of 2-isopropylquinazolin-4(3H)-one (3a)a. |
|
|
Table 2 Optimization of solvent on the synthesis of 2-isopropylquinazolin-4(3H)-one (3a) in the presence of GO/Fe3O4–CuI as catalysta. |
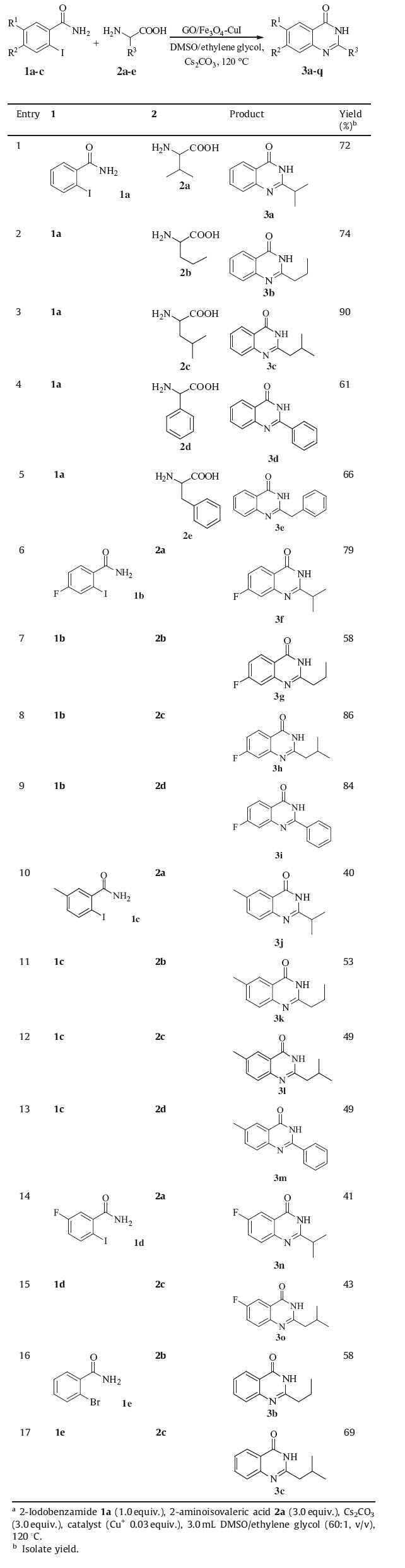
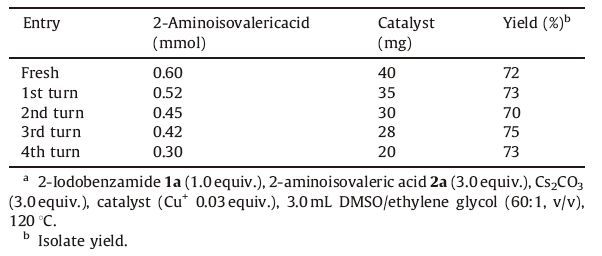
The scope of GO/Fe3O4-CuI-catalyzed cascade reactions of halide benzamideand with amino acids was investigated under the optimized conditions: GO/Fe3O4-CuI (Cu+ 0.03 equiv.) as the catalyst, 3 equiv. of Cs2CO3 as the base, DMSO/ethylene glycol (60:1, v/v) as the solvent at 120 ℃. All the examined substrates provided moderate to excellent yields (Table 3). Importantly, the catalyst recycled by permanent magnet retained their catalytic activity for the synthesis of 2-isopropylquinazolin-4(3H)-one (3a) under the same reaction conditions, with the corresponding yields not significantly reduced (Table 4).
|
|
Table 3 Scope of 2-iodobenzamide and amino acid in synthesis of quinazolinone derivativesa. |
|
|
Table 4 Reuse the catalyst for the synthesis of 3aa. |
4. Conclusion
In summary, we have successfully prepared the magnetically recyclable heterogeneous catalyst GO/Fe3O4-CuI, which exhibited high catalytic activity for the synthesis of quinazolinones derivatives by the condensation of halide benzamide with amino acid. Due to its unique planar structure, huge surface area, magnetism, and chemicals features, the catalyst shows high catalytic efficiency, magnetically recoverability, and reusability, such that it can be used several times to give desired products without any obvious loss of catalytic activity. The magnetically recyclable catalyst GO/Fe3O4-CuI shows its applicability as a reusable and promising catalyst for quinazolinones synthesis.
AcknowledgmentThis work was supported by the Research Foundation of Tongji University.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.01.055.
| [1] |
(a) S. Laclef, M. Harari, J. Godeau, et al., Ligand-free Pd-catalyzed and copperassisted C-H arylation of quinazolin-4-ones with aryl iodides under microwave heating, Org. Lett. 17 (2015) 1700-1703; (b) A. Kumar, A.K. Bishnoi, Nanoparticle mediated organic synthesis (NAMOsynthesis): CuI-NP catalyzed ligand free amidation of aryl halides, RSC Adv. 4 (2014) 41631-41635. |
| [2] | F. Wang, H.X. Liu, H. Fu, Y.Y. Jiang, Y.F. Zhao. An efficient one-pot copper-catalyzed approach to isoquinolin-1(2H)-one derivatives. Org. Lett. 11 (2009) 2469–2472. |
| [3] | W. He, H. Zhao, R.Y. Yao, M.Z. Cai. A highly efficient heterogeneous coppercatalyzed cascade reaction of, 2-halobenzoic acids and amidines leading to quinazolinones. RSC Adv. 4 (2014) 50285–50294. |
| [4] |
(a) H.X. Chai, J.R. Li, L.P. Yang, et al., Copper-catalyzed tandem N-arylatio/condensation: synthesis of quinazolin-4 (3H)-ones from 2-halobenzonitriles and amides, RSC Adv. 4 (2014) 44811-44814; (b) R.J. Abdel-Jalil, W.G. Voelter, M. Saeed, A novel method for the synthesis of 4 (3H)-quinazolinones, Tetrahedron Lett. 45 (2004) 3475-3476; (c) N.Y. Kim, C.H. Cheon, Synthesis of quinazolinones from anthranilamides and aldehydes via metal-free aerobic oxidation in DMSO, Tetrahedron Lett. 55 (2014) 2340-2344; (d) X.W. Liu, H. Fu, Y.Y. Jiang, Y.F. Zhao, A simple and efficient approach to quinazolinones under mild copper-catalyzed conditions, Angew. Chem. Int. Ed. 48 (2009) 348-351; (e) K.M. Khan, S.M. Saad, N.N. Shaikh, et al., Synthesis and b-glucuronidase inhibitory activity of 2-arylquinazolin-4 (3H)-ones, Bioorg. Med. Chem. 22 (2014) 3449-3454. |
| [5] | A. Nasreen, R.M. Borik. Cobalt(Ⅱ) chloride catalyzed one pot synthesis of, 2-substituted and 3-substituted-4 (3H)-quinazolinones. Orient. J. Chem. 30 (2014) 761–768. |
| [6] | W. Xu, Y.B. Jin, H.X. Liu, Y.Y. Jiang, H. Fu. Copper-catalyzed domino synthesis of quinazolinones via ullmann-type coupling and aerobic oxidative C-H amidation. Org. Lett. 13 (2011) 1274–1277. |
| [7] | L. Yu, M. Wang, P.H. Li, L. Wang. Fe3O4 nanoparticle-supported copper(I): magnetically recoverable and reusable catalyst for the synthesis of quinazolinones and bicyclic pyrimidinones. Appl. Organomet. Chem. 26 (2012) 576–582. |
| [8] | W.J. Zhang, X.H. Shi, Y.X. Zhang, et al. , Synthesis of water-soluble magnetic graphene nanocomposites adsorbents for recyclable removal of heavy metal ions. J. Mater. Chem. A 1 (2013) 1745–1753. |
| [9] |
(a) K.M. Ho, P. Li, Design and synthesis of novel magnetic core-shell polymeric particles, Langmuir 24 (2008) 1801-1807; (b) P.D. Stevens, J. Fan, H.M.R. Gardimalla, M. Yen, Y. Gao, Superparamagnetic nanoparticle-supported catalysis of suzuki cross-coupling reactions, Org. Lett. 7 (2005) 2085-2088. |
| [10] | S.M.A.H. Siddiki, K. Kon, A.S. Touchy, K .I. Shimizu. Direct synthesis of quinazolinones by acceptorless dehydrogenative coupling of o-aminobenzamide and alcohols by heterogeneous Pt catalysts. Catal. Sci. Technol. 4 (2014) 1716–1719. |
| [11] |
(a) M.C. Hu, K.S. Hui, K.N. Hui, Role of graphene in MnO2/graphene composite for catalytic ozonation of gaseous toluene, Chem. Eng. J. 254 (2014) 237-244; (b) S. Rostamizadeh, M. Nojavan, R. Aryan, E. Isapoor, M. Azad, Amino acid-based ionic liquid immobilized on a-Fe2O3-MCM-41: an efficient magnetic nanocatalyst and recyclable reaction media for the synthesis of quinazolin-4 (3H)-one derivatives, J. Mol. Catal. A: Chem. 374-375 (2013) 102-110. |
| [12] |
(a) N.A. Zubir, C. Yacou, J. Motuzas, X.W. Zhang, J.C.D.D. Costa, Structural and functional investigation of graphene oxide-Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction, Sci. Rep. 4 (2014) 4594; (b) S.M. Baghbanian, M. Farhang, CuFe2O4 nanoparticles: a magnetically recoverable and reusable catalyst for the synthesis of quinoline and quinazoline derivatives in aqueous media, RSC Adv. 4 (2014) 11624-11633. |
| [13] | J. Li, S.W. Zhang, C.L. Chen, et al. , Removal of Cu(Ⅱ) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 4 (2012) 4991–5000. |
| [14] | J. Li, Z.Y. Shao, C.L. Chen, X.K. Wang. Hierarchical GOs/Fe3O4/PANI magnetic composites as adsorbent for ionic dye pollution treatment. RSC Adv 4 (2014) 38192–38198. |
| [15] |
(a) J.H. Yang, B. Ramaraj, K.R. Yoon, Preparation and characterization of superparamagnetic graphene oxide nanohybrids anchored with Fe3O4 nanoparticles, J. Alloys Compd. 583 (2014) 128-133; (b) Ö. Metin, Ş. Aydoğan, K. Meral, A new route for the synthesis of graphene oxide-Fe3O4 (GO-Fe3O4) nanocomposites and their Schottky diode applications, J. Alloys Compd. 585 (2014) 681-688. |
| [16] | C.L. Su, K.P. Loh. Loh, Carbocatalysts: graphene oxide and its derivatives. Acc. Chem. Res. 46 (2013) 2275–2285. |
| [17] |
(a) J.X. Chen, D.Z. Wu, F. He, et al., Gallium(Ⅲ) triflate-catalyzed one-pot selective synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones and quinazolin-4 (3H)-ones, Tetrahedron Lett. 49 (2008) 3814-3818; (b) X.W. Liu, H. Fu, Y.Y. Jiang, Y.F. Zhao, A simple and efficient approach to quinazolinones under mild copper-catalyzed conditions, Angew. Chem. 121 (2009) 354-357. |
| [18] | A. Sergi, F. Shemirani, M. Alvand, A. Tajbakhshian. Graphene oxide magnetic nanocomposites for the preconcentration of trace amounts of malachite green from fish and water samples prior to determination by fiber optic-linear array detection spectrophotometry. Anal. Methods 6 (2014) 7744–7751. |
| [19] | Y.L. Dong, H.G. Zhang, Z.U. Rahman, et al. , Graphene oxide-Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale 4 (2012) 3969–3976. |
| [20] | L.Z. Bai, D.L. Zhao, Y. Xu, et al. , Inductive heating property of graphene oxide-Fe3O4 nanoparticles hybrid in an AC magnetic field for localized hyperthermia. Mater. Lett. 68 (2012) 399–401. |
| [21] | J.M. Shen, F.Y. Gao, L.P. Guan, et al. , Graphene oxide-Fe3O4 nanocomposite for combination of dual-drug chemotherapy with photothermal therapy. RSC Adv. 4 (2014) 18473–18484. |
| [22] | L.L. Li, H.M. Duan, X.J. Wang, C.N. Luo. Adsorption property of Cr(VI) on magnetic mesoporous titanium dioxide-graphene oxide core-shell microspheres. New J. Chem. 38 (2014) 6008–6016. |
 2016, Vol. 27
2016, Vol. 27