b Department of Environmental Science, College of Environment and Resources, Jilin University, Changchun 130021, China ;
c College of Electronic Science and Engineering, Jilin University, Changchun 130021, China ;
d Institut für Experimentelle und Angewandte Physik, Christian-Albrechts-Universität zu Kiel, D-24098 Kiel, Germany ;
e Institut für Physik, Technische Universität Ilmenau, D-98693 Ilmenau, Germany ;
f Beida Information Research (BIR), Tianjin 300457, China
Supramolecular chemistry plays an important role in molecular catalysis, recognition, medicine, data storage and processing as well as artificial photosynthetic devices. In a similar vein, twodimensional molecular arrays are being investigated on singlecrystal surfaces [1-16]. Significant progress has been made in preparing extended networks, a field that has comprehensively been reviewed [1-4]. Bridging the gap between single molecules and molecular monolayers, isolated supramolecular aggregates on surfaces and their physicochemical properties deserve particular interest [17, 18].
The formation of supramolecular patterns involves weak, selective and directional non-covalent interactions such as hydrogen bonding (C-H...N [19], O...H-O [20-27]), halogen bonding [28], metal-organic coordination [4] and electrostatic multipole interactions [29, 30]. Moreover, van der Waals forces play an important role [31]. Finally, the interaction with the substrate affects the pattern formation, be it in the form of direct molecule-substrate interactions [5, 32] or through indirect intermolecular interactions that are mediated by the substrate [33-35].
A number of routes maybe chosen to prepare isolated aggregates rather than continuous layers. Controlling the kinetics of nucleation and growth by adjusting deposition rate and temperature, an approach that has been successfully used for atomic adsorbates [36], can be extended to molecules. Patterns of the substrate, e.g. the herringbone reconstruction of Au(111) or step patterns on vicinal surfaces [5, 32], may be used to confine molecules at low temperatures. Finally, the molecular structure may be designed to favor isolated structures over extended growth.
Below, examples are summarized that have recently demonstrated the above concepts. By choosing substrates, molecular building blocks and coverages, oligomers, wire-like assemblies and fractals have been obtained. Scanning tunneling microscopy (STM) has been instrumental in determining the structure of these supramolecular assemblies.
2. Molecular design 2.1. Supramolecular pentamersDesigning suitable molecules appears to be the most natural approach to creating isolated supramolecules. While this is a difficult task in solution chemistry, the presence of a surface further complicates this problem. Molecule-substrate interactions come into play and the intermolecular interactions are modified. In addition, the substrate may mediate new intramolecular interactions through lattice distortion or scattering of surface electronic states [33-35]. Nevertheless, a number of examples can be reported.
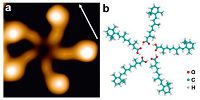
Molecules that provide a single attractive site are obvious candidates for the formation of confined structures. Early examples were tetramers and chiral decamers of 1-nitronaphthalin [29, 30]. The electrical dipole moment of the molecule and its shape favored the formation of these structures. More recently, alltrans- retinoic acid (ReA) was used to prepare supramolecular pentamers on Au(111) (Fig. 1a) [37, 38]. The model in Fig. 1b shows that the pentamer is formed via cyclic O. . .H-O hydrogen bonds at the center. At different coverages different numbers of enantiomers were found. Such pentamers were preponderantly observed in the coverage range 0.01 ML <θ< 0.4 ML. In addition to hydrogen bonding, reducing the molecule-substrate interaction is decisive for forming pentamers. This was demonstrated by depositing ReA on the more reactive Ag(111) surface. Using the same preparation conditions as those used for Au(111) no pentamers were observed on Ag. In contrast, on top of a complete ReA monolayer acting as a buffer pentamers did form. Finally the shape of ReA molecule is another key factor for pentamer formation. From cis-retinoic acid no pentamers were found, presumably owing to the steric hindrance that arises in forming closed cycles of O...H-O hydrogen bonds [37].
|
Download:
|
| Figure 1. (a) Image of a ReA pentamer, observed close to a step on Au(111) at an average coverage θ≈0:2 ML. In this STM images an arrow indicates a [110] direction and its length corresponds to 1.5 nm. (b) Optimized geometry of five ReA molecules, confined to a plane. Adapted with permission from Ref. [37] © 2013 American Chemical Society. | |
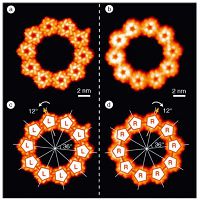
The ReA pentamer is one of the few examples of artificial C5 symmetric supramolecules on surfaces. Chiral pentamers were previously observed from rubrene on Ag(111) [31]. The pattern formation has been interpreted in terms of repulsive interaction between vertical dipoles, which result from charging of the molecules, and attractive van der Waals interactions at short distance [39]. A particularly intriguing aspect of the rubrene system is the observation of decamers of the supramolecular pentamers (Fig. 2). In addition, the chirality of the original molecular building block is present on both levels, namely pentamers and decamers of perntamers (Fig. 2).
|
Download:
|
| Figure 2. Formation of chiral supramolecular decagons of pentagonal supermolecules. (a and b) STM images of a left-handed and a right-handed homochiral decagon; (c and d) Construction of the decagons out of ten counterclockwise rotated L-type pentagons and clockwise rotated R-type pentagons. Adapted with permission from Ref. [31]. © 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | |
2.2. Supramolecular dimers, trimers and tetramers
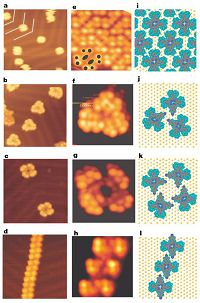
The substituted porphyrin (H2-TBPP) consists of a free-base porphyrin core and four di-tertiarybutylphenyl (tBP) substituents [40]. H2-TBPP molecules are dispersed isolated on Au(111) (Fig. 3a) at low coverages. H2-TBPP molecules exhibit a close-packed arrangement due to van der Waals interaction between the tBP substituents at high coverages (Fig. 3e and i). In a next step (cyanophenyl)-tris (di-tertiarybutylphenyl) porphyrin (CTBPP) was adsorbed [40]. In this molecule a tBP group of H2-TBPP is replaced with a cyanophenyl substituent, bis (cyanophenyl)-bis (di-tertiarybutylphenyl) porphyrin (BCTBPP). The substituent contains two cyanophenyl groups that are substituted at the cis position or transposition to ensure the characteristic interaction of the cyano substituents. As shown in Fig. 3b, the majority of CTBPP molecules arrange themselves into trimers, which are located individually at the elbows of the herringbone reconstruction at low coverage [40]. The structural details of the CTBPP trimer are shown in Fig. 3f, while Fig. 3j is a sketch of the proposed cyclic configuration. If the tectonis (phenyl)-tris (di- tertiarybutylphenyl)porphyrinwhere the cyano substituent is removed from the CTBPP molecule, a random arrangement of themolecules is observed on the surface [40]. Therefore, cyano groups play an important role in the formation of supramolecular aggregation. Fig. 3c shows the supramolecular tetramer comprising four cis-BCTBPP molecules. There are four antiparallel intermolecular connections to form a molecular ring (Fig. 3g and k). Fig. 3d and hshowsthat supramolecularwiresmaybe formed uponincreasing the coverage of trans-BCTBPP (Fig. 3l). There are also antiparallel configurations, andthemaximumlength of wire exceeds 100 nm [40].
|
Download:
|
| Figure 3. Scanning tunneling microscope images (63 K) of supramolecular aggregations induced by cyano groups. At low coverage, individual molecules or clusters are preferentially positioned at the elbows of the herringbone reconstruction of the Au(111) surface (20 × 20 nm2): (a) H2-TBPP; (b) CTBPP; (c) cis-BCTBPP; and (d) trans-BCTBPP. High-resolution STM image (5.3 × 5.3 nm2) and sketch of the proposed adsorption geometry: (e and i) H2-TBPP island; (f and j) CTBPP trimer; (g and k) cis-BCTBPP tetramer; (h and l) trans-BCTBPP wire. As indicated by black dots in (e), the STM image of the single H2-TBPP molecule is composed of four paired lobes surrounding two oblong protrusions, which are assigned to, respectively, the tertiarybutyl substituents and the central porphyrin. Adapted with permission from Ref. [40]. © 2001 Macmillan Magazines Ltd. | |
The antiparallel or cyclic configurations of the cyano substituents formthose aggregations. TheCH...NCcontact corresponds to aweak interaction. It isweaker than the interactionencountered in covalent bonds, electronic coupling and energy transfer [41] in biological systems [42, 43]. The bulky tBP shape is likely to minimize the molecule-substrate interaction. Consequently, these assemblies may exhibit generic molecular physical and chemical properties. Moreover, the observed aggregation sizes and shapes are mainly controlled by molecular properties.
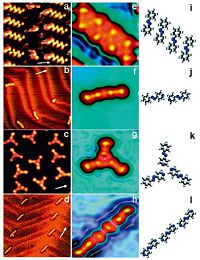
3. Substrate-induced confinement 3.1. Strained layersStrained atomic layers occur in epitaxy and also at some pristine surfaces, Au(111) being the most well-known example [44]. The resulting lattice mismatch between the substrate and the surface layer can result in regular arrays of dislocations, which may be used to guide the self-assembly of molecules. Fig. 4 shows a recent example [45]. Weak C-H...N hydrogen bonds are used to arrange azo molecules into small aggregates. Fig. 4a, e and i shows that straight azobenzene tetramer arrays form in the face-centered cubic (fcc) stacking region of the 22 × ffiffiffi 3 p surface reconstruction of Au(111) at a coverage of θ≈0:2 ML. They are arranged side-by-side, and there are six weak C-H...N hydrogen bonds in a tetramer. At even lower coverage, θ≈0:02 ML, the 4-phenylazopyridine assembles into dimers at elbow sites (Fig. 4b). They exhibit a head-tohead arrangement, and there are two weak C-H...N hydrogen bonds (Fig. 4f and j). At higher coverage, θ≈0:3 ML, the 4- phenylazopyridine molecules form triangular trimers (Fig. 4c). The cyclic configuration with three weak C-H...N hydrogen bonds (Fig. 4g and k) is more stable than the dimer. Fig. 2d shows that 4, 40-azopyridine forms linear trimers at elbow sites in head-tohead arrangement with four weak C-H...N hydrogen bonds (Fig. 4h and l). To prepare dimers, triangular trimers, linear trimers and tetramers the strength and position of C-H...N hydrogen bonds as well as molecule-surface interactions were modified. Together with the molecule coverage the observed supramolecules reflect the subtle balance between these interactions [44].
|
Download:
|
| Figure 4. Pseudo-three-dimensional STM topographs and optimized models of supramolecules on Au(111). Azobenzene tetramers ((a), 12 × 12 nm2) and 4-phenylazopyridine dimers ((b), 19 × 19 nm2) adopt side-by-side and head-to-head configurations, respectively. 4-Phenylazopyridine trimers ((c), 12 × 11 nm2) and 4, 40-azopyridine trimers ((d), 32 × 32 nm2) arrange in the triangular and linear way separately. High-resolution STM images and optimized molecular models: azobenzene tetramer ((e and i), 3.1 × 3.1 nm2); 4-phenylazopyridine dimer ((f and j), 4.2 × 4.2 nm2); 4-phenylazopyridine trimer ((g and k), 3.8 × 3.8 nm2); 4, 40-azopyridine trimer ((h), l, 3.7 × 3.7 nm2). White arrows indicate the [110] direction. I = 0.06 nA, V = -0.8 V for 4-phenylazopyridine and V = 1.0 V elsewhere. Adapted with permission from Ref. [45].© 2008 American Chemical Society. | |
3.2. Vicinal surfaces
Vicinal surfaces [32], i.e., surfaces whose orientation slightly deviates from those of the most symmetric crystallographic planes, provide regular arrays of atomic steps and kinks on the nanometer scale. These arrays can be adjusted to some extent by choosing suitable surface orientations and thus enabled the fabrication of nanowires [46-48] or dots [49, 50] from atoms. This approach has been extended to molecules. Fig. 5a displays an STM topograph of a Au(788) surface. Its regular sequence of steps along with the alternating sequence of fcc and hcp stacked areas provides two dimensional confinement. Upon deposition of C60 (Fig. 5b), almost the entire crystal surface is covered with nearly identical clusters of 19 molecules.

|
Download:
|
| Figure 5. (a) Constant-current image of Au(788). Image size: 14 nm × 14 nm. To improve the visibility of discommensuration lines the corrugation due to atomic terrace levels has been subtracted. Steps ascend from the bottom left to the top right. (b) Pseudo-three dimensional STM topograph of half a monolayer of C60 on Au(788). Image size: 190 nm × 190 nm. Steps of the substrate ascend from left to right. Adapted from Ref. [5]. © 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | |
4. Controlling the size of supramolecules through growth kinetics
The nucleation and the growth of two-dimensional clusters from atoms deposited on single crystal surfaces have been studied in great detail [36]. The cluster densities and sizes may be controlled by varying the temperature of the substrate, the deposition rate and the deposited amount. While this approach can be straightforwardly extended to molecules, the complexity of the assembly processes is drastically increased. Neither the shape of a molecule nor its various interactions are as isotropic as in the case of atomic adsorbates. For instance, ReA molecules could form energy-unfavorable trimers if the Au(111) substrate was kept at around 80 K while deposition [37].
A much more complex example is the preparation of molecular fractals with different sizes by controlling growth kinetics [14, 15, 51, 52]. Self-similar fractal patterns are of interest in mathematics, natural sciences and engineering and often are visually appealing. Fractals assembled from molecules via strong intermolecular interactions have been reported [53-58]. To improve the degree of perfection of the patterns, using weak molecular interactions appears to be a viable path. Along this line, Shang et al. [51] recently prepared molecular Sierpin´ ski triangles (ST) on Ag(111) employing halogen bonding and synergistic hydrogen bonding [59-61]. The structures of the molecules used, B3PB and B4PB, are depicted in Fig. 6a. After depositing molecules, the Ag(111) sample was cooled by liquid helium or liquid nitrogen and imaged at 63 or 4.4 K. Figs. 6b and c show B3PB-ST-2 (2 is the order [51] of the molecular ST) and B4PB-ST-3 on Ag(111) . The Hausdorff dimension [62] of B4PB-ST-3 deduced by the ‘boxcounting’ method is around 1.68 (Fig. 6d) [63]. The molecular ST family is chiral. There is an angle of 88 deviation between B4PB-STCW for clockwise chirality and B4PB-ST-CCW for their anticlockwise counterparts. The formation of molecular ST requires several prerequisites to be fulfilled. An asymmetric molecular shape with 1208 backbone and weak intermolecular interactions are required. Additionally, a three-fold symmetry of the substrate lattice is crucial to form molecular ST, because it matches well the threefold cyclic halogen-bonding nodes of the building block. Moreover, a moderate molecule-substrate interaction is important. Indeed, B4PB turned out to be the most suitable molecule. While B3PB exhibit an enhanced diffusion on Ag(111) but still form fractal ST, B5PB molecules are too immobile. More importantly, ST structures with different sizes could be prepared by controlling the cooling rate in the unit of K/min. For example, the slow cooling rate 0.02 K/ min from 78 K to 63 K led to the formation large B4PB-ST-3 structures while the fast cooling rate resulted in smaller molecular STs [51].

|
Download:
|
| Figure 6. Typical individual molecular STs at the surfaces. (a) Chemical structures of the B3PB and B4PB molecules with indicated geometric parameters. (b and c) Highresolution STM images of individual B3PB-ST-2 (13 × 11 nm2) and B4PB-ST-3 (33 × 29 nm2) on Ag(111). The raw STM images are rotated, cropped and recolored to produce the images of the individual STs shown here. (d) Plot of log(N(r)) versus log (r). The molecular fractal pattern in (c) B4PB-ST-3, was converted into the black–white mode, as shown in the inset. A total of 50 data sets (r, N(r)) were acquired by counting the number of black pixels N(r) in the shaded area with a lateral size of r (pixel). Imaging conditions: constant height, Vbias = 20 mV, I = 1 nA. Adapted with permission from Ref. [51]. © 2015 Macmillan Publishers Limited. | |
5. Conclusions and prospects
Examples of isolated supramolecular aggregates on surfaces have been presented and some factors involved in their assembly have been highlighted. A moderate molecule-substrate interaction appears to be crucial to enable sufficient mobility of the adsorbed molecules. Inert surfaces and buffer layers were employed on the substrate side. As to the adsorbed molecules, suitable functional groups were used to the same end. Confinement to isolated structures was achieved through template effects of the substrate, by using weakly interacting molecules with suitable shape, and by adjusting growth conditions. The structures prepared range from small oligomers to complex fractal arrangements. In only a few cases, successful modelling of the aggregate structures has been reported. Ab-initio methods presently still tend to be too numerically expensive to treat molecular clusters on surfaces. Consequently, despite the encouraging results presented, knowledge- based design of suitable systems is not yet in reach. For the time being, experimental work on promising systems remains the most suitable approach to prepare isolated supramolecules and to investigate their magnetic properties, chemical reactivity and catalytic activity.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21522301, 21373020, 21403008, 61321001, 21433011, 61271050). the Ministry of Science and Technology (Nos. 2014CB239302 and 2013CB933404), and the Research Fund for the Doctoral Program of Higher Education (No. 20130001110029). R. B. acknowledges financial support from the Deutsche Forschungsgemeinschaft (DFG) via the SFB 677.
| [1] | L.J. Wan. Fabricating and controlling molecular self-organization at solid surfaces: studies by scanning tunneling microscopy. Acc. Chem. Res. 39 (2006) 334–342. |
| [2] | R.K. Smith, P.A. Lewis, P.S. Weiss. Patterning self-assembled monolayers. Prog. Surf. Sci. 75 (2004) 1–68. |
| [3] | S. De Feyter, F.C. De Schryver. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 32 (2003) 139–150. |
| [4] | N. Lin, S. Stepanow, M. Ruben, J.V. Barth. Surface-confined supramolecular coordination chemistry, in: P. Broekmann, K.H. Dötz, C.A. Schalley (Eds.). Templates in Chemistry III: Topics in Current Chemistry, , 287, Springer, Berlin, Heidelberg. 2009, pp., 1-44. |
| [5] | N. Néel, J. Kröger, R. Berndt. Highly periodic fullerene nanomesh. Adv. Mater. 18 (2006) 174–177. |
| [6] | D. Bléger, D. Kreher, F. Mathevet, et al. , Surface noncovalent bonding for rational design of hierarchical molecular self-assemblies. Angew. Chem. 119 (2007) 7548–7551. |
| [7] | J. Xu, Q.D. Zeng. Construction of two-dimensional (2D) H-bonded supramolecular nanostructures studied by STM. Chin. Chem. Lett. 24 (2013) 177–182. |
| [8] | C. Xiao, W.Y. Zhao, D.Y. Zhou, et al. , Recent advance of photochromic diarylethenescontaining supramolecular systems. Chin. Chem. Lett. 26 (2015) 817–824. |
| [9] | L. Chen, Y.C. Zhang, W.K. Wang, et al. , Conjugated radical cation dimerizationdriven generation of supramolecular architectures. Chin. Chem. Lett. 26 (2015) 811–816. |
| [10] | T.T. Cao, X.Y. Yao, J. Zhang, Q.C. Wang, X. Ma. A cucurbit[8]uril recognized rigid supramolecular polymer with photo-stimulated responsiveness. Chin. Chem. Lett. 26 (2015) 867–871. |
| [11] | M. Han, G.C. Wang, H.Q. Duan. Construction of supramolecular nanofibers through electrostatic interaction between perylene and cholesterol derivatives. Chin. Chem. Lett. 25 (2014) 51–54. |
| [12] | F. Gao, J.B. Zhang, C.P. Li, T.R. Huo, X.H. Wei. Supramolecular binding of amines with functional magnesium tetraphenylporphyrin for CO2 capture. Chin. Chem. Lett. 24 (2013) 249–252. |
| [13] | J.K. Ouyang, L.J. Chen, L. Xu, C.H. Wang, H.B. Yang. A new family of supramolecular multiferrocenyl rhomboids: synthesis, characterization, and their electrochemical behavior. Chin Chem. Lett. 24 (2013) 471–474. |
| [14] | X. Zhang, N. Li, G.C. Gu, et al. , Controlling molecular growth between fractals and crystals on surfaces. ACS Nano 9 (2015) 11909–11915. |
| [15] | N. Li, X. Zhang, G.C. Gu, et al. Sierpiński-triangle fractal crystals with the C3v point group. Chin. Chem. Lett 26 (2015) 1198–1202. |
| [16] | N. Néel, J. Kröger, R. Berndt. Fullerene nanowires on a vicinal gold surface. Appl. Phys. Lett. 88 (2006) 163101. |
| [17] | N. Wintjes, D. Bonifazi, F.Y. Cheng, et al. , A supramolecular multiposition rotary device. Angew. Chem. Int. Ed. 46 (2007) 4089–4092. |
| [18] | Y.F. Wang, X. Ge, G. Schull, et al. , Switching single azopyridine supramolecules in ordered arrays on Au(111). J. Am. Chem. Soc. 132 (2010) 1196–1197. |
| [19] | B.F. King, F. Weinhold. Structure and spectroscopy of (HCN)n clusters: cooperative and electronic delocalization effects in C-H N hydrogen bonding. J. Chem. Phys. 103 (1995) 333–347. |
| [20] | G. Pawin, K.L. Wong, K.Y. Kwon, L. Bartels. A homomolecular porous network at a Cu(111) surface. Science 313 (2006) 961–962. |
| [21] | Y.C. Ye, W. Sun, Y.F. Wang, et al. , A unified model: self-assembly of trimesic acid on gold. J. Phys. Chem. C 111 (2007) 10138–10141. |
| [22] | R. Otero, M. Lukas, R.E.A. Kelly, et al. , Elementary structural motifs in a random network of cytosine adsorbed on a gold(111) surface. Science 319 (2008) 312–315. |
| [23] | S. Griessl, M. Lackinger, M. Edelwirth, M. Hietschold, W.M. Heckl. Self-assembled two-dimensional molecular host-guest architectures from trimesic acid. Single Mol. 3 (2002) 25–31. |
| [24] | M. Stöhr, M. Wahl, C.H. Galka, et al. , Controlling molecular assembly in two dimensions: the concentration dependence of thermally induced, 2D aggregation of molecules on a metal surface. Angew. Chem. Int. Ed. 44 (2005) 7394–7398. |
| [25] | Q.Y. Liu, Q.Y. Jia, J.Q. Zhu, et al. , Highly ordered arrangement of meso-tetrakis(4-aminophenyl)porphyrin in self-assembled nanoaggregates via hydrogen bonding. Chin. Chem. Lett. 25 (2014) 752–756. |
| [26] | H.Y. Guo, F.F. Yang, Z.Y. Jiao, J.R. Lin. Click synthesis and dye extraction properties of novel thiacalix[4]arene derivatives with triazolyl and hydrogen bonding groups. Chin. Chem. Lett. 24 (2013) 450–452. |
| [27] | X. Meng, Q.G. He, H.M. Cao, J.G. Cheng. A novel chemosensor-bipyridyl end capped hyperbranched conjugated polymer. Chin. Chem. Lett. 22 (2011) 725–728. |
| [28] | P. Metrangolo, F. Meyer, T. Pilati, G. Resnati, G. Terraneo. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 47 (2008) 6114–6127. |
| [29] | M. Böhringer, K. Morgenstern, W.D. Schneider, R. Berndt. Trennung eines racemischen gemisches zweidimensionaler molekularer cluster mit dem rastertunnelmikroskop. Angew. Chem. 111 (1999) 832–834. |
| [30] | M. Böhringer, K. Morgenstern, W.D. Schneider, et al. , Two-dimensional self-assembly of supramolecular clusters and chains. Phys. Rev. Lett. 83 (1999) 324–327. |
| [31] | M.C. Blüm, E. Ćavar, M. Pivetta, F. Patthey, W.D. Schneider. Conservation of chirality in a hierarchical supramolecular self-assembled structure with pentagonal symmetry. Angew. Chem. Int. Ed. 44 (2005) 5334–5337. |
| [32] | C. Tegenkamp. Vicinal surfaces for functional nanostructures. J. Phys. Condens. Matter 21 (2009) 013002. |
| [33] | M.M. Kamna, S.J. Stranick, P.S. Weiss. Imaging substrate-mediated interactions. Science 274 (1996) 118–119. |
| [34] | A. Schiffrin, A. Riemann, W. Auwärter, et al. , Zwitterionic self-assembly of L-methionine nanogratings on the Ag(111) surface. Proc. Natl. Acad. Sci. USA 104 (2007) 5279–5284. |
| [35] | Y.F. Wang, X. Ge, C. Manzano, et al. , Supramolecular patterns controlled by electron interference and direct intermolecular interactions. J. Am. Chem. Soc. 131 (2009) 10400–10402. |
| [36] | H. Brune. Microscopic view of epitaxial metal growth: nucleation and aggregation. Surf. Sci. Rep. 31 (1998) 125–229. |
| [37] | S. Karan, Y.F. Wang, R. Robles, N. Lorente, R. Berndt. Surface-supported supramolecular pentamers. J. Am. Chem. Soc. 135 (2013) 14004–14007. |
| [38] | S. Karan, N. Li, Y.J. Zhang, et al. , Spin manipulation by creation of single-molecule radical cations. Phys. Rev. Lett. 116 (2016) 027201. |
| [39] | G. Tomba, M. Stengel, W.D. Schneider, A. Baldereschi, A. De Vita. Supramolecular self-assembly driven by electrostatic repulsion: the, 1d aggregation of rubrene pentagons on Au(111). ACS Nano 4 (2010) 7545–7551. |
| [40] | T. Yokoyama, S. Yokoyama, T. Kamikado, Y. Okuno, S. Mashiko. Selective assembly on a surface of supramolecular aggregates with controlled size and shape. Nature 413 (2001) 619–621. |
| [41] | P.J. De Rege, S.A. Williams, M.J. Therien. Direct evaluation of electronic coupling mediated by hydrogen bonds: implications for biological electron transfer. Science 269 (1995) 1409–1413. |
| [42] | H.W. Fink, C. Schönenberger. Electrical conduction through DNA molecules. Nature 398 (1999) 407–410. |
| [43] | L.T. Cai, H. Tabata, T. Kawai. Self-assembled DNA networks and their electrical conductivity. Appl. Phys. Lett 77 (2000) 3105–3106. |
| [44] | D.D. Chambliss, R.J. Wilson, S. Chiang. Nucleation of ordered Ni island arrays on Au (111) by surface-lattice dislocations. Phys. Rev. Lett. 66 (1991) 1721–1724. |
| [45] | Y.F. Wang, X. Ge, G. Schull, et al. , Azo supramolecules on Au(111) with controlled size and shape. J. Am. Chem. Soc. 130 (2008) 4218–4219. |
| [46] | J. Shen, R. Skomski, M. Klaua, et al. , Magnetism in one dimension: Fe on Cu(111). Phys. Rev. B 56 (1997) 2340–2343. |
| [47] | P. Gambardella, M. Blanc, H. Brune, K. Kuhnke, K. Kern. One-dimensional metal chains on Pt vicinal surfaces. Phys. Rev. B 61 (2000) 2254–2262. |
| [48] | J.L. Lin, D.Y. Petrovykh, A. Kirakosian, et al. , Self-assembled Fe nanowires using organometallic chemical vapor deposition and CaF2 masks on stepped Si(111). Appl. Phys. Lett. 78 (2001) 829–831. |
| [49] | V. Repain, G. Baudot, H. Ellmer, S. Rousset. Two-dimensional long-range-ordered growth of uniform cobalt nanostructures on a Au(111) vicinal template. Europhys. Lett. 58 (2002) 730–736. |
| [50] | S.Y. Ohno, K. Yagyuu, K. Nakatsuji, F. Komori. One-dimensional self-organized patterns on vicinal Cu(001)-c(2×2)N surfaces. Jpn. J. Appl. Phys. 41 (2002) L1243–L1246. |
| [51] | J. Shang, Y.F. Wang, M. Chen, et al. , Assembling molecular Sierpiński triangle fractals. Nat. Chem. 7 (2015) 389–393. |
| [52] | G.C. Gu, N. Li, X. Zhang, et al. Sierpiński triangle fractal structures investigated by STM. Acta Phys. Chim. Sin 32 (2016) 195–200. |
| [53] | G.R. Newkome, C. Shreiner. Dendrimers derived from, 1 !3 branching motifs. Chem. Rev. 110 (2010) 6338–6442. |
| [54] | K.I. Sugiura, H. Tanaka, T. Matsumoto, T. Kawai, Y. Sakata. A mandala-patterned bandanna-shaped porphyrin oligomer, C1244H1350N84Ni20O88, having a unique size and geometry. Chem Lett. 28 (1999) 1193–1194. |
| [55] | G.R. Newkome, P.S. Wang, C.N. Moorefield, et al. , Nanoassembly of a fractal polymer: a molecular "Sierpinski hexagonal gasket". Science 312 (2006) 1782–1785. |
| [56] | K. Fujibayashi, R. Hariadi, S.H. Park, E. Winfree, S. Murata. Toward reliable algorithmic self-assembly of DNA tiles: a fixed-width cellular automaton pattern. Nano Lett. 8 (2008) 1791–1797. |
| [57] | R. Sarkar, R. Sarkar, K. Guo, C.N. Moorefield, et al. , One-step multicomponent selfassembly of a first-generation Sierpiński triangle: from fractal design to chemical reality. Angew. Chem. Int. Ed. 53 (2014) 12182–12185. |
| [58] | M. Wang, C. Wang, X.Q. Hao, et al. , Hexagon wreaths: self-assembly of discrete supramolecular fractal architectures using multitopic terpyridine ligands. J. Am. Chem. Soc. 136 (2014) 6664–6671. |
| [59] | H. Walch, R. Gutzler, T. Sirtl, G. Eder, M. Lackinger. Material-and orientationdependent reactivity for heterogeneously catalyzed carbon-bromine bond hemolysis. J. Phys. Chem. C 114 (2010) 12604–12609. |
| [60] | K.H. Chung, J. Park, K.Y. Kim, et al. , Polymorphic porous supramolecular networks mediated by halogen bonds on Ag(111). Chem. Commun. 47 (2011) 11492–11494. |
| [61] | W.H. Wang, X.Q. Shi, S.Y. Wang, M.A. Van Hove, N. Lin. Single-molecule resolution of an organometallic intermediate in a surface-supported Ullmann coupling reaction. J. Am. Chem. Soc. 133 (2011) 13264–13267. |
| [62] | B.B. Mandelbrot, The Fractal Geometry of Nature, W.H. Freeman, New York, 1982. |
| [63] | M. Alfonseca, A. Ortega. Determination of fractal dimensions from equivalent L systems. IBM J. Res. Dev. 45 (2001) 797–805. |
 2016, Vol. 27
2016, Vol. 27 


