b School of Chemistry and Environmental Engineering, Wuhan Institute of Technology, Wuhan 430073, China
Human activities,besides their contributions to the global civilization,also introduce considerable pollutants,such as heavy metals,organic compounds,and other hazardous materials,into the environment [1]. Heavy metal pollution of water through the discharge of industrial wastewater is a particularly intractable problem [2]. The high volatility of Hg and its compounds,and their toxicity,accumulative,and persistent features in the ecosystem as well as the biomagnifications along the food chain has been considered as a serious health threat to human beings [3]. Therefore,highly effective removal of mercury ions from wastewater has been a long-standing goal for industrial process and environmental remediation [4]. Many separation technologies have been utilized for effectively reducing mercury concentrations from wastewater,including chemical precipitation,adsorption,ion exchange,membrane separation,solvent extraction,and electrochemical treatment [5],etc. Among these separation processes,adsorption using functionalized adsorbents is considered to be one of the most common techniques due to the advantages of simplicity of operation,applicability toward dilute solutions and economical feasibility [6].
Porous carbons with large surface areas,suitable mechanical strength,and good acid and alkali resistance show important applications in pollutant removal. However,their adsorption of soft,heavy metals (e.g.,Hg) is usually limited because of their relatively inert surface (i.e[1TD$DIF].,lack of active functional groups). This shortage causes difficulties in the widespread use of porous carbon materials as acceptable adsorbents for the removal of mercury ions from wastewater. Therefore,it is important to modify or functionalize porous carbons by heteroatoms,such as sulfur and nitrogen,for finally achieving the modifications/adjustments in which efficient and effective adsorption of mercury becomes a reality [2c,2d,7]. In general,however,post-synthesis treatment suffers the disadvantage of unstable functionalized groups and relatively complicated preparation processes. Therefore,to prepare functionalized porous carbon materials using one-step synthesis process is a preferred choice [8].
On the other hand,the present carbon adsorbents are mainly powdered materials,which are difficult for efficient separation and recycling. Carbon micro- or nanospheres with regular morphology and adjustable porosity and diameter show enhanced mechanical strength and improved adsorption,separation,and recycling performance compared with carbon powders or flakes [9]. Furthermore,the common separation methods for adsorbents from aqueous solution are precipitation,filtration,and centrifugation which are inconvenient,uneconomical,and inefficient. Magnetic materials could be conveniently separated from environmental water with an external magnetic field,exhibiting promising prospects for heavy metal removal [10]. For example,Peng et al. prepared carbon nanotube-iron oxide magnetic composites as an adsorbent for removal of aqueous Pb2+ and Cu2+ ions [10c]. Liu et al. developed humic acid coated Fe3O4 nanoparticles by a co-precipitation procedure for the removal of toxic Hg(Ⅱ),Pb(Ⅱ),Cd(Ⅱ),and Cu(Ⅱ) from water [10d].
Taking into account all the above-mentioned criteria,carbonbased materials with a combination of porous structure,regular functional groups,and a magnetic separation feature would represent innovative materials and are expected to be particularly suitable as sorbents. As a target toward that goal,herein,we demonstrate the synthesis of magnetically separated and N,S codoped mesoporous carbon microspheres (N/S-MCMs/Fe3O4) for the removal of mercury ions. N/S-MCMs/Fe3O4 shows the advantages of high specific surface area,dual mesopore sizes,microspherical shape,sulfur and nitrogen heteroatoms,and superparamagnetic Fe3O4. N/S-MCMs/Fe3O4 has an Hg2+ adsorption capacity of 74.5 mg/g,and the adsorbent can be rapidly separated from the aqueous phase with an external magnetic field. This study highlights the great potential to fabricate well-designed,carbonbased adsorbents for the removal of aqueous mercury ions.
2. Experimental100 mL of distilled water,40 mL of ethanol,and 1.5 mL of 25 wt% ammonia solution were mixed under stirring. Then 2.0 g of tetraethyl orthosilicate (TEOS) was added into the mixed solution and stirred for 30 min to prepare silica nanoparticles. Into the solution,1.0 g of resorcinol and 1.0 g of cysteine were added under stirring for 10 min. Then,1.5 g of formaldehyde (37-40 wt%) solution was slowly added to the above mixed solution and stirred for 24 h at 30 ℃. After that,the whole mixture was transferred to a Teflon autoclave at 100 ℃ for 24 h to fabricate SiO2 nanoparticles embedded N,S co-doped polymer microspheres (N/S-PMs). N/SPMs were subjected to thermal treatment at 550 ℃ for 4 h with a heating rate of 3 ℃/min under N2 flow,followed by etching SiO2 template with 3 mol/L NaOH solution to prepare N,S co-doped mesoporous carbon microspheres (N/S-MCMs). For comparison,pure mesoporous carbon microspheres (MCMs) were also obtained using similar procedure without cysteine. Finally,Fe(NO3)3 9H2O and N/S-MCMs were mixed with a mass ratio of 1:2,and ethanol was added to dissolve ferric nitrate. The mixture was agitated by rotation at 200 ± 5 rpm in a rotator oscillator at r.t. for 24 h,followed by natural evaporation of ethanol. The sample was heated to 400 ℃ under N2 atmosphere for 1 h to fabricate Fe3O4 and N/S-doped carbon microspheres (labeled as N/S-MCMs/Fe3O4). The samples were characterized by SEM,XRD,Raman spectra,XPS,N2 adsorption,and a vibrating sample magnetometer.
The batch adsorption experiments of adsorbents were performed using Hg(NO3)2 as a model pollutant. In a typical experiment,20 mg of as-prepared adsorbent was diluted with 50 mL of Hg(NO3)2 solution and the concentration of Hg2+ was varied from 10 mg/L to 50 mg/L (pH 6.0 ± 0.05). Kinetic study was conducted using 20 mg of adsorbent in contact with 50 mL Hg(NO3)2 solution (CHg = 50 mg/L). The solution was stirred by rotation at 200 ± 5 rpm at 25 ℃ until equilibrium was reached. Separate experiments were done using rotation time intervals from 10 min to 150 min. Before analysis,the suspension was separated using a 0.45 mm membrane filter,and the adsorption capacities of Hg2+ were measured using the supernatant. The concentrations of Hg2+ were determined using an atom absorption spectroscopy. The amounts of Hg2+ adsorbed (Qe in mg/g) were calculated according to the following equation,
| ${Q_{\text{e}}} = \frac{{\left( {{C_0} - {C_{\text{e}}}} \right)V}}{W}$ | (1) |
where,C0 and Ce are the initial and equilibrium concentrations of mercury ions (mg/L),respectively; V is the volume of mercury nitrate solution (L) and W is the weight of the adsorbent (g).
3. Results and discussionFig. 1 shows SEM images of MCMs,N/S-MCMs,and N/S-CMs/ Fe3O4. MCMs exhibit regular spherical geometry with a uniform diameter of about 580 nm,as shown in Fig. 1a. The diameters of carbon-based microspheres are constant (Fig. 1b). Cysteine which provides N,S sources can be introduced into the carbon framework and does not affect the ethanol/water/ammonia system to generate polymer microspheres. After the introduction of Fe3O4 into the N/S-MCMs,the surface roughness and luster were altered (Fig. 1c).

|
Download:
|
| Fig. 1. SEM images of MCMs (a), N/S-MCMs (b), and N/S-CMs/Fe3O4 (c). | |
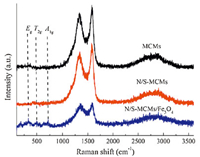
Fig. 2 shows Raman spectra of MCMs,N/S-MCMs,and N/SMCMs/ Fe3O4. The spectra have a distinct pair of peaks located at about 1350 cm-1 (D band) and 1580 cm-1 (G band). The D band is a typical characteristic of disordered graphite or crystal defects,and the G band is ascribed to an ideal graphitic lattice vibration mode with E2g symmetry [11]. Additionally,the peak located at ~2700 cm-1 denotes G' band,corresponding to undisturbed or highly ordered graphitic lattices [12]. In the Raman spectrum of N/ S-MCMs/Fe3O4,three dominant Raman bands at about 360,500,and 700 cm-1 reflect the Eg,T2g,and A1g mode of magnetite [13]. The Fe(NO3)3 9H2O in N/S-MCMs occurs a decomposition reaction to transfer into g-Fe2O3 at a temperature of 350 ℃ [14],and then g-Fe2O3 was transfer into Fe3O4 by carbothermal reduction,which matches the result obtained from XRD analysis (Fig. S1 in Supporting information).
|
Download:
|
| Fig. 2. Raman spectra of MCMs, N/S-MCMs, and N/S-MCMs/Fe3O4. | |
The XPS spectrum of N/S-MCMs/Fe3O4,shown in Fig. 3a,exhibits signals for the binding energy of S 2p,C 1s,N 1s,O 1s,and Fe 2p. There are six peaks in N 1s spectrum (Fig. 3b) at the binding energy of 398.6,400.0,400.7,401.2,402.6,and 404.1 eV which are ascribed to the chemical state of N atoms corresponding to pyridine (N-6),amine,pyrrole or pyridine (N-5),quaternary nitrogen (N-Q),pyridine-N-oxide or amino,and chemisorbed nitrogen oxides (N-Ox) [15]. The S 2p spectrum (Fig. 3c) exhibits three peaks at 163.8,168.0,and 164.9 eV attributed to the binding energy of aliphatic -SH and oxidized sulfur thiophenic-type sulfur [16]. The Fe 2p spectra of N/S-MCMs/Fe3O4 (Fig. 3d) shows two peaks at 711.4 and 724.4 eV,assigned to the binding energy of Fe 2p3/2 and Fe 2p1/2 in γ-Fe2O3 or Fe3O4 [14, 17]. A key feature which distinguishes γ-Fe2O3 from Fe3O4 is the presence of the satellite peaks at the binding energy of about 718.8 and 729.5 eV [17]. There is no such peak structure in Fig. 4d,which would indicate the existence of Fe3O4,and this result can be further confirmed by the peak position of Fe2+ at 709.0 eV and Fe3+ at 711.0 eV.

|
Download:
|
| Fig. 3. Wide-scan XPS spectrum of N/S-MCMs/Fe3O4 (a), and fitted high-resolution XPS spectra of N 1s (b) S 2p (c), and Fe 2p (d). | |

|
Download:
|
| Fig. 4. Nitrogen adsorption/desorption isotherms (a) and pore size distributions (b) of MCMs, N/S-MCMs, and N/S-MCMs/Fe3O4. | |
Table 1 shows the elemental composition of N/S-PMs,N/SMCMs,and N/S-MCMs/Fe3O4. Compared with N/S-PMs,the carbon content in N/S-MCMs was increased from 61.24 wt% to 86.89 wt%,while the weight ratio of oxygen was decreased from 28.74 to 10.32% due to the decomposition of the polymer. The nitrogen content of N/S-MCMs is 1.30 wt%,not obvious smaller than that of N/S-PMs,indicating that the C-N covalent bonds are well retained after carbonization. The weight ratio of sulfur in N/S-MCMs is only 0.73%,much lower than that of N/S-PMs (2.72%). This is because the C-S covalent bond has relatively low bond energy among the C-C,C-S,and C-N covalent bonds,with the majority of C-S bonds lost during carbonization. Compared with N/S-MCMs,N/S-MCMs/ Fe3O4 has a lower carbon content of 76.94 wt%,which is ascribed to the carbon loss during the carbothermal reduction of γ-Fe2O3 to Fe3O4. Correspondingly,the weight of O,N,and S was obviously increased. Also,N/S-MCMs/Fe3O4 shows an iron content of 4.7 wt%.
|
|
Table 1 Elemental composition of N/S-PMs, N/S-MCMs, and N/S-MCMs/Fe3O4. |
Fig. 4 shows N2 adsorption-desorption isotherms and pore size distribution curves of MCMs,N/S-MCMs,and N/S-MCMs/Fe3O4. All samples exhibit type Ⅳ isotherm curves with hysteresis loops at a relative pressure P/P0 of 0.5-1.0 (Fig. 4a),which is a characteristic of capillary condensation occurring in mesoporous solids [11]. MCMs,N/S-MCMs,and N/S-MCMs/Fe3O4 have dual mesopore size of ~5.0 nm and ~16 nm,as shown in Fig. 4b. The smaller mesopores come from the removal of SiO2 nanoparticles which were prepared using a similar condition reported in our previous work [18]. While the larger mesopores can be ascribed to interparticle cavities resulted from the stacking of carbon microspheres. The specific surface areas for MCMs,N/S-MCMs,and N/SMCMs/ Fe3O4 are 564,502,and 542 m2/g,respectively. On the one hand,the similar surface areas and pore sizes among these samples suggest that the mesoporous structure of the microspheres has been well-kept. High-surface-areas are maintained after the introduction of Fe3O4 into the mesopores,which is similar to that reported in our previous work,where the mesopore size was basically retained after MnO2 insertion into the carbon microspheres [11]. On the other hand,the surface area of N/S-MCMs/ Fe3O4 is somewhat increased compared with N/S-MCMs as a result of carbothermal reduction of Ⅳ-Fe2O3 to Fe3O4.
The Stöber method is a classic strategy for synthesizing silica spheres by ammonia-catalyzed hydrolysis and condensation of TEOS in ethanol-water mixture [19]. Liu et al. first extended the Stöber method to prepare monodisperse R/F polymer and carbon microspheres with uniform and tunable particle sizes [20]. Subsequently,themodified Stöbermethod highlighted newopportunities for the synthesis of micro- and/or mesoporous carbon spheres [18, 21]. The schematic illustration for the fabrication of N/S-MCMs/ Fe3O4 is shown in Fig. S2 in Supporting information. The SiO2 nanoparticles were synthesized using the Stöber method,and have hydroxyl groupswhich can formstableH-bonds with resorcinol and formaldehyde,and thus the carbon source polymerizes on the surface of such colloidal seeds to formpolymer microspheres under the modified Stöber condition. Cysteine can serve as a particle stabilizer and a source of heteroatoms (nitrogen and sulfur),and can be introduced into the framework of phenolic resin-based spheres [22]. The SiO2/N,S-doped polymer microspheres were carbonized,followed by etching of silica colloids to obtain N/S-MCMs. Fe(NO3)3 9H2Owas introduced into themesopores of N/S-MCMs,followed by heat treatment at 400 ℃ to fabricate N/S-MCMs/Fe3O4.
The adsorption performance of the samples was evaluated by capacity and kinetic studies using the target adsorbate,mercury nitrate solution. The adsorption capacity of N/S-MCMs is 68.1 mg/g (Table 2),2.4 times more than that of MCMs (27.9 mg/g),which can be ascribed to N/S heteroatoms which allow effective adsorption of Hg2+. Besides,N/S-MCMs/Fe3O4 shows a higher adsorption capacity of 74.5 mg/g compared with N/S-MCMs. Due to the structure similarity between N/S-MCMs and N/S-MCMs/Fe3O4,a higher surface area leads to a higher adsorption capacity. And the magnetization loop of N/S-MCMs/Fe3O4 indicates that the sample is super-paramagnetic at r.t. (Fig. S3 in Supporting information). The saturation magnetization value of N/S-MCMs/Fe3O4 is much smaller than that of the bulk Fe3O4 (92 emu/g) [17],which can be attributed to only 4.7 wt% Fe content (6.5 wt% Fe3O4) and the major existence of carbon in the sample. Super-paramagnetization of N/ S-MCMs/Fe3O4 enables the adsorbent to be easily separated from aqueous solution under a magnetic field in less than 30 s (Fig. S3 in Supporting information).
|
|
Table 2 Pseudo-first-order and pseudo-second-order kinetics parameters of MCMs, N/SMCMs, and N/S-MCMs/Fe3O4. |
The pseudo-first-order and pseudo-second-order kinetic studies were performed to evaluate the adsorption kinetics and calculate the rate constants,initial adsorption rates and adsorption capacities. The pseudo-first-order and pseudo-second-order kinetics equation are shown in Eqs. (2) and (3),respectively:
| ${Q_{\text{t}}} = {Q_{\text{e}}}\left( {1 - {e^{ - {k_1}t}}} \right)$ | (2) |
| ${Q_{\text{t}}} = \frac{{Q_{_{\text{e}}}^2{k_2}t}}{{1 + {Q_{\text{e}}}{k_2}t}}$ | (3) |
where,k1 (min-1) and k2 (g/mg min) are the rate constants of pseudo-first-order and pseudo-second-order adsorption,respectively; Qe (mg/g) is the equilibrium adsorption capacity,Qt (mg/g) is the amounts of mercury ions adsorbed at time t. The pseudo-firstorder and pseudo-second-order kinetic plots are shown in Fig. 5. Kinetic parameters and correlation coefficients for the adsorption of Hg2+ ions by MCMs,N/S-MCMs,and N/S-MCMs/ Fe3O4 are shown in Table 2. The correlation coefficient value (R2) obtained from the pseudo-second-order model is 0.996 or 0.997,much higher than that obtained from the pseudo-first-second order model. This result indicates that a pseudo-second-order model accurately fits the adsorption behavior of mercury ions on the adsorbents.

|
Download:
|
| Fig. 5. Pseudo-first-order (a) and pseudo-second-order kinetic plots (b) of MCMs, N/S-MCMs, and N/S-MCMs/Fe3O4 | |
4. Conclusion
In conclusion,we demonstrate the synthesis of magnetically separated N/S-MCMs/Fe3O4 and an initial application of this carbon-based adsorbent for the removal of mercury ions from wastewater. N/S-MCMs/Fe3O4 show regular morphology (~580 nm in diameter),high surface area (542 m2/g) and mesoporous structure,which provide the potential as an adsorbent. Furthermore,N/S heteroatoms grafted into the carbon matrix endow enhanced Hg2+ adsorption capacity of 74.5 mg/g,while the introduction of Fe3O4 benefit convenient and fast separation of the adsorbent from aqueous solution using an external magnetic field. Our methodology creates a mode for the synthesis of wellstructured carbon-based materials as a promising adsorbent to achieve efficient removal of mercury ions from wastewater.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.038.
| [1] |
(a) J.H. Zhu, S.Y. Wei, H.B. Gu, et al., One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal, Environ. Sci. Technol. 46(2011) 977-985; (b) H.B. Gu, S.B. Rapole, J. Sharma, et al., Magnetic polyaniline nanocomposites toward toxic hexavalent chromium removal, RSC Adv. 2(2012) 11007-11018; (c) P.K. Tripathi, M.X. Liu, Y.H. Zhao, et al., Enlargement of uniform micropores in hierarchically ordered micro-mesoporous carbon for high level decontamination of bisphenol A, J. Mater. Chem. A 2(2014) 8534-8544; (d) M. Cegłowski, G. Schroeder, Preparation of porous resin with Schiff base chelating groups for removal of heavy metal ions from aqueous solutions, Chem. Eng. J. 263(2015) 402-411; (e) S.Y. Lin, H.J. Zhu, W.J. Xu, G.M. Wang, N.Y. Fu, A squaraine based fluorescent probe for mercury ion via coordination induced deaggregation signaling, Chin. Chem. Lett. 25(2014) 1291-1295. |
| [2] |
(a) J.H. Zhu, H.B. Gu, J. Guo, et al., Mesoporous magnetic carbon nanocomposite fabrics for highly efficient Cr (VI) removal, J. Mater. Chem. A 2(2014) 2256-2265; (b) S.X. Zhang, Y.Y. Zhang, J.S. Liu, et al., Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal, Chem. Eng. J. 226(2013) 30-38; (c) F.L. Fu, Q. Wang, Removal of heavy metal ions from wastewaters:a review, J. Environ. Manag. 92(2011) 407-418; (d) A.H. Chen, S.C. Liu, C.Y. Chen, C.Y. Chen, Comparative adsorption of Cu (Ⅱ), Zn (Ⅱ), and Pb (Ⅱ) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin, J. Hazard. Mater. 154(2008) 184-191; (e) Y. Liu, E.B. Yang, R.H. Han, et al., A new rhodamine-based fluorescent chemosensor for mercury in aqueous media, Chin. Chem. Lett. 25(2014) 1065-1068. |
| [3] |
(a) M. Hadavifar, N. Bahramifar, H. Younesi, Q. Li, Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multiwalled carbon nanotube with both amino and thiolated groups, Chem. Eng. J. 237(2014) 217-228; (b) A.M. Starvin, T.P. Rao, Removal and recovery of mercury (Ⅱ) from hazardous wastes using 1-(2-thiazolylazo)-2-naphthol functionalized activated carbon as solid phase extractant, J. Hazard. Mater. 113(2004) 75-79; (c) J.Z. Zhu, B.L. Deng, J. Yang, D.C. Gang, Modifying activated carbon with hybrid ligands for enhancing aqueous mercury removal, Carbon 47(2009) 2014-2025. |
| [4] | A.A. Ismaiel, M.K. Aroua, R. Yusoff. Palm shell activated carbon impregnated with task-specific ionic-liquids as a novel adsorbent for the removal of mercury from contaminated water. Chem. Eng. J. 225 (2013) 306–314 |
| [5] |
(a) M.M. Matlock, B.S. Howerton, D.A. Atwood, Chemical precipitation of heavy metals from acid mine drainage, Water Res. 36(2002) 4757-4764; (b) J. Aguado, J.M. Arsuaga, A. Arencibia, Adsorption of aqueous mercury (Ⅱ) on propylthiol-functionalized mesoporous silica obtained by cocondensation, Ind. Eng. Chem. Res. 44(2005) 3665-3671; (c) C. Jeon, W.H. Höll, Chemical modification of chitosan and equilibrium study for mercury ion removal, Water Res. 37(2003) 4770-4780; (d) S. Chaturabul, W. Srirachat, T. Wannachod, et al., Separation of mercury (Ⅱ) from petroleum produced water via hollow fiber supported liquid membrane and mass transfer modeling, Chem. Eng. J. 265(2015) 34-46; (e) H. Pietilä, P. Perämä ki, J. Piispanen, et al., Determination of low methylmercury concentrations in peat soil samples by isotope dilution GC-ICP-MS using distillation and solvent extraction methods, Chemosphere 124(2015) 47-53; (f) S. Vasudevan, M.A. Oturan, Electrochemistry:as cause and cure in water pollution-an overview, Environ. Chem. Lett. 12(2014) 97-108. |
| [6] |
(a) S. Babel, T.A. Kurniawan, Low-cost adsorbents for heavy metals uptake from contaminated water:a review, J. Hazard. Mater. 97(2003) 219-243; (b) Y. Kikuchi, Q. Qian, M. Machida, et al., Effect of ZnO loading to activated carbon on Pb (Ⅱ) adsorption from aqueous solution, Carbon 44(2006) 195-202; (c) Y.Y. Xu, Z.H. Hao, H. Chen, J.M. Sun, D.J. Wang, Preparation of polyacrylonitrile initiated by modified corn starch and adsorption for mercury after modification, Ind. Eng. Chem. Res. 53(2014) 4871-4877. |
| [7] |
(a) Y. Shin, G.E. Fryxell, W. Um, et al., Sulfur-functionalized mesoporous carbon, Adv. Funct. Mater. 17(2007) 2897-2901; (b) G. Zolfaghari, A. Esmaili-Sari, M. Anbia, et al., Taguchi optimization approach for Pb (Ⅱ) and Hg (Ⅱ) removal from aqueous solutions using modified mesoporous carbon, J. Hazard. Mater. 192(2011) 1046-1055. |
| [8] | L.F. Lai, G.M. Huang, X.F. Wang, J. Weng. Solvothermal syntheses of hollow carbon microspheres modified with-NH2 and-OH groups in one-step process. Carbon 48 (2010) 3145–3156 |
| [9] |
(a) X.B. Wang, J. Liu, W.Z. Xu, One-step hydrothermal preparation of aminofunctionalized carbon spheres at low temperature and their enhanced adsorption performance towards Cr (VI) for water purification, Colloids Surf. A 415(2012) 288-294; (b) X.H. Song, P. Gunawan, R.R. Jiang, et al., Surface activated carbon nanospheres for fast adsorption of silver ions from aqueous solutions, J. Hazard. Mater. 194(2011) 162-168; (c) X. Zhao, W. Li, S.S. Zhang, L.H. Liu, S.X. Liu, Hierarchically tunable porous carbon spheres derived from larch sawdust and application for efficiently removing Cr (Ⅲ) and Pb (Ⅱ), Mater. Chem. Phys. 155(2015) 52-58; (d) C.M. Zhang, W. Song, G.H. Sun, et al., Synthesis, characterization, and evaluation of activated carbon spheres for removal of dibenzothiophene from model diesel fuel, Ind. Eng. Chem. Res. 53(2014) 4271-4276. |
| [10] |
(a) L.C.A. Oliveira, D.I. Petkowicz, A. Smaniotto, S.B.C. Pergher, Magnetic zeolites:a new adsorbent for removal of metallic contaminants from water, Water Res. 38(2004) 3699-3704; (b) Z.G. Jia, L.L. Yang, J.H. Liu, Q.Z. Wang, R.S. Zhu, Preparation of magnetic carbon spheres derived form 8-quinoliolato Fe (Ⅲ) complexe and its application in water treatment, J. Ind. Eng. Chem. 21(2015) 111-117; (c) X.J. Peng, Z.K. Luan, Z.C. Di, Z.G. Zhang, C.L. Zhu, Carbon nanotubes-iron oxides magnetic composites as adsorbent for removal of Pb (Ⅱ) and Cu (Ⅱ) from water, Carbon 43(2005) 880-883; (d) J.F. Liu, Z.S. Zhao, G.B. Jiang, Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water, Environ. Sci. Technol. 42(2008) 6949-6954. |
| [11] | M.X. Liu, L.H. Gan, W. Xiong, et al. Development of MnO2/porous carbon microspheres with a partially graphitic structure for high performance supercapacitor electrodes. J. Mater. Chem. A 2 (2014) 2555–2562 |
| [12] | A. Sadezky, H. Muckenhuber, H. Grothe, R. Niessner, U. Pöschl. Raman microspectroscopy of soot and related carbonaceous materials:spectral analysis and structural information. Carbon 43 (2005) 1731–1742 |
| [13] |
(a) D.L.A. de Faria, S.V. Silva, M.T. de Oliveira, Raman microspectroscopy of some iron oxides and oxyhydroxides, J. Raman Spectrosc. 28(1997) 873-878; (b) C.F. Guo, Y. Hu, H.S. Qian, J.Q. Ning, S.J. Xu, Magnetite (Fe3O4) tetrakaidecahedral microcrystals:synthesis, characterization, and micro-Raman study, Mater. Charact. 62(2011) 148-151. |
| [14] | S.Y. Lee, D.H. Kim, S.C. Choi, et al. Porous multi-walled carbon nanotubes by using catalytic oxidation via transition metal oxide. Microporous Mesoporous Mater. 194 (2014) 46–51 |
| [15] |
(a) Y.H. Zhao, M.X. Liu, X.X. Deng, et al., Nitrogen-functionalized microporous carbon nanoparticles for high performance supercapacitor electrode, Electrochim. Acta 153(2015) 448-455; (b) D.Z. Zhu, Y.W. Wang, L.H. Gan, et al., Nitrogen-containing carbon microspheres for supercapacitor electrodes, Electrochim. Acta 158(2015) 166-174. |
| [16] |
(a) S.A. Wohlgemuth, F. Vilela, M.M. Titirici, M. Antonietti, A one-pot hydrothermal synthesis of tunable dual heteroatom-doped carbon microspheres, Green Chem. 14(2012) 741-749; (b) S.A. Wohlgemuth, R.J. White, M.G. Willinger, M.M. Titirici, M. Antonietti, A one-pot hydrothermal synthesis of sulfur and nitrogen doped carbon aerogels with enhanced electrocatalytic activity in the oxygen reduction reaction, Green Chem. 14(2012) 1515-1523. |
| [17] | J.N. Gao, X.Z. Ran, C.M. Shi, et al. One-step solvothermal synthesis of highly watersoluble, negatively charged superparamagnetic Fe3O4 colloidal nanocrystal clusters. Nanoscale 5 (2013) 7026–7033 |
| [18] | X.M. Ma, L.H. Gan, M.X. Liu, et al. Mesoporous size controllable carbon microspheres and their electrochemical performances for supercapacitor electrodes. J. Mater. Chem. A 2 (2014) 8407–8415 |
| [19] | W. Stöber, A. Fink, E. Bohn. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 26 (1968) 62–69 |
| [20] | J. Liu, S.Z. Qiao, H. Liu, et al. Extension of the Stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres. Angew. Chem. Int. Ed. 50 (2011) 5947–5951 |
| [21] |
(a) J. Choma, D. Jamioła, K. Augustynek, et al., New opportunities in Stöber synthesis:preparation of microporous and mesoporous carbon spheres, J. Mater. Chem. 22(2012) 12636-12642; (b) A.B. Fuertes, P. Valle-Vigón, M. Sevilla, One-step synthesis of silica@resorcinol-formaldehyde spheres and their application for the fabrication of polymer and carbon capsules, Chem. Commun. 48(2012) 6124-6126; (c) J. Liu, T.Y. Yang, D.W. Wang, et al., A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres, Nat. Commun. 4(2013) 2798; (d) M.X. Liu, X.M. Ma, L.H. Gan, et al., A facile synthesis of a novel mesoporous Ge@C sphere anode with stable and high capacity for lithium ion batteries, J. Mater. Chem. A 2(2014) 17107-17114; (e) M.X. Liu, J.H. Qian, Y.H. Zhao, et al., Core-shell ultramicroporous@microporous carbon nanospheres as advanced supercapacitor electrodes, J. Mater. Chem. A 3(2015) 11517-11526. |
| [22] | N.P. Wickramaratne, V.S. Perera, J.M. Ralph, S.D. Huang, M. Jaroniec. Cysteineassisted tailoring of adsorption properties and particle size of polymer and carbon spheres. Langmuir 29 (2013) 4032–4038 |
 2016, Vol. 27
2016, Vol. 27