b College of Chemistry and Chemical Engineering, Yantai University, Yantai 264005, China
Many naturally occurring [(bacterio)chlorophylls,(B)Chls] have a highly reactive formyl group attached directly to their tetrapyrrole macrocycles,such as Chl-b,Chl-d,and BChl-e. It is well known that these carbon-oxygen double bonds are readily modified to build various unique chemical structures capable of influencing the fundamental properties. From many quantitative structure-activity relationship (QSAR) studies it has been shown that the presence,variety and position of the substituents in the parent molecule made a remarkable difference in biological activity [1, 2, 3, 4, 5]. Introducing active functional groups around the porphyrin core has become an important strategy to produce useful tetrapyrrolic macrocycles. Among these,the transformation of an aldehyde into a nitrile group is a highly valued reaction because of the versatility of nitriles as starting materials for the synthesis of different heterocyclic structures generating a broad spectrum of biological activities [6, 7]. However,relevant reports related to chlorophyllous chlorin are scarce except the synthesis of cyano-pyropheophorbide-α by a two-step reaction including oximation and cyanidation at the 12-positions in our early works [8],and at the 3-position in other’s [9]. With regard to these works,chlorin oximes were firstly prepared from chlorophyll derivatives bearing an aldehyde group by condensation with hydroxylamine hydrochloride,then underwent the Beckmann rearrangement upon treatment with 2,4,6-trichloro-[1, 3, 5]triazine (TCT) in DMF or ethyl dichlorophosphate (EtOPOCl) and 1,8- diazabicyclo[5.5.0]undes-7-ene (DBU) in dichloromethane to afford cyanochlorins. Nevertheless,the reaction procedures suffer from various limitations,such as costly reagents,intricate manipulation and/or harsh reaction conditions. To expand and simplify this peripheral cyanation reaction of chlorin,herein,we report an efficient synthesis for chlorin nitriles from chlorin aldehydes by a tandem procedure combining an oximation reaction with a Beckmann rearrangement.
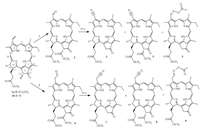
2. ExperimentalIn this study,the transformation of an aldehyde group linked to the chlorin chromophore into a nitrile was carried out by a one-pot,two-step reaction. As a starting material,methyl pheophorbide-α (MPa) 1a,isolated from Spirulina pacifica [10],was oxidized with osmium (Ⅷ) oxide in tetrahydrofuran containing a catalytic amount of pyridine at 0 ℃ followed by the glycol cleavage with sodium periodate in aqueous tetrahydrofuran to give the methyl pheophorbide-d (MPd) 2 [11]. The oximation with hydroxylammonium chloride in acetic anhydride and the subsequent cyanation under reflux were combined into one step to give a complicated mixture from which the cyano-pyropheophorbide-α 3 was isolated in low yield (9%). This one-pot reaction implies that some reaction conditions for the oximation may be inappropriate such as temperature and acidity,therefore we attempted new protocol for the oximation via a modified procedure. As shown in Scheme 1,chlorin aldehyde 2 reacted with hydroxylammonium chloride in methyl alcohol in the presence of triethylamine at room temperature. After adding acetic anhydride the obtained crude product,upon being subjected to the Beckmann rearrangement by stirring at 90 ℃ for 6 h,was converted into cyano-chlorin 3 (47%) as a major product. In addition to this,acetylated chlorin oxime 5 (12%) and an inseparable mixture including 3 and a small amount C3-cyanized pheophorbide-α 4 were also obtained. The cyanation of 3-formymethyl pheophorbide-α 6 bearing an aliphatic aldehyde group at the 3-position,prepared from MPα 1a by a thallium nitrate oxidation in tetrahydrofuran followed by hydrolysis in 88% formic acid [12],was implemented under same reaction condition to produce similar reaction result. Besides major product 3-cyano pyropheophorbide-α 7 (45%),trace amounts of pheophorbide-α 8 along with 7 and acetylated chlorin oxime 9 (9%) was separated from the reaction system.
|
Download:
|
| Scheme. 1. Reagent and conditions: (a) OsO4/Pyr/NaIO4; (b) NH2OH•HCl/MeOH/TEA; (c) Ac2O/90 8C; (d) TI(NO3)3/88% formic acid | |
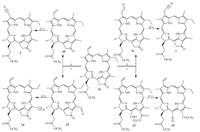
Whether in one-pot reaction or tandem reaction,the methoxyformyl group at the 132-position was removed by decarbomethoxylation in acidic medium. Based on this result,we next attempted,using chlorin aldehyde derived from methyl pyropheophorbide- α 1b (MPPα) which has no substituted groups in its exocyclic ketone [10],to form the oximido intermediate in situ. Aromatic chlorin aldehydes 10 (MPPd) and adipose homolog 11 were obtained from 1b by the same oxidation procedure using OsO4/NaIO4 [13, 14] and TI(NO3)3 as the oxidizing agents [15],respectively. 12-Formylpyropheophorbide-α 12 and 15-formylchlorin- f dimethyl eaters 13 also were separated from a mixture from the isomerization of MPPα 1b upon the treatment with saturated methanol solution of LiOH in the presence of oxygen (exposure of the reaction mixture to air) followed,by the acidification with AcOH and methylation with diazomethane [16, 17]. The transformation of these carbon-oxygen double bonds to the carbon-nitrogen triple bonds were performed under the same in-situ conditions as the transformation of chlorin aldehyde 2 to homologic chlorin nitriles 3 (53%),7 (58%),14 (52%) and 15 (61%),respectively (Scheme 2).
|
Download:
|
| Scheme. 2. Regeant and conditions: (a) OsO4/Pyr/NaIO4; (b) TI(NO3)3/88% formic acid; (c) LiOH/air/MeOH; (d) NH2OH•HCl/MeOH/TEA; (e) Ac2O, 90 8C. | |
All reactions were carried out under an argon atmosphere with dry solvents under anhydrous conditions. Reagents were purchased at the highest commercial quality and used without further purification. Reactions were monitored by thin layer chromatography (TLC). 1H NMR spectra were recorded on a Bruker AV-400 instrument and calibrated using residual undeuterated chloroform (δH = 7.26) as an internal reference. IR spectra were recorded on a Thermo Scientific Nicolet 380 FT-IR spectrometer. Mass spectra (MS) were recorded on a GC-MS Automass 120 or on a Kratos Concept instrument.
Representative procedure for the tandem cyanation reaction of chlorin aldehyde: An oven-dried 25 mL round-bottomed flask equipped with a magnetic stirring bar and a three-way stopcock was charged with chlorin aldehyde (0.3 mmol). The flask was evacuated and flushed with argon (three times),and then absolute methyl alcohol (2 mL) was added. To the solution were successively added grinded hydroxylamine hydrochloride (0.6 mmol) and triethylamine (0.5 mL),followed by stirring at room temperature. The oximation was complete within 1.5 h,monitored by TLC analysis,and then acetic anhydride (20 mL) was added to the reaction solution,which was directly subjected to Beckmann rearrangement by stirring at 90 ℃ for 6 h. The resulted mixture was poured into brine and extracted with dichloromethane (3 × 15 mL). The organic layers were combined,washed with water,concentrated in vacuo,and purified by chromatography on silica gel using EtOAc/petroleum ether (1:5-1:3) as an eluent to give the corresponding chlorin nitriles. All the compounds reported herein showed spectral data consistent with the assigned structures. Selected data are as follows:
For 3: mp 191-194 ℃; UV-vis (CH2Cl2) λmax(e): 416 (9.32 × 104),514 (1.51 × 104),568 (1.02 × 103),645 (6.30 × 103),686 (7.06 × 104) nm; IR (KBr,cm-1 ): v 3452 (N-H),2957 (C-H),1732,1726 (C=O),1608 (C=C),1520 (chlorin skeleton),1434,1363,1256,1089,908; 1H NMR (400 MHz,CDCl3): δ 9.47,9.41,8.74 (each s,each 1H,meso-H),5.53 (d,1H,J = 20.0 Hz,132-H),5.18 (d,1H,J = 20.0 Hz,132-H),4.58 (q,1H,J = 7.2 Hz,18-H),4.39 (d,1H,J = 8.0 Hz,17-H),3.62 (q,2H,J = 7.6 Hz,8a-H),3.65,3.63,3.62,3.17 (each s,each 3H,all 12H,CH3 + OCH3),2.56-2.77 (m,2H,17a + 17b-H),2.25-2.36 (m,2H,17a + 17b-H),1.86 (d,3H,J = 7.2 Hz,18-CH3),1.64 (t,3H,J = 7.6 Hz,8b-CH3),-0.50 (br s,1H,NH),-2.38 (br s,1H,NH); EI-MS m/z: 548.7 (MH+); Anal. Calcd. for C33H33N5O3:C 72.37,H 6.07,N 12.79; found: C 72.57,H 6.15,N 12.64;
For 5: mp 207-210 ℃; UV-vis (CH2Cl2) λmax(e): 385 (1.24 × 105 ),410 (9.31 × 104),514 (1.11 × 103),545 (1.02 × 103),617 (5.67 × 103),677 (5.12 × 104) nm; IR (KBr,cm-1 ): v 3469(O-H),3344 (N-H),2862,2927 (C-H),1749,1747,1703 (C=O),1629 (C=C),1591 (chlorin skeleton),1491,1448,1263,1087,1041; 1H NMR (400 MHz,CDCl3): δ 9.73,9.28,8.58 (each s,each 1H,meso-H),9.39 (s,1H,3a-H),5.35 (d,1H,J = 20.0 Hz,132-H),5.13 (d,1H,J = 20.0 Hz,132-H),4.67 (q,1H,J = 7.2 Hz,18- H),4.29 (d,1H,J = 8.0 Hz,17-H),3.49 (q,2H,J = 7.6 Hz,8a-H),3.63,3.55,3.43,3.06 (each s,each 3H,all 12H,CH3 + OCH3),2.55-2.77 (m,2H,17a + 17b-H),2.47 (s,3H,3a-NOCOCH3),2.18-2.30 (m,2H,17a + 17b-H),1.81 (d,3H,J = 7.2 Hz,18-CH3),1.58 (t,3H,J = 7.6 Hz,8b-CH3),-0.15 (br s,1H,NH),-2.19 (br s,1H,NH); 13C NMR (100 Hz,CDCl3): δ 187.31,173.54,171.43,161.80,154.78,150.44,149.65,149.10,145.00,142.00,141.93,136.91,136.51,135.96, 131.14,128.62,128.46,112.28,106.64,104.52,97.04,93.31,51.96,51.43,49.79,31.24,29.31,23.54,19. 47,19.42,17.50,17.00,12.20,11.29,10.98; EI-MS m/z: 608.0 (MH+); Anal. Calcd. for C35H37N5O5:C 69.18,H 6.14,N 11.52; found: C 69.07,H 6.19,N 11.69;
For 7: mp 194~197 ℃; UV-vis (CH2Cl2) λmax(e): 316 (1.84 × 103 ),406 (1.53 × 104),504 (9.18 × 102),534 (8.35 × 102),608 (7.65 × 102),664 (1.19 × 104) nm; IR (KBr,cm-1): v 3445 (N-H),2958,2835 (C-H),1736,1689 (C=O),1655 (C=C),1527 (chlorin skeleton),1400,1286,1189,1094; 1H NMR(400 MHz,CDCl3): δ 9.51,9.20,8.59 (each s,each 1H,meso-H),5.27 (d,1H,J = 19.8 Hz,132-H),5.12 (d,1H,J = 19.8 Hz,132-H),4.81 (s,2H,3a-H),4.53 (q,1H,J = 7.2 Hz,18-H),4.31 (d,1H,J = 8.6 Hz,17-H),3.66 (q,2H,J = 7.6 Hz,8a-H),3.65,3.62,3.39,3.25 (each s,each 3H,all 12H,CH3 + OCH3),2.66-2.74 (m,1H,17a + 17b-H),2.53~2.61 (m,2H,17a + 17b-H),2.25~2.33 (m,1H,17a + 17b-H),1.83 (d,3H,J = 7.2 Hz,18-CH3),1.69 (t,3H,J = 7.6 Hz,8b-CH3),0.16 (br s,1H,NH),-1.88 (br s,1H,NH); 13C NMR (100 Hz,CDCl3): δ 197.27,173.63,172.77,160.81,156.24,145.34,137.69,136.99,136.28,136.24,132.20,128.38,128.27,122.93,113.78,113. 35,106.43,104.56,97.41,93.03,53.93,51.92,50.77,50.26,33.48,30.40,29.75,23.47,20.96,19.33,17.40,12.11,12.04,11 .31; EI-MS m/z: 562.5 (MH+); Anal. Calcd. for C34H35N5O3:C 72.71,H 6.28,N 12.47; found: C 72.59,H 6.32,N 12.30;
35,106.43,104.56,97.41,93.03,53.93,51.92,50.77,50.26,33.48,30.40,29.75,23.47,20.96,19.33,17.40,12.11,12. 04,11.31; EI-MS m/z: 562.5 (MH+); Anal. Calcd. for C34H35N5O3:C 72.71,H 6.28,N 12.47; found: C 72.59,H 6.32,N 12.30;For 14: mp 188-191 ℃; UV-vis (CH2Cl2) λmax(e): 401 (1.19 × 104 ),518 (5.95 × 102),564 (2.62 × 103),614 (7.14 × 102),670 (3.33 × 103) nm; IR (KBr,cm-1): v 3434 (N-H),2858 (C-H),1739,1689 (C=O),1623 (C=C),1562 (chlorin skeleton),1460,1343,1218,1160,1027,905; 1H NMR (400 MHz,CDCl3): d 8.63,8.51,8.18 (each s,each 1H,meso-H),7.70 (dd,1H,J = 17.8,11.6 Hz,3a-H),6.19 (d,1H,J = 17.8 Hz,trans- 3b-H),6.15 (d,1H,J = 11.6 Hz,cis-3b-H),5.01 (d,1H,J = 19.6 Hz,132-H),4.27 (dq,1H,J = 7.4,1.9 Hz,132-H),4.27 (dq,1H,J = 7.4,1.9 Hz,18-H),4.03 (td,1H,J = 9.4,1.9 Hz,17-H),3.16 (q,2H,J = 7.6 Hz,8a-H),3.67,3.24,2.80 (each s,each 3H,all 9H,CH3 + OCH3),2.54-2.66 (m,1H,17a + 17b-H),2.33-2.43 (m,2H,17a + 17b-H),2.13-2.23 (m,1H,17a + 17b-H),1.80 (d,3H,J = 7.2 Hz,18-CH3),1.42 (t,3H,J = 7.6 Hz,8b-CH3),0.72 (br s,1H,NH),-0.49 (br s,1H,NH); 13C NMR (100 Hz,CDCl3): δ 192.11,182.27,175.49,173.73,166.66,152.41,143.70,142.83,137.18,137.06,136.27,134.77,131.28,128.87,128.59, 128.58,128.40,127.20,123.19,115.84,103.25,102.21,101.05,95.27,52.64,51.78,49.38,32.24,31.76,23.96, 19.01,17.05,11.91,10.97; EI-MS m/z: 560.3 (MH+); Anal. Calcd. for C34H35N5O3:C 72.97,H 5.94,N 12.51; found: C 73.06,H 6.12,N 12.40;
For 15: mp 178-181 ℃; UV-vis (CH2Cl2) λmax(e): 385 (5.23 × 103 ),422 (1.07 × 104),512 (9.42 × 102),561 (5.01 × 103),688 (9.34 × 103) nm; IR (KBr,cm-1 ): v 33419 (N- H),2915,2847 (C-H),1733,1691 (C=O),1604 (C=C),1497 (chlorin skeleton),1437,1365,1199,1111,981; 1H NMR (400 MHz,CDCl3): d 9.63,9.52,8.71 (each s,each 1H,meso-H),7.94 (dd,1H,J = 17.8,11.5 Hz,3a-H),6.28 (d,1H,J = 17.8 Hz,trans-3b-H),6.14 (d,1H,J = 11. 5 Hz,cis-3b-H),4.75 (dd,1H,J = 9.0,3.0 Hz,17-H),4.51 (q,1H,J = 7.2 Hz,18-H),3.64 (q,2H,J = 7.6 Hz,8a-H),4.37,3.59,3.57,3.40,3.18 (each s,each 3H,all 15H,CH3 + OCH3),2.67-2.75 (m,1H,17a + 17b-H),2.53~2.62 (m,2H,17a + 17b-H),2.24-2.32 (m,1H,17a + 17b-H),1.79 (d,3H,J = 7.2 Hz,18-CH3),1.65 (t,3H,J = 7.6 Hz,8b-CH3),-1.15 (br s,2H,NH); 13C NMR (100 Hz,CDCl3): δ 196.32,173.38,171.75,159.61,169.68,148.90,146.53,144.82,144.69,139.56,138.94,135.94,134.00,132.61,131.84, 131.66,118.93,106.65,104.92,90.98,51.75,51.51,49.24,48.55,30.97,29.80,29.77,22.19,20.54,19.39,16.94, 16.39,15.49,15.40,11.14; EI-MS m/z: 592.5 (MH+); Anal. Calcd. for C35H37N5O4:C 71.05,H 6.30,N 11.84; found: C 71.21,H 6.19,N 11.69.
3. Results and discussionThe dehydration of chlorin oximes under reflux in acetic anhydride to produce chlorin nitriles was inefficient (their yields were lower than 12% in general). This is largely as a result of the known sensitivity of chlorophyll derivatives toward heat in acidic or alkalic medium [18, 19, 20, 21]. We have managed to substantially increase the yields (up to [4TD$DIF]53%-61%) of the target molecules by carrying out the acetic anhydride-mediated dehydration of oximes at a lower reaction temperature (90 ℃) and over a longer period of time (6 h). If the cyanation reaction was terminated prematurely,O-acetylated oxime mingled with chlorin nitrile to result in reduced yields and difficulty for separation. On the basis of this result,the formation of cyanochlorin was apparently preceded by the acylation of the starting oximes. Take 16,obtained from MPPd 10,for example,the intermediate acetates 5 was firstly formed,and then subjected to the elimination reaction to leave an acetic acid by stirring in hot medium for 6 h. During the preliminary 4 h the contents of oxime 16 and acetylated chlorin 5 showed changes that were consistent with the reaction process shown in Scheme 3.

|
Download:
|
| Scheme. 3. Regeant and conditions: (a) NH2OH•HCl/MeOH/TEA; (b) Ac2O/90 8C. | |
Differing from benzaldehydes,the oximation of the peripheral formyl groups linked to chlorin produced only single E-type oxime 16. The reason for lacking of the Z-type isomer 160 probably is because of the crowded atmosphere between the oxime-hydroxyl with the adjacent substituents,namely,with C5-meso-hydrogen or C3-methyl group as shown in Scheme 3. While,the oximation for aliphatic aldehyde 6 gave a pair of E-Z isomers due to that the oxime moiety connected to the 3a-position is far apart from the macrocyclic chromophore,thereby avoiding the spatial repulsion with the vicinal substitutes. The 1 H NMR spectrum of the oximated product exhibited coupled chemical shifts for methyl groups and meso-hydrogen protons,indicating that the resulted mixture was composed of E-Z-isomers. It is noteworthy that the stereochemistry of the oxime influenced the subsequent rearrangement reaction,and the acetylation for the oxime hydroxyl group was prone to decarboxylation at the temperature of hydrolysis. In this step,O-acetylchlorin oxime 5 lost an acetic acid through a cyclic transition state to give the expected chlorin nitrile 3. Comparatively speaking,the decarboxylation for the Z-type acetylated oxime was more difficult on due to the lack of a steady intermediate state for compound 5 in the rearrangement process. Maybe this reason resulted in requiring a longer period of time for 11 (8 h) during cyanation reaction,compared with 2 (6 h).
In the 1H NMR spectra of all the formyl-substituted chlorins,their singlet signals,attributed to the protons of aromatic aldehyde,clearly appeared in downfield (δH 11.31-11.32). The corresponding absorption peak for 12,for example,occurs at 11.31 ppm in the screenshot B in Fig. 1. The aliphatic aldehyde protons in 6 and 11 appeared at 9.85 and 9.85 ppm. It was found that these characteristic absorptions for aldehyde groups vanished altogether after the transformation from aldehyde to nitrile,and other chemical shifts basically presented at diagnostic regions,indicating that the original structures were intact. As shown in the screenshots A and C in Fig. 1,the former,related to chlorin nitrile 14,reveals an unambiguous vinyl signal at 7.00 ppm for 3a-H and 6.35 (6.13) ppm for 3b-H. The latter,as the screenshot of chlorin nitrile 7,showed no vinyl group peaks because it has been converted into the cyanomethyl group.

|
Download:
|
| Fig. 1. The comparative 1H NMR spectra (CDCl3, 400 MHz) in the region 6.0–11.4 ppm of chlorin 14, 12, and 7. | |
In the UV-vis spectra,the position and intensity of the Soret band as well as the Qy bands of the products in different reactive stage changed significantly. The introduction of a formyl group in the chlorophyll degradation products causes an obvious bathochromic shift of the Qy peak maxima in dichloromethane,whose ΔQy were from 28 nm to 32 nm. The transformation of aldehyde into nitrile reduced the Qy absorption wavelength. The cyanized chlorins showed their Qy bands between 680 [5TD$DIF]nm and 690 nm to form obvious differences with chlorin aldehydes in their UV-vis spectrum. Taking cyanation at 12-position as an example as shown in Fig. 2,The Qy peak for the starting material 1b appeared at 668 nm,while the homologous absorption band for oxidized product 12 shifted bathochromicly to 690 nm. After the Beckmann rearrangement the Qy peak for resulted chlorin nitrile 14 fell back to 670 nm.

|
Download:
|
| Fig. 2. UV–vis spectra of MPPα 1b, 12-formylpheophorbide-α 12 and 12-cyanopheophorbide-α 14 in CH2Cl2. | |
Comparing with C3-formylation,the bathochromic effect resulted from the introduction of the formyl group to the 12- position was relatively low. The repellency with C131-carbonyl group disrupted the co-planarity of the C12-carbon-oxygen double bond with chlorin chromophore and weaken their conjugation. On the contrary,the C3-formymethylation caused hypochromatic shift for the Qy absorption in contrast to the precursors (1a and 1b) of the oxidized products (6 and 11). The reason that Qy peaks formed at shorter wavelength regions should be that the C3-aliphatic aldehyde group is unable to conjugate with macrocycle π-system.
4. ConclusionWe have developed a facile tandem synthesis for cyanochlorins related to chlorophyll from formyl-substituted chlorins. These readily available macrocylic aldehydes are assembled together with hydroxylamine hydrochloride to produce relevant chlorin nitriles in good yields using in-situ procedure including oximation and Beckmann rearrangement. This simple method not only is applicable to aromatic and aliphatic aldehydes,but also to different positions around the periphery. The structural transformation for chlorin nitrile represents a synthetic approach for acquiring novel chlorophyllous chlorins. Further studies toward constructing different heterocyclic structures by chemical modification of the cyano group attached to chlorin ring are currently underway.
| [1] | G. Zhang, W.R. Piotter, S.H. Camacho, et al. Synthesis, photophysical properities, tumor uptake, and preliminary in vivo photpsensitizing efficacy of a homologous series of 3-(1'-alkyloxy)ethyl-3-devinylpurpurin-18-N-alkylimides with variable lipophilicity. J. Med. Chem. 44 (2001) 1540–1559 |
| [2] | M. Ethirajan, Y.H. Chen, P. Joshi, et al. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 40 (2011) 340–362 |
| [3] | J.Z. Li, J.J. Wang, I. Yoon, et al. Synthesis of novel wavelength cationic shlorins via strereoselective aldol-like condensation. Bioorg. Med. Chem. Lett. 22 (2012) 1846–1849 |
| [4] | Y. Liu, X.X. Sen, J.Z. Li, et al. Cyclopropylation of chlorophyllous degradation products and synthesis of chlorin derivatives. Chin. J. Org. Chem. 34 (2014) 552–560 |
| [5] | P. Joshi, M. Ethirsjsn, L.N. Goswami, et al. Synthesis, spectroscopic, and in vitro photosensitizing efficacy of ketobacteriochlorins derived from ring-B and ring-D reduced chlorins via pinacol-pinacolone rearrangement. J. Org. Chem. 76 (2011) 8629–8640 |
| [6] | B.A. Trofimov, A.M. Vasiltsov, A.I. Mikhaleva, et al. Synthesis of 1-vinylpyrrole-2-carbonitriles. Tetrahedron Lett. 50 (2009) 97–100 |
| [7] | L.D. Luca, G. Giacomelli, A. Porcheddu. Beckmann rearrangement of oxime under very mild conditions. J. Org. Chem. 67 (2002) 6272–6274 |
| [8] | J.J. Wang, P. Zhang, J.Z. Li, et al. Synthesis of novel C12-nonmethylated chlorophyll derivatives from methyl pyropheophorbide-Ⅱ by allomerization and functionalization. Bull. Korean Chem. Soc. 32 (2011) 3473–3476 |
| [9] | S. Sasaki, K. Mizutani, M. Kunieda, et al. Synthesis and optical properties of C3-ethynlated chlorin and ǐ-extended chlorophyll dyads. Tetrahedron 67 (2011) 6065–6072 |
| [10] | K.M. Smith, D.A. Goff, D.J. Simpson. Meso substitution of chlorophyll derivatives:direct route for transformation of bacteriopheophorbide-d into bacteriopheophorbide-c. J. Am. Chem. Soc. 107 (1985) 4946–4951 |
| [11] | G.F. Han, J.J. Wang, Y. Qu, et al. Chemical modification of derivatives of purpurin-18 imide. Chin. J. Org. Chem. 26 (2006) 43–50 |
| [12] | G.F. Han, J.J. Wang, Y. Qu, et al. Synthesis of purpurin-18 imide derivatives. Chin. J. Org. Chem. 25 (2005) 319–326 |
| [13] | J.J. Wang, Y. Zhao, X.R. Wu, et al. Protection of the exocyclic carbonyl group of 2-acyl pyropheophorbide a methyl ester and their reactions with Grignard reagents. Chin. J. Org. Chem. 22 (2002) 565–570 |
| [14] | J.J. Wang, H.G. Fan, X.R. Wu, et al. Bromination reaction of methyl pyropheophorbide-Ⅱ. Chin. J. Org. Chem. 24 (2004) 537–542 |
| [15] | J.J. Wang, H.G. Fan, J.G. Yin, et al. Chemical modification along N21-N23 axis in methyl (pyro)pheophorbide-Ⅱ and effect on the visible spectra. Acta Chim. Sin. 61 (2003) 907–916 |
| [16] | J.Y. Ji, J.G. Yin, Q. Zhang, et al. C(12)-nomethylation of pyropheophorbide-Ⅱ and synthesis of chlorophyllous chlorin derivatives. Chin. J. Org. Chem. 34 (2014) 2047–2056 |
| [17] | J.Z. Li, W.H. Liu, F.G. Li, et al. Air oxidation and rearrangement reactions of methyl (pyro)pheophorbide-Ⅱ in the presence of lithiumhydroxide. Chin. J. Org. Chem. 27 (2007) 1594–1599 |
| [18] | J.J. Wang. Progress in the chemical reactions of chlorophyll-Ⅱ derivatives and synthesis of polysubstituted chlorin or porphyrin. Chin. J. Org. Chem. 25 (2005) 1353–1371 |
| [19] | J.Z. Li, Y. Liu, X. X.S, et al. Highly efficient synthesis of novel methyl 132-methylene mesopyropheophorbide-Ⅱ and its stereoselective Michael addition reaction. Org. Biomol. Chem. 13 (2015) 1992–1995 |
| [20] | H. Tamiaki, Y. Okamoto, Y. Mikata, et al. Photooxidative cleavage of zinc 20-substituted chlorophyll derivatives:conformationally P-helix-favored formation of regioselectively 19-20 opened linear tetrapyrroles. Photochem. Photobiol. Sci. 11 (2012) 898–907 |
| [21] | M. Ethirajan, P. Joshi, W.H. Wiloliam, et al. Remarkable regioselective position-10 bromination of bacteriopyropheophorbide-Ⅱ and ring-B reduced pyropheophorbide-Ⅱ. Org. Lett. 13 (2011) 1956–1959 |
 2016, Vol. 27
2016, Vol. 27 


