Numerous studies have shown that TiO2 has been extensively investigated as the promising state-of-the-art photocatalysts due to their strong oxidizing ability,low toxicity,low cost,and facile synthesis since it was reported in 1972 [1, 2]. However,the photocatalytic efficiency of TiO2 must overcome the poor quantum yield of the catalyst,which was caused by the rapid recombination of the photogenerated electrons and holes [3, 4]. Several research approaches have committed to exploring the more efficient and active photocatalysts.
Recently,BiOBr catalysts have attracted much attention because the hybridization between O2p and Bi6s states narrows the band gap well and enables BiOBr make use of visible light [5]. Furthermore,it has good chemical stability and being eco-friendly [6, 7]. BiOBr was a significantly V-VI-VII ternary semiconductor with layer structure characterized by [Bi2O2]2+ slabs that interleaved by double halide atoms layers. Several studies have been reported for the application and preparation of BiOBrmicro/nanostructures. Deng et al. reported the synthesis of one-dimensional (1D) BiOBr nanowires and nanotubes using a cationic surfactant cetyltrimethylammonium bromide (CTAB) as the bromine source [8]. Two-dimensional (2D) single-crystalline BiOBr nanoplates,nanosheets,and microsheets were obtained by hydrogen peroxide oxidation of bulk metal Bi particles in a surfactant-mediated solution [9]. Fabrication of threedimensional (3D) BiOBr materials are more sophisticated. Zhang et al. successfully synthesized the 3D flower-like hollow microsphere, which showed a more excellent photoactivity under visible light than the BiOBr bulk plates [10]. In addition,the applications of BiOBr are also various. Jiang et al. designed flake-like BiOBr using a HAc-assisted hydrothermal route for photocatalytic degradation of MO [11]. Mesoporous 3D BiOBr microspheres were got by a facile solvothermal method for photodecomposition of toluene [12]. Ai and co-workers found the BiOBr microspheres,which displayed efficient photocatalytic activity,can remove the textileNOspecies in indoor air [13]. Thus,preparing the BiOBr with different structures that possess visible photocatalytic activity is a newtrend nowadays.
Ionic liquids (ILs) are non-volatile and non-flammable organic salts with lowmelting points [14]. Because of its unique properties, such as extremely low volatility,high ionic conductivity,good dissolving ability and designable structures,it has been widely used as solvents,templates,or reactants for the synthesis of inorganic nanomaterials [15, 16, 17, 18]. In 2000,Dai and co-workers first observed the synthesis of SiO2 aerogel using ionic liquids,which exhibited superior capability for solvation [19]. Khatri et al. reported that ultrafine monodisperse gold nanoparticles (AuNPs) were synthesized by an elegant sputtering of gold onto 1-n-butyl-3-methylimidazoliumhexafluorophosphate ionic liquid [20]. Xia et al. initially prepared the hollow and porous BiOBr materials with ionic liquids, which expanded the methods of the preparation of BiOBr [21].
Although there is much improvement in the area of BiOBr assisted by ionic liquids,the development of more environmental friendly and convenient synthesis method is still significantly important for BiOBr. Hence,synthetic conditions remain to be expanded. In addition,it is of great realistic meaning to boost the photocatalytic performance of BiOBr by exploring the conditions of prepared.
In this work,we have synthesized the BiOBr catalysts via an ionic liquid-assisted solvothermal approach to explore the best preparation conditions of dyes removal. Based on this background, BiOBr catalysts have been acquired from different kinds of ionic liquids,which demonstrates that the chain length of ILs have a farreaching influence on the visible light photocatalytic activity of BiOBr.
2. Experimental 2.1. Materials and physical measurementsBismuth nitrate pentahydrate (Bi(NO3)3•5H2O) was obtained from Tianjin Bodi Chemical Co.,Ltd.; Absolute ethanol,glycol ether were bought from Tinjin Fuyu Chemical Co.,Ltd.; ionic liquids was purchased from Shanghai Chengjie Co.,Ltd. All of the chemicals are analytic grade and used as received without purification.
X-ray powder diffraction (XRD) analysis was recorded with a Bruker advanced D8 powder diffractometer with a Cu-Kα radiation source. The scan ranges were 10°-80° with 0.02°s-1,respectively. The morphologies of BiOBr catalysts were observed on a Transmission electron microscopy (SEM). To study the recombination of photo induced charge carriers,photoluminescence (PL,Hitachi F-4500,250 nm) spectra was collected. Electron Paramagnetic Resonance (EPR) tests were carried out in a magnetic field modulation of 100 kHz using a Bruker,ER 200-SRC spectrometer at 77 K.
2.2. Synthesis of catalystsTypically,BiOBr was prepared under the conditions of ionic liquids (ILs). The procedure can be concluded as follows. Solution A was formed by the dissolution of 2 mmol of bismuth nitrate pentahydrate in 10 mL of glycol ether. In another process,2 mmol of 1-butyl-3-methylimidazolium bromide was dissolved in 20 mL of glycol ether. It was denoted as solution B. Solutions A and B were mixed together drop by drop with continuous stirring. When the color of the solution was changed from yellow green to clear and the BiOBr precursor solution was formed,1 mmol of 1-butyl-3- methylimidazolium bromide was introduced. After stirring for another 1 h,the mixture was transferred into a 50 mL Teflon reactor inside a stainless-steel vessel and heated at 433 K for 8 h. The resulting precipitates were collected,thoroughly washed with deionized water and ethanol three times. After dried at 353 K in the air,the products were got. The particles were denoted as BiOBr- C4. Whereas,the course of BiOBr synthesized by ionic liquids of different chain length was followed by the same procedure except that 1-butyl-3-methylimidazolium bromide were substituted by 1-octyl-3-methylimidazolium bromide,1-dodecyl-3-methylimidazolium bromide and 1-cetyl-3-methylimidazolium bromide in both solution A and B,respectively. The products were marked as BiOBr-C8,BiOBr-C12 and BiOBr-C16,respectively.
2.3. Photodegradation of dyeThe photocatalytic activities of the as-prepared BiOBr samples were tested by photodegradation of methyl orange (MO). Experiments were carried out in a photocatalytic reactor irradiated by a 300W Xenon lamp with a 420 nm UV light cut filter. In a typical procedure,0.1 g BiOBr photocatalysts were added into 100 mL of MO aqueous of 10 mg/L. Prior to photocatalytic reaction, the suspension was magnetically stirred for 30 min in dark to reach an adsorption-desorption equilibrium. Then the mixture was exposed to light irradiation under magnetic stirring. About 4 mL of the suspension was sampled and filtered to remove the photocatalysts every 15 min. The supernatant solution was analyzed by a UV spectrophotometer.
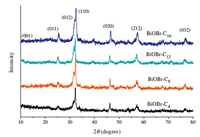
3. Results and discussionThe purity and phase of the samples were determined by X-ray powder diffraction (XRD). Fig. 1 shows the typical diffraction patterns of the as-prepared BiOBr samples under different conditions. For the samples,all the peaks of the as-prepared BiOBr at 25.38 (0 1 1),31.8° (0 1 2),32.3° (1 1 0),46.3° (0 2 0),57.3° (2 1 2) and 76.7° (0 3 2) were readily indexed to the tetragonal phase whose lattice parameters are a = 0.3915 nm,c = 0.8076 nm (space group: P4/nmm,JCPDS card no. 73-2061). It was indicated that the BiOBr catalysts were synthesized successfully. Furthermore,the samples synthesized by 1-butyl-3-methylimidazolium bromide were also characterized by SEM,which were exhibited in Fig. 2. It can be seen from SEMimages that BiOBr-C4 with aggregated,tremella-ball-like structures were formed,which was similar to the BiOBr reported early [13]. The samples’ surfaces were comprised of numerous layered-interacross BiOBr,which was different from the traditional layered BiOBr that synthesized by NaBr or KBr. This special morphology can make full use of the light,which might due to the multiple reflections among the layered-interacross appearance. From those results,the BiOBr samples have been successfully prepared by different kinds of the ILs,especially by 1-butyl-3- methylimidazolium bromide. In the procedure of the preparation, the ILs played not only the role of the bromide sources,but also the templates and the solvents of the fabrication of BiOBr,which exhibited the microflowers morphology.
|
Download:
|
| Fig. 1. XRD patterns for BiOBr samples prepared under different kinds of ionic liquids. | |

|
Download:
|
| Fig. 2. SEM images of BiOBr–C4 synthesized by ionic liquids | |
UV-vis diffuse reflectance spectroscopy was conducted to measure the BiOBr samples’ optical properties. The band gap energy (Eg) of BiOBr prepared with different kinds of ILs can be estimated according to the Eq. (1) and the diagram was exhibited in Fig. 3a and b.

|
Download:
|
| Fig. 3. (a) UV–vis absorption spectra and (b) band energy image of BiOBr synthesized by ionic liquids | |
| $ahv = k{\left( {hv - Eg} \right)^{1/n}}$ | (1) |
where,k represents a constant,a,h,v and Eg is absorption coefficient,Plank constant,light frequency,and band gap, respectively. By extrapolating the linear region,the band gap energies of BiOBr-C4,BiOBr-C8,BiOBr-C12 and BiOBr-C16 were calculated to be 2.31,2.30,2.27,2.21 eV,respectively. Moreover, those as-prepared catalysts have proper band gaps to be activated by visible light for photocatalytic degradation of organic pollutants, which were manifested in Fig. 3a.
Methyl orange (MO),a dye of widely used,was select as the model contaminant. The photocatalytic performance of the BiOBr samples were measured from the variation of the color in the reaction system by evaluating the maximum adsorption intensity of MO chromophoric group at the maximum wavelength of 463 nm under Xe lamp with a 420 nm UV light cut filter. As shown in Fig. 4,it can be found that the degradation of MO by BiOBr-C4 can reach to 94.0% in 270 min,which was more efficient than that of BiOBr-C8 (85.1%),BiOBr-C12 (83.5%) and BiOBr-C16 (69.2%) under the same conditions,respectively.

|
Download:
|
| Fig. 4. Photocatalytic activity of BiOBr prepared under different kinds of ionic liquids for degradation of MO under visible irradiation | |
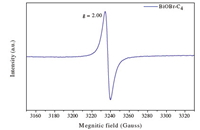
In order to investigate the effects of different kinds of ILs functionalized the BiOBr samples,photoluminescence (PL) spectra was used and presented in Fig. 5. Itwas depicted that the intensity of the maximum emission peaks gradually decreased as the chain length of ILs increased. Jing et al. [21] have argued that the stronger the PL intensity,the higher photocatalytic activity can be achieved. It was the reason that PL spectra resulted from the surface oxygen vacancies during the photoluminescence process,while surface oxygen vacancies were favorable for the photocatalytic oxidation reaction since it can trap the photoelectrons to prolong the combination time of the photogenic charge carrier [22]. From Fig. 6,oxygen vacancies were tested from BiOBr-C4,which can be inferred to BiOBr-C8,BiOBr-C12 and BiOBr-C16 since all samples were prepared by the same procedure and oxygen vacancies were originated from O-atom,which derived from the reagents. Consequently,the rules exhibited by the MO degradation experiment, which belongs to the photocatalytic oxidation reaction, suggesting that the results of the degradation ofMOwere consistent with the PL experiments.

|
Download:
|
| Fig. 5. Photoluminescence spectra of BiOBr synthesized by different kinds of ionic liquids. | |
|
Download:
|
| Fig. 6. EPR spectra of BiOBr–C4 samples at 77 K. | |
4. Conclusion
In summary,we have synthesized the hierarchical BiOBr microspheres with an average diameter of 2-4 mm via solvothermal process in the presence of reactable ionic liquids. The experimental results revealed that BiOBr prepared by the ionic liquids with short chain length exhibited a better performance in the degradation of MO. Consequently,BiOBr-C4 shows better photocatalytic activities than other BiOBr in terms of dyes removal. This finding provides a new insight into the fabrication and design of the advanced photocatalytic BiOBr catalysts,which can be applied for dyes removal.
| [1] | A. Fujishima, K. Honda. Electrochemical photolysis of water at a semiconductor electrode. Nature 238 (1972) 37–38 |
| [2] | S.Y. Guo, S. Han, H.F. Mao, et al. Structurally controlled ZnO/TiO2 heterostructures as efficient photocatalysts for hydrogen generation from water without noble metals:The role of microporous amorphous/crystalline composite structure. J. Power Sources 245 (2014) 979–985 |
| [3] | Z. Song, Q. Li, L. Gao. Preparation and properties of nano-TiO2 powders. Mater. Sci. Technol. 13 (1997) 321–323 |
| [4] | Z.D. Wei, R. Wang. Preparation and photocatalytic activities of nanocomposites of MCNTs/TiO2 and MCNTs-phosphotungstic acid/TiO2. Petroleum and Coal 56 (2014) 475–479 |
| [5] | D. Zhang, J. Li, Q.G. Wang, Q.G. Wu. High {001} facets dominated BiOBr lamellas:facile hydrolysis preparation and selective visible-light photocatalytic activity. J. Mater. Chem. A 1 (2013) 8622–8629 |
| [6] | Y.F. Fang, Y.P. Huang, J. Yang, P. Wang, G.W. Cheng. Unique ability of BiOBr to decarboxylate D-Glu and D-MeAsp in the photocatalytic degradation of microcystin-LR in water. Environ. Sci. Technol. 45 (2011) 1593–1600 |
| [7] | X. Zhang, Z. Ai, F. Jia, L. Zhang. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X=Cl, Br, I) nanoplate microsheres. J. Phys. Chem. C 112 (2008) 747–753 |
| [8] | Z.T. Deng, D. Chen, B. Peng, F.Q. Tang. From bulk metal Bi to two-dimensional wellcrystallized BiOX (X=Cl, Br) micro- and nanostructures:synthesis and characterization. Cryst. Growth Des. 8 (2008) 2995–3003 |
| [9] | J.W. Wang, Y.D. Li. Synthesis of single-crystalline nanobelts of ternary bismuth oxide bromide with different compositions. Chem. Commun. 18 (2003) 2320–2321 |
| [10] |
(a) J. Zhang, F.J. Shi, J. Lin, et al., Self-assembled 3-D architectures of BiOBr as a visible light-driven photocatalyst, Chem. Mater. 20(2008) 2937-2941; (b) Z. Jiang, F. Yang, G.D. Yang, et al., The hydrothermal synthesis of BiOBr flakes for visible-light-responsive photocatalytic degradation of methyl orange, J. Photochem. Photobiol. A 212(2010) 8-13. |
| [11] | Y.C. Feng, L. Li, J.W. Li, J.F. Wang, L. Liu. Synthesis of mesoporous BiOBr 3D microspheres and their photodecomposition for toluene. J. Hazard. Mater. 192 (2011) 538–544 |
| [12] | Z.H. Ai, W.K. Ho, S.C. Lee, L.Z. Zhang. Efficient photocatalytic removal of NO in indoor air with hierarchical bismuth oxybromide nanoplate microspheres under visible light. Environ. Sci. Technol. 43 (2009) 4143–4150 |
| [13] | D.Q. Zhang, M.C. Wen, B. Jiang, G.S. Li, J.C. Yu. Ionothermal synthesis of hierarchical BiOBr microspheres for water treatment. J. Hazard. Mater. 211- 212 (2012) 104–111 |
| [14] | Y.N. Wang, K.J. Deng, L.Z. Zhang. Visible light photocatalysis of BiOI and its photocatalytic activity enhancement by in situ ionic liquid modification. J. Phys. Chem. C 115 (2011) 14300–14308 |
| [15] | F. Caruso. Nanoengineering of particle surfaces. Adv. Mater. 13 (2001) 11–22 |
| [16] | R. Katoh, M. Hara, S. Tsuzuki, Ion pair formation in[bmim]I ionic liquids, J. Phys. Chem. B 112(2008) 15426-15430. |
| [17] | S.J. Guo, S.J. Dong, E.K. Wang. Constructing carbon nanotube/Pt nanoparticle hybrids using an imidazolium-salt-based ionic liquid as a linker. Adv. Mater. 22 (2010) 1269–1272 |
| [18] | D.J. Mao, X.M. Lü, Z.F. Jiang, et al. Ionic liquid-assisted hydrothermal synthesis of square BiOBr nanoplates with highly efficient photocatalytic activity. Mater. Lett. 118 (2014) 154–157 |
| [19] | O.P. Khatri, K. Adachi, K. Murase, et al. Self-assembly of ionic liquid (BMI-PF6)-stabilized gold nanoparticles on a silicon surface:chemical and structural aspects. Langmuir 24 (2008) 7785–7792 |
| [20] | J.X. Xia, S. Yin, H.M. Li, et al. Improved visible light photocatalytic activity of sphere-like BiOBr hollow and porous structures synthesized via a reactable ionic liquid. Dalton Trans. 40 (2011) 5249–5258 |
| [21] | L.Q. Jing, B.F. Xin, D.J. Wang, et al. Relationships between photoluminescence performance and photocatalytic activity of ZnO and TiO2 nanoparticles. Chem. J. Chin. Univ. 26 (2005) 111–115 |
| [22] | H. Li, J. Shang, Z.H. Ai, L.Z. Zhang. Efficient visible light nitrogen fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} facets. J. Am. Chem. Soc. 137 (2015) 6393–6399 |
 2016, Vol. 27
2016, Vol. 27 


