b School of Physics and Optoelectronics Technology Dalian University of Technology Dalian 116024,China
Phthalocyanine and phthalocyanine complexes have a special two-dimensional conjugated π electronic structure and strong π-π electronic interactions caused by their conjugate ring structures,giving this variety of compounds their characteristic optical,electrical,and magnetic properties. Thus,they have potential applications in nonlinear optical materials [1],optical limiting complex materials [2, 3],molecular electronic components [4, 5],electrochromic materials [6, 7],liquid crystal displaymaterials [8, 9] and optical dynamic anticancer treatment drugs [10, 11, 12]. Phthalocyanine complex single crystals are accessible and have been extensively usedinOLEDs as the hole injectionlayermaterial [13, 14] or as a hole buffer layer located between the ITO anode and the hole transport layer to improve device half-life. Yoshino et al. studied the near-infrared (NIR) emission spectroscopy of several phthalocyanine complex single crystals and their corresponding evaporation deposited films [15]. Zinc phthalocyanine single crystal has fluorescence peaks at 760 nm and 1000 nm,and a sharp phosphorescent peak at 1150 nm. Copper phthalocyanine single crystal has only a sharp phosphorescent peak around 1120 nm.
The photoluminescence properties in near-infrared region of phthalocyanine indicated that it could be used in organic NIR electroluminescent (EL) devices as the light-emitting layer material. Assour et al. have examined the fluorescence of phthalocyanine (H2Pc) in 1-hydrogen chloride naphthalene solution [16],and observed three peaks located at 699 nm,735 nm,and 778 nm. Fujii et al. reported single-layer H2Pc light-emitting devices with emission of near-infrared light at 920 nm[17]. Despite these advances,phthalocyanine (H2Pc) single crystals are difficult to obtain,and their corresponding fundamental solubility and optical properties have until now not been rigorously investigated.
Here,we reported a facile solvothermal synthesis to prepare single-crystalline H2Pc. It gives poor solubility in various organic solvents,such as 1-chlorine naphthalene. UV-vis spectroscopy and PL spectroscopy of solid-state H2Pc single crystals show wide absorption in the range from 620 nm to 679 nm and a wide emission near 922 nm. The strong aggregations of H2Pc single crystals weaken fluorescence intensity due to concentration quenching. Thus,H2Pc was doped with Alq3 as a light-emitting layer to fabricate multilayer EL devices by a vacuum deposition method with a structure of ITO/NPB/Alq3:H2Pc/BCP/Alq3/Al. The emission center is at 936 nm when the H2Pc SC doping concentration is 20 wt%. The H2Pc doping concentrations influence emission intensity and blue shift the emission center.
2. Experimental 2.1. Materials and equipmentsSolvents were purified according to standard procedures. All chemicals were obtained commercially and used without further purification.
Absorption spectra were taken on a UV-3600 230 VCE recording spectrophotometer (Shimadzu,Japan). Versus voltage (V) measurements were obtained using a Keithley 2400 current-voltage source. NIR PL spectra were measured on a PL 9000.
Photoluminescence System (Bio-Rad Micromeasurements Ltd.,UK); the NIR EL signals were focused into a monochromator and detected with a liquid-nitrogen-cooled Ge detector,using standard lock-in techniques. All the measurements were performed in air at room temperature.
2.2. Synthesis of H2Pc single crystalThe mixture of phthalonitrile (0.051 g,0.4 mmol),ammonium molybdate (0.022 g,0.1 mmol),and urea (0.024 g,0.4 mmol) in quinoline (20 mL) was kept at 180 ℃ in the autoclave for 8 h,and then cooled back to room temperature (Scheme 1). After removing the solvent,the target product of H2Pc single crystal (0.0246 g,48.24% yield) was obtained as a purple crystal.
|
Download:
|
| Scheme. 1. Synthesis of H2Pc single crystal. | |
2.3. H2Pc electroluminescent devices
Organic film was prepared by a multiple source organic molecule vapor deposition system,and the instrument was produced by the Shenyang institute of Sida vacuum technology. The gas was pumped with a vacuum pump at a pressure of 4 × 10-4 Pa. For the evaporation of organic materials,the vacuum needs to be maintained at less than 1 × 10-3 Pa. The evaporation rate and film thickness were monitored in real-time through a quartz vibrator film thickness gauge.
The samples were stimulated by a He-Cd laser UV light with emission wavelength at 325 nm. Emitted light was focused by two lenses,passed through the slit of a grating monochromator,then detected by a photomultiplier tube to be recorded by the data acquisition system. The signalwas enlarged by using standard phaselocked amplifier technology. When the near infrared electroluminescence spectrum of the device was tested,a JT-1 transistor characteristic tracer generated a 100 Hz saw tooth wave to launch 100 Hz pulse light. The light was aimed at the optical grating monochromator slits,and the signal was detected by liquid nitrogen cooling germanium detectors after passing through a monochromator spectrometer,and the data was recorded by a characteristic X-Y recorder after a phase-locked amplifier.When the samplewas tested in the near-infrared range,532 nm Nd YAG green laser was used as the excitation light source,and chopped into 25 Hz pulses of light. The light emitted by the samples was focused by two lenses,passed through the slit of a gratingmonochromator,and detected by a liquid nitrogen cooled germanium detector. The data was recorded by characteristic X-Y recorder after a phase-locked amplifier finally. I-V characteristic of the device was tested on Keithley 2400 current- voltage source.
Metal aluminum electrode was evaporated in a vacuum deposition machine of DM-300B type,which was produced in Beijing scientific instrument factory. We usually evaporate aluminum film when the vacuum reaches 2 × 10-3 Pa,and its thickness was also monitored by quartz vibrator film thickness gauge.
3. Results and discussion 3.1. Synthesis and structure determinationQuinoline is a high boiling point solvent which can be used as both the solvent for solvothermal synthesis and the culture solution for single crystal growth. Here,the H2Pc single crystal was synthesized with phthalonitrile as the original material.
The single crystal with size of 0.08mm× 0.02mm × 0.01mm was chosen for test by four-circle X-ray single crystal diffractometer. H2Pc single crystal belongs to monoclinic system with formula of N℃32H16,space group of P2(1)/n,and crystal cell parameters as follows: a = 14.768(3)Å ,b = 4.7209(9)Å ,c = 17.331(3)Å ,α = 908,β = 104.240(3)8,γ = 908,V = 1171.2(4)Å 3,R1 = 0.0468,wR2 = 0.1209.
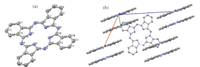
The structure of H2Pc is shown in Fig. 1. The central 16-member ring consists of eight N atoms and eight C atoms,and the C-N bonds range from 1.320(2)Å to 1.370(2)Å ,which are similar to those observed in other H2Pc structures. In H2Pc ring,the distances between two relative N1 atoms is 3.928(3)Å and the distances between two N3 atoms is 3.926(3)Å . The packing diagram of H2Pc is shown in Fig. 2,the H2Pc rings are stacked in a herringbone fashion,similar to that of the unsubstituted phthalocyanine. It is found that the intermolecular π-π stacking is present between two H2Pc molecules with central-to-central distances of 4.721(2)Å along the crystallographic b axis. As one of important types of supramolecular force,π-π stacking shows a specific structural requirement for substrate recognition or the arrangement of complicated architectures.
|
Download:
|
| Fig. 1. Structure of H2Pc single crystal (a) and crystal-packing diagram of H2Pc (b). | |
|
Download:
|
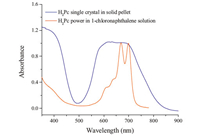
| Fig. 2. UV–vis absorption spectra of H2Pc single crystal (blue curve) and H2Pc power1-chlorine naphthalene as reference (red curve). | |
3.2. The solubility of H2Pc single crystal
H2Pc single crystal is insoluble in common organic solvents such as CHCl3,CH2Cl2,DMF,DMSO,THF,toluene,and pyridine,because it has no substituent introduced and no center metal ion coordinated. Aggregation occurs due to the π-conjugate system interactions between phthalocyanine molecules,which results in even poor solubility in large π electron conjugated system and strong polar 1-chlorine naphthalene solvents.
3.3. The optical properties of the H2Pc single crystalAs shown in Fig. 2 wide absorption peak from 620 nm to 679 nm was observed for H2Pc single crystal in the solid-state ultraviolet-visible (UV-vis) spectra,corresponding to the two Q zone (664 nm and 699 nm) of H2Pc powder in 1-chlorine naphthalene (reference). The blue shift and broadening of the peaks in the single-crystalline H2Pc compared to the dilute solution are the result of interactions that arise from aggregated molecules compared to dissolved monomers [18]. Another strong absorption peak is located at 335 nm,corresponding to the B zone in the UV-vis spectra of the H2Pc solution.
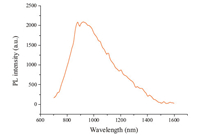
PL spectra were obtained from single-crystal H2Pc ground with KBr and pressed into pellets,and the spectra showed a broad emission band at 922 nm (Fig. 3). This 223 nm red shift and peak broadening compared to the dilute solution in 1-chlorine naphthalene is again due to the different state of the molecules. The fluorescence in dilute 1-chlorine naphthalene solution is from H2Pc monomers,but in the single crystal,it is from the molecules packing state. H2Pc molecules are closely stacked,and the effective distances between molecules are very small. Strong intermolecular forces between molecules influence molecular behavior and restrain molecules. Thus,the emission was derived from the H2Pc condensed state,not produced by any individual molecules.
|
Download:
|
| Fig. 3. Photoluminescence (PL) spectra of H2Pc single crystal. | |
The most common aggregation is composed of two molecules. When two identical molecules interact and emit a photon,the two molecules form an excimer,which can generate a new,stronger and wider emission peak at a longer wavelength range. Single crystal PL spectra may be from the H2Pc excimers. As the luminescence wavelength for H2Pc single crystal was longer than the corresponding monomers,it is more suitable for applications in near infrared organic light-emitting diodes (OLED) [19].
3.4. Electroluminescent performanceITO glass was cleaned in acetone and ethanol,then subsequently sonicated in acetone,anhydrous ethanol,and deionized (DI) water for 5 min each. After drying under nitrogen,it was immediately placed in the multi-source organic molecule vacuum deposition system. The system was pumped at a pressure below 1 × 10-3 Pa,and the material was heated to 50 ℃ to remove moisture. After 15 min,temperatures were adjusted to evaporate and deposit the organic layers in order. Growth thickness and rate were monitored in real-time by a quartz crystal oscillator film thickness gauge. The doping process was implemented by growing two materials at the same time,according to the designed concentration. Growth rate of each material in the evaporation process can be regulated by adjusting the source temperature until they meet the required ratio. A 2 mm × 2 mmopen mask plate was covered on the organic layers after evaporation to control the lightemitting area of the device. Various properties were tested at room temperature immediately after finishing the device fabrication.
Fig. 4 shows the structure of the device,where Alq3 was employed as the host material,H2Pc as a light-emitting layer (30 nm),the ITO as the anode,and aluminum (Al,120 nm) as the cathode. N,N'-di-1-naphthyl-N,N'-diphenylbenzidine (NPB) and 2,9-dimethyl-4,7-diphenyl-1,10-phenanthrolien (BCP) work as hole transport layer (30 nm) and a hole blocking layer (20 nm),respectively. Alq3 functions as electron transfer and injection layer (20 nm).

|
Download:
|
| Fig. 4. Structures of device with H2Pc as light-emitting active material. | |
Fig. 5 shows electroluminescent (EL) spectra of the device in near infrared at 8 mA working current with different H2Pc doping concentrations. When the doping concentration is 20 wt%,a wide and strong emission band with the center of emission wavelength of near 936 nm was observed,which is similar to the PL peak. Therefore,the emission band can be derived from H2Pc molecular aggregation. EL intensity is also affected by the doping concentration. The EL intensity decreased as the doping concentrations of H2Pc decreased from 10 wt% to 1 wt%. As for the EL spectra,a blue shift of the emission wavelength was observed at the same time. The blue shift may be induced by the various aggregation degrees of H2Pc molecular at different doping concentrations [20].
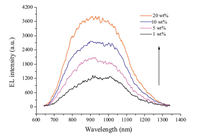
|
Download:
|
| Fig. 5. The near infrared (NIR) electroluminescence (EL) spectra of devices with different H2Pc concentrations. | |
When the device is powered,the excitons formed in the lightemitting layer will convey the energy to H2Pc molecular aggregates,and radiative transitions in the aggregates gives luminescence. When doping H2Pc into Alq3,Alq3 works as the diluent. When the H2Pc concentration is high,the distribution of H2Pc molecules is dense,and the molecule interactions are strong. With decreasing H2Pc concentration,the distribution is looser,and the interaction between molecules is weaker as the distance between the molecules becomes bigger. Therefore,H2Pc doping concentrations give rise to the blue shift of the emission wavelength and the weakening of EL intensity.
H2Pc electroluminescence (EL) mechanism in OLED devices might be a space charge limited mechanism. H2Pc works as both the hole trapping center where the holes are injected from ITO electrode and transmitted into each functional layer,and the electron[3TD$DIF] trapping center where the electrons pass through Al electrodes into each functional layer. The captured electrons and holes on H2Pc directly form excitons,which gives luminescence after excited state transition to the ground state. Fo¨ rster energy transfer is mainly from the dipole interaction of donor and receptor,which depends on the distance between the donor and acceptor molecules. The occurrence probability is proportional to the degree of overlap between donor emission spectra and receptor absorption spectra. Due to the small overlap between absorption spectrum of H2Pc and Alq3 light spectrum (Fig. 1),Förster energy transfer gives little contribution into the system.
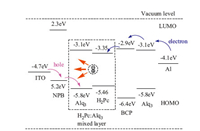
In the energy level diagrams in Fig. 6,the HOMO and LUMO of H2Pc are 5.46 eV and 3.35 eV,respectively,[21] which is located within the Alq3 forbidden band. This result indicates that the H2Pc in the device can work as both the hole trap and electron trap,which determines the direct space charge limited mechanism. Holes were injected from the ITO,passed through NPB layer,and captured by H2Pc,while electrons were injected from Al cathode,penetrated into BCP,and captured by H2Pc. The trapped holes and electrons on H2Pc generate H2Pc excited state. Fig. 7 shows the current density-voltage (I-V) characteristics for the device with different H2Pc doping concentrations. The results illustrate a low driving voltage of around 4 V. The driving voltage for the device decreases as H2Pc doping concentrations increases from 1 wt% to 20 wt%. This originated from the close effective distances and enhanced interactions of densely distributed H2Pc molecules for high doping concentration,which also supports the direct charge space charge limited mechanism in device luminescence.
|
Download:
|
| Fig. 6. Energy level diagram of the device. | |

|
Download:
|
| Fig. 7. Current density–voltage characteristics of device. | |
4. Conclusion
H2Pc single crystal was successfully synthesized by one-step solvothermal method. Compared with absorption spectroscopy and optical luminescence of H2Pc solution,H2Pc single crystal exhibits a broadening absorption peak with a large blue shift,and a broadening PL peak with a significant red shift at around 922 nm. With H2Pc doped Alq3 as a light-emitting layer,the EL device gives an emission center at 936 with doping concentration of 20 wt%. The device can be driven at low voltage of 4 V. The EL emission intensity and blue shift are affected by H2Pc doping concentration in Alq3. The facile synthesis of H2Pc crystal and the near-infrared EL properties of H2Pc based light-emitting layer will enhance the applications of H2Pc crystal in OLEDs.
| [1] | D.K.P. Ng, J.Z. Jiang. Sandwich-type heteroleptic phthalocyaninato and porphyrinato metal complexes. Chem. Soc. Rev. 26 (1997) 433–442 |
| [2] | S.M. LeCours, H.W. Guan, S.G. Dimagno, C.H. Wang, M.J. Therien. Push-pull arylethynyl porphyrins:new chromophores that exhibit large molecular firstorder hyperpolarizabilities. J. Am. Chem. Soc. 118 (1996) 1497–1503 |
| [3] | S. Priyadarshy, M.J. Therien, D.N. Beratan. Acetylenyl-linked,porphyrin-bridged,donor-acceptor molecules:a theoretical analysis of the molecular first hyperpolarizability in highly conjugated push-pull chromophore structures. J. Am. Chem. Soc. 118 (1996) 1504–1510 |
| [4] | T. Toupance, V. Ahsen, J. Simon. Ionoelectronics. Cation-induced nonlinear complexation:crown ether- and poly (ethylene oxide)-substituted lutetium bisphthalocyanines. J. Am. Chem. Soc. 116 (1994) 5352–5361 |
| [5] | T. Toupance, H. Benoit, D. Sarzain, J. Simon. Ionoelectronics. Pillarlike aggregates formed via highly nonlinear complexation processes. A light-scattering study,. J. Am. Chem. Soc. 119 (1997) 9191–9197 |
| [6] | G. De La Torre, P. Vázquez, F. Agulló-López, T. Torres. Phthalocyanines and related compounds:organic targets for nonlinear optical applications. J. Mater. Chem. 8 (1998) 1671–1683 |
| [7] | P.N. Moskalev, I.S. Kirin. Spectrophotometric study of the sulphonated diphthalocyanine complexes of yttrium,gadolinium,and luthetium in aqueous solution. Russ. J. Inorg. Chem. 16 (1971) 57–60 |
| [8] | Z. Belarbi, M. Maitrot, K. Ohta, et al. Electrical properties of condensed phases of the mesogen bis(octa-octadecyloxymethylphthalocyaninato) lutetium. Chem. Phys. Lett. 143 (1988) 400–403 |
| [9] | T. Toupance, P. Bassoul, L. Mineau, J. Simon. Poly(oxyethylene)-substituted copper and lutetium phthalocyanines. J. Phys. Chem. 100 (1996) 11704–11710 |
| [10] | I. Rosenthal. Phthalocyanines as photodynamic sensitizers. Photochem. Photobiol. 53 (1991) 859–870 |
| [11] | D. Wöhrle, N. Iskandar, G. Graschew, et al. Synthesis of positively charged phthalocyanines and their activity in the photodynamic therapy of cancer cells. Photochem. Photobiol. 51 (1990) 351–356 |
| [12] | D. Wöhrle, A. Hirth, T. Bogdahn-Rai, G. Schnurpfeil, M. Shopova. Photodynamic therapy of cancer:second and third generations of photosensitizers. Russ. Chem. Bull. 47 (1998) 807–816 |
| [13] | S.A. Van Slyke, C.H. Chen, C.W. Tang. Organic electroluminescent devices with improved stability. Appl. Phys. Lett. 69 (1996) 2160–2162 |
| [14] | Z.L. Zhang, X.Y. Jiang, S.H. Xu, T. Nagatomo, O. Omoto. Stability enhancement of organic electroluminescent diode through buffer layer or rubrene doping in holetransporting laye. Synth. Met. 91 (1997) 131–132 |
| [15] | K. Yoshino, M. Hikida, K. Tatsuno, K. Kaneto, Y. Inuishi. Emission spectra of phthalocyanine crystals. J. Phys. Soc. Jpn. 34 (1973) 441–445 |
| [16] | J.M. Assour, S.E. Harrison. On the optical absorptions of phthalocyanines. J. Am. Chem. Soc. 87 (1965) 651–652 |
| [17] | A. Fujii, M. Yoshida, Y. Ohmori, K. Yoshino. Two-band electroluminescent emission in organic electroluminescent diode with phthalocyanine film. Jpn. J. Appl. Phys. 35 (1996) L37–L39 |
| [18] | M. Rębarz, M. Wojdyła, W. Bała, Z. Łukasiak. Study of excited states in thin films of perylene derivatives by photoluminescence and absorption spectroscopy. Opt. Mater. 30 (2008) 774–776 |
| [19] | M. Cocchi, D. Virgili, V. Fattori, J.A.G. Williams, J. Kalinowski. Highly efficient nearinfrared organic excimer electrophosphorescent diodes. Appl. Phys. Lett. 90 (2007) |
| [20] | C.W. Tang, S.A. VanSlyke, C.H. Chen. Electroluminescence of doped organic thin films. J. Appl. Phys. 65 (1989) 3610–3616 |
| [21] | R.O. Loutfy, J.H. Sharp. Electrode behaviour of insoluble suspensions of metal-free phthalocyanines in methylene chloride. J. Appl. Electrochem. 7 (1977) 315–321 |
 2016, Vol. 27
2016, Vol. 27 


