Aconitum sinomontanum Nakai,a plant mainly distributed in western China,has long been employed as a folk medicine for the treatment of injuries,rheumatism,acute or chronic bacterial dysentery,and enteritis [1]. Investigation on the chemical components of A. sinomontanum were carried out by many research groups [2, 3, 4, 5, 6, 7, 8],leading to the isolation of a number of C18-diterpenoid alkaloids,with sporadic C19-counterparts,only two C20-diterpenoid alkaloids lepenine [7] and septatisine [4]. As a continuous research program to explore the diterpenoid alkaloids from Aconitum and Delphinium species [9, 10, 11, 12, 13, 14],we were interested in the alkaloidal constituents of A. sinomontanum [4, 5, 6]. The present study of a re-collection of this medicinal herb resulted in the identification of two new C20-diterpenoid alkaloids sinomontanidines A (1) and B (2) (Fig. 1). Herein,we report the isolation and structure elucidation of the new alkaloids.

|
Download:
|
| Fig. 1. The structures of C20-diterpenoid alkaloids 1–2. | |
2. Experimental 2.1. General
ESI-HR-MS was measured on a Bruker BioTOFQ mass spectrometer. IR spectra were recorded on a Nicolet 200 SXV spectrometer. Optical rotations were obtained in a 1.0 dm cell with a Perkin-Elmer 341 polarimeter at 20 ± 1 ℃. NMR spectra were recorded on Varian INOVA-400/54 and Agilent DD2-600/54 instruments including tetramethylsilane as the internal standard. Column chromatography was performed on silica gel (200-300 mesh,Qingdao Marine Chemical Factory,China). Thin layer chromatography (TLC) was carried out on silica gel GF254 plates (GF254,Qingdao Marine Chemical Co.,Ltd.,Qingdao,China) visualized by spraying a solution of potassium bismuth iodide (Dragendorff’s reagent),or by means of potassium permanganate.
2.2. Plant materialsThe roots of A. sinomontanum were collected in Gucheng county of Gansu Province,China,in May 2013. The materials were identified by Professor Hao Zhang at West China College of Pharmacy,Sichuan University. A voucher specimen (No. 130501) has been deposited at West China College of Pharmacy as well.
2.3. Extraction and isolationThe air dried roots (10 kg) of A. sinomontanum were chopped and percolated with 0.1 mol/L HCl (150 L). The acidic solution was filtrated and alkalized with 10% NH4OH to pH >9 and then extracted with ethyl acetate (50 L × 3). The combined extracts were concentrated in vacuum to yield the total crude alkaloids (155.4 g),which were chromatographed over silica gel column using a step-gradient eluting with petroleum ether-acetone (50:1-0:1,v:v) to obtain nine fractions: A (7.1 g),B (8.3 g),C (2.1 g),D (27.0 g),E (44.9 g),F (1.9 g),G (2.7 g),H (9.4 g),and I (13.8 g). Fraction B was eluted with CH2Cl2-MeOH (40:1),to give sinomontanidine A (1,13.7 mg). Fraction C was eluted with CHCl3- MeOH (100:1,v:v,1% diethylamine) to afford sinomontanidine B (2,18.0 mg).
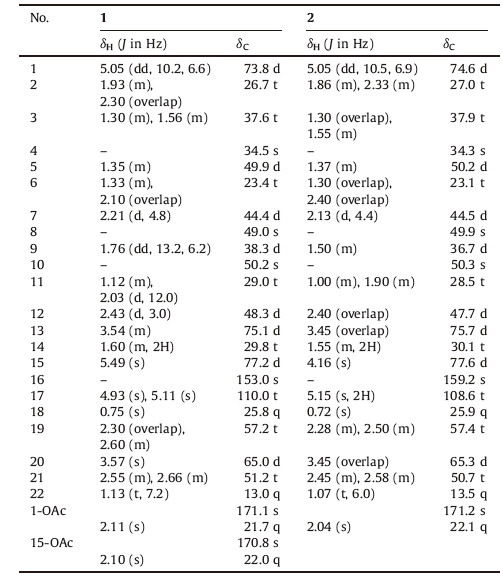
3. Results and discussionCompound 1 was obtained as a white amorphous powder. [α]D20 -46:5 (c 0.48,CHCl3). Its molecular formula was determined to be C26H37NO5 by HR-ESI-MS (m/z 444.2785 [M + H]+,calcd. for 444.2750),indicating nine degrees of unsaturation. The IR spectrum displayed the absorption of hydroxyl (3429 cm-1) and carbonyl (1728 cm-1) groups. Based on the NMR spectroscopic data,compound 1 contained four methyl groups,eight methylenes (one sp2 methylene carbon),eight methines (three oxygenated),in addition to six quaternary carbons (two ester carbonyls and one exocyclic double bond) (Table 1). The aforementioned spectral features along with the biogenetic considerations suggested that compound 1 could be a denudatine- or hetidine-type C20- diterpenoid alkaloids [15]. A C-7 to C-20 linkage was revealed by the crucial HMBC correlations (Fig. 2) from H-7 to C-5 and C-10,and from H-5 to C-7,C-18,C-19,and C-20,which proved that 1 possessed a denudatine-type skeleton. Three oxygenated groups were located at C-1,C-13,and C-15,respectively,according to the HMBC cross peaks at H-1 (δH 5.05) with C-2 (δC 26.7),C-9 (δC 38.3),C-10 (δC 50.2),C-20 (δC 65.0),and 1-OAc (δC 171.1); H-13 (dH 3.54) with C-8 (δC 49.0),C-12 (δC 48.3),C-11 (δC 29.0),C-14 (δC 29.8),C- 16 (δC 153.0),and C-17 (δC 110.0); H-15 (dH 5.49) with C-9 (δC 38.3),C-12 (δC 48.3),C-16 (δC 153.0),C-17 (δC 110.0),and 15-OAc (δC 170.8). There was a strong cross peak signal between H-13 and H-11,moreover,the observed NOE (Fig. 2) between H-13 with H- 11 and H-17b provided further evidence for positioning the only hydroxyl group at C-13,the relative stereochemistry of which was assigned to be β-orientation accordingly. Furthermore,H-1β and H-15α were deduced from the NOEDS experiments by the correlation between H-1 with H-5,H-9,as well as between H- 15 with H-7 and H-17a,respectively. The structure of 1 was thus established,and given the trivial name sinomontanidine A.

|
Download:
|
| Fig. 2. Key 1H,1H-COSY, HMBC, and NOE correlations of compound 1. | |
|
|
Table 1 NMR spectroscopic data for compounds 1 and 2 (600MHz for 1H, 150MHz for 13C, CDCl3, δ in ppm). |
Compound 2,a white amorphous powder,[α]D20 -10:6 (c 0.76,CHCl3),has the molecular formula of C24H35NO4 based on the pseudomolecular ion at m/z 402.2647 [M + H]+ (calcd. for 402.2644) in its HR-ESI-MS. Compound 2 exhibited nearly identical 1H NMR and 13 [5TD$DIF]C NMR resonances to those of 1,with the only difference by the absence of one acetyl substituent in 2,thus validating 42 mass units less found by mass spectrometry. Remarkably,replacement of the 15-OAc in 1 with the 15-OH was evident that the d-value of H-15 (δH 4.16) was significantly shifted up-field by comparison with that of 1 (H-15,δH 5.49). Finally,unambiguous assignments of 1H- and 13C-chemical shifts for compound 2 (Table 1) were accomplished using the 2D NMR techniques (1H,1H-COSY,HMQC,and HMBC). All available evidence suggested the structure of 2 as shown in Fig. 1,which was named sinomontanidine B.
4. ConclusionTwo new denudatine-type C20-diterpenoid alkaloids,sinomontanidines A (1) and B (2) were isolated from the roots of A. sinomontanum,the structures of which were elucidated based on the interpretation of NMR and HR-ESI-MS data. While the majority of diterpenoid alkaloids found from this species fell into the C18- category,isolation of two rare C20-diterpenoid alkaloids in the present investigation might provide further clues for understanding the chemotaxonomic significance of A. sinomontanum.
| [1] | Wang W.T., Xiao P.G.. Aconitum sinomontanum Nakai, in:Flora of China, Vol. 27[M]. Beijing: Science Press , 1979 : 168 -170. |
| [2] | S.Y. Chen, Y.Q. Liu, C.R. Yang. Chemical constituent of Aconitum sinomontanum Nakai. Acta Bot. Yunnan 2 (1980) 473–475 |
| [3] | B.Y. Wei, X.W. Kong, Z.Y. Zhao, et al. The research of Chinese Aconitum XVIII. Zhong Yao Tong Bao 6 (1981) 26–28 |
| [4] | C.S. Peng, J.Z. Wang, X.X. Jan, et al. Alkaloids of Aconitum sinomontanum and Aconitum racemulosum franch var. pengzhouense. Nat. Prod. Res. Dev. 12 (2000) 45–51 |
| [5] | C.S. Peng, F.P. Wang, J.Z. Wang, et al. Two new bisnorditerpenoid alkaloids sinomontanines D and E from Aconitum sinomontanum. Acta Pharm. Sin. 35 (2000) 201–203 |
| [6] | C.S. Peng, D.L. Chen, Q.H. Chen, F.P. Wang. New diterpenoid alkaloids from roots of Aconitum sinomontanum. Chin. J. Org. Chem. 25 (2005) 1235–1239 |
| [7] | C.L. Yuan, X.L. Wang. Isolation of active substances and bioactivity of Aconitum sinomontanum Nakai. Nat. Prod. Res. 26 (2012) 2099–2102 |
| [8] | B. Xu, J. Xue, J. Tan, et al. Two new alkaloids from the roots of Aconitum sinomontanum Nakai. Helv. Chim. Acta 97 (2014) 727–732 |
| [9] | F.P. Wang, Q.H. Chen, X.Y. Liu. Diterpenoid alkaloids,. Nat. Prod. Rep. 27 (2010) 529–570 |
| [10] | X.Y. Liu, Q.H. Chen, F.P. Wang. Three new C20-diterpenoid alkaloids from Delphinium anthriscifolium var. savatieri,. Chin. Chem. Lett. 20 (2009) 698–701 |
| [11] | L. Lin, D.L. Chen, X.Y. Liu, et al. Trichocarpinine, a novel hetidine-hetisine type bisditerpenoid alkaloid from Aconitum tanguticum var. trichocarpum. Helv. Chim. Acta 93 (2010) 118–122 |
| [12] | D.L. Chen, P. Tang, Q.H. Chen, F.P. Wang. New C20-diterpenoid alkaloids from Delphinium laxicymosum var. pilostachyum,. Nat. Prod. Commun. 9 (2014) 623–625 |
| [13] | Z.T. Zhang, X.Y. Liu, D.L. Chen, F.P. Wang. Three new C20-diterpenoid alkaloids from Aconitum tanguticum var. trichocarpum. Nat. Prod. Commun. 10 (2015) 861–862 |
| [14] | Q. Mei, Y.N. Wang, M. Zhao, et al. 1H-15N HMBC spectra of C18-diterpenoid alkaloids. Chin. Chem. Lett. 26 (2015) 804–806 |
| [15] | F.P. Wang, X.T. Liang. C20-diterpenoid alkaloids, in:G.A Cordell (Ed.), The Alkaloids:Chemistry and Biology, Vol. 59, Elsevier,. New York (2002) 1–280 |
 2016, Vol. 27
2016, Vol. 27