b Technical Center for Industrial Products and Raw Materials Inspection and Testing, Shanghai Entry-Exit Inspection &Inspection and Quarantine Bureau, Shanghai 200135, China
Currently,many primary aromatic amines (PAAs) are classified as toxic compounds or suspected human carcinogens [1, 2, 3]. They are widely used as starting substances or intermediates in pesticides,polymers,pharmaceuticals,cosmetics,and azo-dyes. However,the reactivity of PAAs has biological implications,with exposure to these compounds being linked to a number of toxicological effects,including carcinogenicity and genotoxicity. Many of them are specified by the National Institute for Occupational Safety and Health (NIOSH) as chemical hazards [4],as well as by the International Agency for Research on Cancer (IARC) [5]. For the proven carcinogenic substances,no detectable exposure levels are allowed for controlling work place exposures.
The published literature shows that a number of different analytical methods have been employed for the detection of PAAs [6, 7, 8, 9]. The simplest approach for the analysis of PAAs is the colorimetric method [10, 11]. However,this approach lacks selectivity and has high risk of false positive results. Various more confirmatory and sensitive methods for PAAs were developed based on mass spectrometry due to its high accuracy,reliability,and selectivity. Gas chromatography-mass spectrometry (GC-MS) after previous derivatization with isobutyl chloroformate is a sensitive method for PAA detection [9]. Currently the most exploited technique is liquid chromatography coupled with mass spectrometry (LC-MS) or tandem mass spectrometry (LC-MS/MS) [12, 13]. Not only do these approaches usually need expensive instruments and sophisticated technicians,but also the process is time-consuming. Therefore,it is urgent and meaningful to develop a simple and sensitive detection method for identification and quantification of PAAs.
Surface-enhanced Raman spectroscopy (SERS) is a rapid and ultrasensitive spectroscopic technique in chemical analysis. Some porous material has been used as SERS substrate because of their high SERS enhanced property. The porous structure might benefit to high SERS enhancement [14, 15, 16, 17]. The objective of this study was to develop a rapid and sensitive SERS sensor for PAAs detection and identification using nanoparticles decorated GMA-EDMA organic porous material. The effectiveness of the SERS sensor was validated using a group of PAAs with different substituent groups. This SERS sensor provides an easy and reliable method for trace PAAs detection.
2. ExperimentalAll reagents were of analytical reagent grade and used without further purification. Glycidyl methacrylate (GMA) was obtained from Tokyo Chemical Industry Co.,Ltd. (Tokyo,Japan). Ethylene dimethacrylate (EDMA),benzoperoxide (BPO),1-dodecanol and cyclohexanol were purchased from Acrosorganics (New Jersey,USA). Benzidine,p-toluidine,p-nitroaniline and 4,4-methylenebis-( 2-chloroaniline) were obtained from J&K Scientific,Ltd. (Beijing,China). The PAAs stock solutions (100 mg/L) were prepared in ethanol. The PAAs working solutions were obtained by diluting the stock solutions.
Glycidyl methacrylate-ethylene dimethacrylate (GMA-EDMA) porous material and AuNPs colloids were synthesized according to literature procedures [18, 19]. Gold colloid was synthesized using the method of Freeman [20]. The synthesized polymeric material was ground with a mortar and sieved with a sieve whose size was 60-80 mesh. GMA-EDMApowder material was modified by amino group. Then,the AuNPs were immobilized on the GMA-EDMA material through the interaction between amino groups and AuNPs. Thus,AuNPs decorated GMA-EDMA material was obtained for further use.
PAAs samples (100 mL) with various concentrations were mixed with appropriate amounts of the above materials,and the mixture was shaken for 1 min. Then,the materials were taken out and put on a quartz plate for SERS detection. A portable Raman spectrometer (i-Raman,B&W Tek Inc.,USA) with 785 nm excitation source was used for the measurement at an integration time of 10 s. The laser power and laser spot were 200mW and 150 mm,respectively.
3. Results and discussionThe resultisng Au-immobilized GMA-EDMA substrate was characterized by UV-vis spectrum and SEM (See Supporting information). Fig. S1(a) in Supporting information demonstrates the UV-vis spectrum of AuNPs decorated GMA-EDMA substrate. The maximum plasmon absorption of the Au colloid is located at 548 nm. Fig. S1(b) shows a SEM image of AuNPs decorated GMAEDMA substrate and the inset is the blank GMA-EDMA materials without NPs. It clearly shows that AuNPs are self-assembled on GMA-EDMA porous materials with high density and homogeneity. Very little stacking of AuNPs is observed on the surface,which ensures the stability and reproducibility of SERS signals in the following measurements.
In order to evaluate SERS performance of the synthesized substrate,the SERS spectra using traditional gold colloid as SERS substrate were collected for comparison. One kind of PAAs-4,4- methylene-bis-(2-chloroaniline) (MOCA) was used as target compound to demonstrate the highly efficient enhancement of SERS signal on synthesized substrate. The spectra in Fig. 1 demonstrated that SERS spectrum of MOCA had some same characteristic peaks as Raman spectra (curve c vs. curve d) and synthesized substrate can obtain higher SERS signal intensity of MOCA at 10-5 mol/L (curve b vs. curve d).
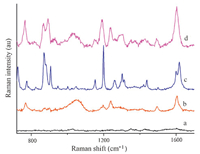
|
Download:
|
| Fig. 1. (a) SERS spectrum of synthesized substrate; (b) MOCA SERS spectrum when Au colloid as substrate; (c) MOCA Raman spectrum; (d) MOCA SERS spectrum on synthesized substrate. The concentrations of MOCA are (a) 0 mol/L; (b) 10-5 mol/L; (c) pure MOCA; (d) 10-5 mol/L. | |
In the present work,four PAAs under investigation and their known carcinogenic risk to humans [5] are listed in Table S1 in Supporting information. They were analyzed by SERS based on immobilized NPs,and their spectra were shown in Fig. S2. For comparison,the Raman spectra of the PAAs standards were also collected. The observed SERS spectra agreed well with the corresponding Raman spectra for PAAs standards. The lack of change in SERS peaks from the Raman spectra implies that the enhancement may be mainly due to the electromagnetic mechanism,which does not require the formation of chemical bonds between the analyte and substrate. To quantify the SERS intensity as a function of the PAAs concentration,the SERS peak which had higher intensity and well-shaped peak was selected for quantification. The Raman shift of quantitative peaks were 730 cm-1 for p-toluidine,851 cm-1 for p-nitroaniline,1183 cm-1 for benzidine,and 1603 cm-1 for MOCA. Their possible peak assignments based on literature data were C-C,out of plane vibration of C-H,in-plane bending vibration of C-H,and stretching mode of C55C,respectively [21, 22, 23].
The PAAs sampleswith various concentrationswere measured and their spectra were shown in Fig. 2. It was demonstrated that the signal intensity of quantitative peaks increased in response to the increasing PAAs concentration. The calibration curves of four PAA samples were linear within certain concentration ranges (2 × 10-9-6 × 10-8 mol/L for p-toluidine,5 × 10-9- 8 × 10-8 mol/L for p-nitroaniline,5 × 10-10-8 × 10-8 mol/L for benzidine,and 1 × 10-9-5 × 10-8 mol/L for 4,4-methylene-bis- (2-chloroaniline)) and their corresponding correlation coefficients squared were 0.9869,0.9830,0.9842,and 0.9852,respectively. The limit of detection was calculated to be 1.9 × 10-10,3.7× 10-10,2.1× 10-11 and 1.5 × 10-10 mol/L for p-toluidine,p-nitroaniline,benzidine and 4,4-methylene-bis-(2- chloroaniline),respectively.

|
Download:
|
| Fig. 2. SERS spectra of PAAs on the substrates at different concentrations and linear correlation between the Raman intensities and sample concentrations. (A) SERS of ptoluidine: (a–f) 2 × 10-9–6 × 10-8 mol/L; (B) linear correlations at the band of 730 cm-1 for p-toluidine; (C) SERS of p-nitroaniline: (a–f) 5 × 10-9–8 × 10-8 mol/L; (D) linear correlations at the band of 851 cm-1 for p-nitroaniline; (E) SERS of benzidine: (a–f) 5 × 10-10–8 × 10-8 mol/L; (F) linear correlations at the band of 1183 cm-1 for benzidine; (G) SERS of 4,4-methylene-bis-(2-chloroaniline): (a–f) 1 × 10-9–5 × 10-8 mol/L; (H) linear correlations at the band of 1603 cm-1 for 4,4-methylene-bis-(2-chloroaniline). | |
Compared with other methods for the determination of p-toluidine,p-nitroaniline,benzidine and 4,4-methylene-bis-(2- chloroaniline),our method has much better LOD than spectrophotometry [24],SPE-CZE [25],LC-MS/MS [26],CFME-HPLC [27],HLLE-IMS [28],etc.,comparable LOD to HPLC [29, 30],but lower LOD than GC-MS [31]. Also,it should be noted that short analysis time and low cost are the advantages of SERS in comparison with GC-MS. The proposed method was applied for the determination of MOCA in tap water in order to test its feasibility for real samples. After pretreatment of precipitation and filtration,samples were detected directly using our method. Results showed that no MOCA could be detected in tap water. Furthermore,two tap water samples spiked with MOCA solution at concentrations of 1.5 × 10-8-5 × 10-8 mol/L were analyzed and the recovery results were 84.2% and 112.6%. Detailed results were shown in Table S2 in Supporting information.
4. ConclusionThe gold nanoparticles decorated GMA-EDMA porous material was used as SERS substrate to detect PAAs compounds. Highly sensitive and rapid analysis was achieved for all four PAAs. The immobilization of AuNPs on the GMA-EDMA material makes the substrate closely packed but not aggregated Au NP arrays. As a result,prominent SERS enhancement can be obtained through the strong interaction between PAAs compounds and Au nanoparticles decorated GMA-EDMA materials. High sensitivity and good linear relationship between SERS signals and concentrations of PAAs are obtained for all four PAAs. Hence,the present approach should have great potential application in the rapid identification and quantification of PAA pollutants in the environment and food contact articles.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.059.
| [1] | R.J. Turesky, L. Le Marchand. Metabolismand biomarkers of heterocyclic aromatic amines in molecular epidemiology studies:lessons learned from aromatic amines. Chem. Res. Toxicol. 24 (2011) 1169–1214 |
| [2] | P. Silar, J. Dairou, A. Cocaign, et al. Fungi as a promising tool for bioremediation of soils contaminated with aromatic amines, a major class of pollutants. Nat. Rev. Microbiol. 9 (2011) 477 |
| [3] | Y.C. Fan, Z.L. Hu, M.L. Chen, C.S. Tu, Y. Zhu. Ionic liquid based dispersive liquidliquid microextraction of aromatic amines in water samples. Chin. Chem. Lett. 19 (2008) 985–987 |
| [4] | Centers for Disease Control and Prevention (CDC), NIOSH pocket guide to chemical hazards, GA, 1992. <http://www.cdc.gov/niosh/npg/nengapdx.html. > |
| [5] | International Agency for Research on Cancer (IARC), IARC monographs on the evaluation of carcinogenic risks to humans, Lyon, 2015. < http://monographs.iarc.fr/ENG/Classification/index.php. > |
| [6] | J. Schubert, O. Kappenstein, A. Luch, T.G. Schulz. Analysis of primary aromatic amines in the mainstream waterpipe smoke using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 1218 (2011) 5628–5637 |
| [7] | B. Jurado-Sánchez, E. Ballesteros, M. Gallego. Gas chromatographic determination of N-nitrosamines, aromatic amines, and melamine in milk and dairy products using an automatic solid-phase extraction system. J. Agric. Food Chem. 59 (2011) 7519–7520 |
| [8] | M. Aznar, E. Canellas, C. Nerín. Quantitative determination of 22 primary aromatic amines by cation-exchange solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 1216 (2009) 5176–5181 |
| [9] | C. Brede, I. Skjevrak, H. Herikstad. Determination of primary aromatic amines in water food simulant using solid-phase analytical derivatization followed by gas chromatography coupled with mass spectrometry. J. Chromatogr. A 983 (2003) 35–42 |
| [10] | R.R. Krishna, C.S.P. Sastry. A new spectrophotometric method for the determination of primary aromatic amines. Talanta 26 (1979) 861–865 |
| [11] | J.T. Stewart, T.D. Shaw, A.B. Ray. Spectrophotometric determination of primary aromatic amines with 9-chloroacridine. Anal. Chem. 41 (1969) 360–362 |
| [12] | S.K. Mortensen, X.T. Trier, A. Foverskov, J.H. Petersen. Specific determination of 20 primary aromatic amines in aqueous food simulants by liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. A 1091 (2005) 40–50 |
| [13] | D. Pezo, M. Fedeli, O. Bosetti, C. Nerín. Aromatic amines from polyurethane adhesives in food packaging:the challenge of identification and pattern recognition using quadrupole-time of flight-mass spectrometry. Anal. Chim. Acta 756 (2012) 49–59 |
| [14] | F. Akbarian, B.S. Dunn, J.I. Zink. Surface-enhanced Raman spectroscopy using photodeposited gold particles in porous sol-gel silicates. J. Phys. Chem. 99 (1995) 3892–3894 |
| [15] | V. Iancu, L. Baia, N. Tarcea, J. Popp, M. Baia. Towards TiO2-Ag porous nanocomposites based SERS sensors for chemical pollutant detection. J. Mol. Struct. 1073 (2014) 51–57 |
| [16] | X.X. Zou, R. Silva, X.X. Huang, J.F. Al-Sharab, T. Asefa. A self-cleaning porous TiO2-Ag core-shell nanocomposite material for surface-enhanced Raman scattering. Chem. Commun. 49 (2013) 382–384 |
| [17] | Q.Q. Li, Y.P. Du, Y. Xu, et al. Rapid and sensitive detection of pesticides by surfaceenhanced Raman spectroscopy technique based on glycidyl methacrylate-ethylene dimethacrylate (GMA-EDMA) porous material. Chin. Chem. Lett. 24 (2013) 332–334 |
| [18] | F. Svec, J.M.J. Frechet. Continuous rods of macroporous polymer as high-performance liquid chromatography separation media. Anal. Chem. 64 (1992) 820–822 |
| [19] | C.L. Yang, Y.L. Wei, Q.H. Zhang, et al. Preparation and evaluation of a large-volume radial flow monolithic column. Talanta 66 (2005) 472–478 |
| [20] | K.C. Grabar, R.G. Freeman, M.B. Hommer, M.J. Natan. Preparation and characterization of Au colloid monolayers. Anal. Chem. 67 (1995) 735–743 |
| [21] | M. Karnan, V. Balachandran, M. Murugan. FT-IR, Raman and DFT study of 5-chloro-4-nitro-o-toluidine and NBO analysis with other halogen (Br, F) substitution. J. Mol. Struct. 1039 (2013) 197–206 |
| [22] | S. Bilal, A.U.H.A. Shah, R. Holze. Raman spectroelectrochemical studies of copolymers of o-phenylenediamine and o-toluidine. Vib. Spectrosc. 53 (2010) 279–284 |
| [23] | T. Tanaka, A. Nakajima, A. Watanabe, T. Ohno, Y. Ozaki. Surface-enhanced Raman scattering spectroscopy and density functional theory calculation studies on adsorption of o-, m-, and p-nitroaniline on silver and gold colloid. J. Mol. Struct. 661- 662 (2003) 437–449 |
| [24] | M. Goodarzil, A.K. Malik, N. Goudarzi. Simultaneous spectrophotometric determination of nitroanilines using genetic-algorithm-based wavelength selection in principal component-artificial neural network. Afr. J. Pharm. Pharmacol. 6 (2012) 135–143 |
| [25] | A. Cavallaro, V. Piangerelli, F. Nerini, S. Cavalli, C. Reschiotto. Selective determination of aromatic amines in water samples by capillary zone electrophoresis and solid-phase extraction. J. Chromatogr. A 709 (1995) 361–366 |
| [26] | P. Sutthivaiyakit, S. Achatz, J. Lintelmann, et al. LC-MS/MS method for the confirmatory determination of aromatic amines and its application in textile analysis. Anal. Bioanal. Chem. 381 (2005) 268–276 |
| [27] | X.J. Liu, X.W. Chen, S. Yang, X.D. Wang. Comparison of continuous-flow microextraction and static liquid-phase microextraction for the determination of ptoluldine in Chlamydomonas reinhardtii. J. Sep. Sci. 30 (2007) 2506–2512 |
| [28] | A. Ashori, A. Sheibani. Homogeneous liquid-liquid extraction coupled to ion mobility spectrometry for the determination of p-toluidine in water samples. Bull. Environ. Contam. Toxicol. 94 (2015) 474–478 |
| [29] | P.F. Xiao, C.L. Bao, Q. Jia, et al. Determination of nitroanilines in hair dye using polymer monolith microextraction coupled with HPLC. J. Sep. Sci. 34 (2011) 675–680 |
| [30] | L. Zhang, J.M. You, G.C. Ping, et al. Analysis of aromatic amines by high-performance liquid chromatography with pre-column derivatization by 2-(9-carbazole)-ethyl-chloroformate. Anal. Chim. Acta 494 (2003) 141–147 |
| [31] | M. Akyüz, S. Ata. Simultaneous determination of aliphatic and aromatic amines in water and sediment samples by ion-pair extraction and gas chromatographymass spectrometry. J. Chromatogr. A 1129 (2006) 88–94 |
 2016, Vol. 27
2016, Vol. 27 


