b University of Chinese Academy of Sciences, Beijing 100190, China
The process of exocytosis is common to many different types of cells and central to understanding of the process of chemical intercellular communication [1, 2, 3, 4, 5]. Neurons secrete transmitter molecules packed in intracellular vesicles of 50-200 nm diameter,wherein membrane bound vesicles,storing transmitter molecules,release their contents into the extracellular space upon stimulation [6, 7, 8, 9]. Measuring transmitter released from individual vesicles with fast time resolution is essential to develop and test mechanistic models of exocytosis and endocytosis [10, 11, 12, 13].
Enormous efforts have been taken to develop new techniques for decades in order to deal with the challenges for determination of exocytosis of a neurotransmitter,particularly,by quantal release [10, 14, 15, 16, 17, 18, 19, 20, 21]. Ralph Adams is generally credited with being the first to implant a carbon fiber microelectrode (CFE) into brain of a rat with the objective of measuring the in vivo concentration of catecholamine [11, 22]. Electrochemical methods based on CFE could be pretreated to allow resolution of the neurotransmitter’s oxidation current,and as a result CFE has been applied to real-time monitoring of the instantaneous neurotransmitters release of single cell [12, 23, 24, 25, 26, 27]. For example,Wightman et al. used CFE with a diameter of ~1 mm to record secretion from a membrane area of < 1 mm2,which reveals that vesicular exocytosis catecholamines easily occurs at certain membrane regions near the sites of Ca2+ entry [23, 28, 29]. Due to the microelectrode fabrication process,the CFE is a manual craft with low-throughput,and the accuracy measured by the coefficient of variation is not good. Moreover,the CEF needs to be close to the aimed cell depending on the means of micromanipulator [15, 30].
The microelectrode array (MEA) fabricated by micro-electro mechanical system technology (MEMS) makes it possible to record cells simultaneously at different sites with high throughput [31, 32, 33, 34, 35, 36, 37, 38, 39]. The microelectrodes have been developed for exocytosis recordings,including chromaffine cells,brain slices and endothelial cells,where,for instance,Spégel et al. used an interdigitated electrode structure for recordings of dopamine exocytosis of PC12 cells [36] and Gillis’s group made an automated targeting of cells to electrochemical electrodes for quantal exocytosis [30, 34, 35, 36, 37, 38, 39, 40]. These works are important to improve the technology of detection of catecholamines releasing from a single cell. However,there are three significant demands requiring improvement using MEA for detecting quantal release; (1) the sensitivity of DA detecting with MEA should be high enough to measure very weak current response of the quantal exocytosis from single cell,(2) it does not bring the microelectrode to the cell with a micromanipulator,but one must bring the cell to the MEA initiatively,(3) it is essential that the electrode should be small (cell sized) enough to have an acceptably low level of current noise and to reduce the occurrence of overlapping spikes so that single spike parameters can be measured. Taking into account these problems,a gold/chromium (Au/Cr) MEA with a polymer film (poly L-lysine and poly dopamine,PLL-PDA-MEA) is rarely used as a sensor for detecting dopamine secreted from single PC12 cell.
In the present study,a novel MEA made by MEMS methods,which is modified with PLL-PDA,was designed for monitoring DA release from chromaffine cells. Calibration results showed the MEAs with good linear relationship and high sensitivity,meanwhile,the MEA was applied successfully for real-time monitoring quantal exocytosis from single PC12 cell.
2 Experimental 2.1. Reagents and instrumentationThe PC12 cells were cultured in the CO2 incubator (SANYO,Japan). Original PC12 cells were provided from Peking Union Medical College Hospital (Cell Resource Center,CAMS/PUMC,China). All reagents were used as received without further purification.Waterwas purified through aMichemUltrapureWater Apparatus (Michem,Chengdu,China). The standard cell buffer solution consisted of (in mmol L-1): 150 NaCl,2 CaCl2,1.2 MgCl2,5 KCl,11 glucose and 10 HEPES,pH7.2,freshly prepared prior to use. The high K+ solution consisted of (in mmol L-1): 150 NaCl,2 CaCl2,1.2 MgCl2,100 KCl,11 glucose and 10 HEPES,pH 7.2,freshly prepared prior to use. The phosphate buffer saline (PBS,0.1 mol L-1 Na2HPO4-NaH2PO4-KCl,pH 7.4) was prepared from a PBS tablet (Sigma). Cyclic voltammetry and I-T were performed on Autolab PGSTAT302N electrochemical work station (Autolab,Switzerland). The signals of exocytosis were recorded on Patch Clamp System (HEKA,Lambrecht,Germany). The analysis softwarewas Igor Pro 6.1 (WaveMatrics,USA).
2.2. Fabrication of PLL-PDA-MEAThe MEA,manufactured using standard lithographic processes,consists of an Au/Cr conductive layer and silicon nitride (Si3N4) insulating layer as shown in Fig. 1c. In brief,a standard commercialized glass was used as the substrate,cleaned in turn by acetone,ethanol,and deionized (DI) water,and then an Au/Cr film (200/30 nm) conductive layer was deposited after the first photolithographic step. The pattern of 60 circular microelectrodes with the diameter of 20 mm and the spacing of 100 mm is formed followed by lift-off process. Subsequently,a Si3N4 insulating layer (800 nm) was grown onto the substrate using plasma enhanced chemical vapor deposition before the microelectrode sites were etched in a SF6 - deep reactive ion etcher (SF6-DRIE) for 10 min at a power of 100 W,and the MEA is displayed in Fig. 1a and b) [30, 33]. The MEAs were irradiated by ultraviolet ray for 30 min incubation in the mixture of poly L-lysine (0.05 g L-1) and poly dopamine (1.0 g L-1).

|
Download:
|
| Fig. 1. The fabricated MEA and its structure. (a) An Au/Cr microelectrode array fabricated by MEMs technology. (b) The enlarged view of 60 working sites in the center of the microelectrode array. (c) Overview MEA structure diagram. | |
2.3. Electrochemical measurements of PLL-PDA-MEA
The MEA,after polymer coating,was electrochemically characterized using dopamine (DA) as a probe. Amperometry [41, 42, 43] was performed for DA. TheMEAwas measured in a Plexiglass ring glued to the MEA with an Autolab workstation connected to a computer. Cyclic voltammetry [44, 45, 46] (CV),amperometry and differential pulse voltammetry [47, 48, 49, 50, 51, 52, 53] (DPV) aremeasured for the performance in detection of quantal exocytosis. CVs are recorded at the MEA modified with a polymer filmin 0.25mmol L-1 K3[Fe(CN)6] solution at 10 mV s-1. Amperometric response of DA was performed on the MEA with PLL-PDA film at the working potential of 650 mV vs. Ag/AgCl. A three-electrode systemwith the selectedmicroelectrode was utilized as the working electrode,with an integrated Ag/AgCl as the reference electrode anda platinumwire as the counter electrode.
2.4. Cell preparationPC12 cells (2 mL) at a density of about 106 cells per mL were originally cultivated in T25 polystyrene cell culture flasks in 6 mL of culture media (Ham’s F12K medium supplemented with 10% fetal bovine serum and 5% horse serum) at 3 ℃ in a humidified atmosphere of 5% CO2/95% air. After 48 h,cells were harvested by trypsinization followed by gentle centrifugation and then was planted on the PLL-PDA-MEA filled with 2 mL culture medium. The PC12 cells were cultivated for 24 h before detection of the quantal exocytosis experiment.
2.5. Cell countingPC12 cells were trypsinized to single cell suspensions,and then 10 mL 0.4% trypan blue solution was added into 90 mL cell suspension. Gently pipetting the mixed cell suspension,10 mL of the mix was transferred to a counting chamber and,subsequently,the cells were observed and calculated under a microscope.
2.6. Measurement and analysis of quantal exocytosisAn EPC-10 patch-clamp amplifier with Patchmaster software was applied for recordings at r.t.,using our fabricated interface for connecting PLL-PDA-MEA with the patch-clamp amplifier. Amperometric measurements were performed at a sampling rate of 10 kHz and the potential of working site was maintained at 650 mV vs. Ag/AgCl after 20 mL K+ stimulation solution (that consists of 150 mmol L-1 NaCl,2 mmol L-1 CaCl2,1.2 mmol L-1 MgCl2,100 mmol L-1 KCl,11 mmol L-1 glucose and 10 mmol L-1 HEPES,pH 7.2) was injected into the MEA. As there are only two probes for our purchased HEKA instrument,another two separate electrodes were immediately measured,while two electrodes recorded. Data analysis of quantal release was performed on IgorProv. 6.1 (Wavematrics,Lake Oswego,OR,USA) using the threshold method. A quantal exocytosis event was defined as an amperometric spike should be six times at least than the standard deviation of the background noise. Several parameters are calculated from amperometric spikes. The T (pulse width for the current response of quantal exocytosis) represents the kinetics of the major DA content releasing from the membrane to the microelectrode surface,whereas Imax (the amplitude of the spike) and Q (the charge generated when neurotransmitter release each time) represent a measure of the vesicular DA content. All parameters were presented as mean ± SEM (standard error of the mean),respectively.
3. Results and discussion 3.1. Electrochemical characterization of the PLL-PDA-MEA 3.1.1. Cyclic voltammetric behaviors at the microelectrodeThe MEA displayed in Fig. 1a and b was electrochemically investigated using cyclic voltammetry (CV),amperometry and differential pulse voltammetry (DPV) for the purpose of optimization in the detection of quantal exocytosis. Fig. 2a shows CVs obtained from Au/Cr microelectrodes (20 mm in diameter) with PLL-PDA film,and without the film in 1 mmol L-1 K3[Fe(CN)6]. Recorded on film,a well behaved reversible redox wave of ferricyanide ion with peak potential separations of 53 mV were obtained. Its oxidation peak current and the reduced peak current were -64.32 nA,62.72 nA,respectively. However,the bare microelectrode does not show obvious oxidation and reduction peaks (as indicated on the inset in Fig. 2a),but a platform curve. Such a distinct response may be attributed that (1) the poly dopamine is pervious to an ion that reduces the difficulty of ferricyanic ions diffusing to the surface of the Au microelectrode; (2) the effective surface area of the microelectrode increased as the coated PLL-PDA film,and thus,contributing more ferricyanic ions to react in the same unit time. The effective surface area of the modified microelectrode was calculated to be 838.4 mm2 using the Randles-Sevcik equation,which is 2.67 times larger than that of the bare electrode (The geometric surface area of bare Au was 314.16 mm2).

|
Download:
|
| Fig. 2. The electrochemical performance of the MEA modified with PLL-PDA film. (a) CVs recorded at the modified microelectrode and the bare electrode in 1 mmol L-1 K3[Fe(CN)6] solution at a scanning rate of 10 mV s-1; (b) amperometric response of DA (whose concentration ranges from 1 mmol L-1 to 10 mmol L-1) on the MEA with PLL-PDA film at the working potential of 650 mV vs. Ag/AgCl. (c) The linearity of response current of DA is measured by the microelectrode with PLL-PDA. | |
3.1.2. Amperometric response of dopamine
The sensitivity of PLL-PDA-MEA for DA was then tested by adding DA solutions of different concentrations. The response current at the fixed potential of was recorded under working voltage of 650 mV vs. Ag/AgCl,as depicted in Fig. 2b. Data in Fig. 2c shows a good linear relationship between the current and DA concentration in the range of 1 mmol L-1 to 10 mmol L-1. The sensitivity of the MEA without the polymer film is 0.05 nA mmol-1 L-1,whereas that of PLL-PDA-MEA reaches as high as 0.14 nA mmol-1 L-1 (12659.24 mA L mmol-1 cm-2). Since the MEA fabrication procedure was highly rigorous,our research has determined that the PLL-PDA-MEA result in a linear regression curve fitted with correlation coefficient of 0.992. Each determination of the DA concentration was replicated 5 times,and results revealed the reproducibility of the amperometric response with the PLL-PDA-MEA. In addition,compared to the MEA without polymer film,the PLL-PDA-MEA has lower background current and higher steady current response throughout the entire injection period of different concentrations of DA. The comparison of the DA determinations on the proposed microelectrodes with other previously reports is summarized in Table 1. The present PLL-PDA-MEA demonstrates improved,or comparable performance for DA detection. The good performance may be attributed to the following: (1) as mentioned before,the effective surface area of the microelectrode increased as with the coated PLL-PDA film,thus contributing more DA to react in the same unit time; (2) The diameter of microelectrode is 20 mm which is similar to the cell’s size,therefore,the calculated current per unit area density will be high when the response current is the same.
|
|
Table 1 Comparison of analytical performance of the proposed modified MEA with previously reported DA electrodes modified with different materials. |
3.2. Selectivity of dopamine,5-hydroxytryptamine and ascorbic acid
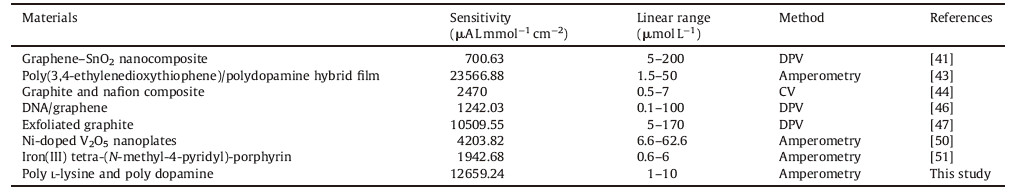
DA and other electro-active molecules such as ascorbic acid (AA) and 5-hydroxytryptamine (5-HT) coexist in the brain and their electrochemical oxidation peaks take place at very close potential [41, 43]. Thus,the determination on selectivity of DA,AA and 5-HT is important to measure the performance of an electrode. Fig. 3a displays DPV responds of different concentrations of DA in the presence of 800 mmol L-1 AA and 500 mmol L-1 5-HT at the PLL-PDA-MEA. Three well-defined oxidation peaks were observed at the potential of -241 mV,67 mV,and 263 mV,corresponding to AA,DA and 5-HT. The calculated peak separations of DA and AA,DA and 5-HT,AA and 5-HT are 308 mV,196 mV,and 504 mV,indicating that the PLL-PDA-MEA is reliable to differentiate DA free from AA and 5-HT. Similarly,the oxidation peak current of DA increased with concentration,increasing in the range of 125 nmol L-1 to 40 mmol L-1,whereas the voltammetric peak current of AA and 5-HT remained the same.
|
Download:
|
| Fig. 3. (a) DPV response of different concentrations of DA in the presence of 800 mmol L-1 AA and 500 mmol L-1 5-HT at the PLL-PDA-MEA. (b) Statistics of the cell density on the substrates of Au, PLL-PDA and AZ1500. | |
3.3. Biocompatibility of PLL-PDA-MEA
Biocompatible evaluation of the PLL-PDA film was addressed by culture of the PC12 cells. The cells were grown on the substrates of Au,PLL-PDA and AZ1500 (A positive photoresist which is frequently used in photolithography step of the MEA fabrication) for 48 h. The statistics of the cell density using counting chambers on the three substrates are shown in Fig. 3. Cells were found to adhere to and proliferate better on the PLL-PDA film than other substrates. Compared to the cells’ number of AZ1500 (5.5 × 105 mL-1),that of PLL-PDA achieves to 1.23 × 106 mL-1. Result indicates that the photoresist is poisonous to PC12 cells and therefore it should be cleaned before the MEA is used with culture cells. In order to determine the capability of capturing cells,we flushed gently PC12 cells from cultured MEA,and then counted cells adhering to the microelectrode. Some 44 among the 60 microelectrodes (73.3%) were covered with PC12 cells when using PLL-PDA. The results demonstrate that theMEA coated with PLL-PDA filmprovides good biocompatibility and capability of capturing cells.
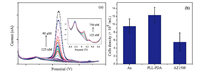
3.4. Monitoring of quantal exocytosisAs can be seen in Fig. 4a,the photomicrograph shows that among 15 micro-electrodes used in the experiment,11 electrodes are occupied by PC12 cells following cell washing with cell buffer solution consisting of 150 mmol L-1 NaCl,2 mmol L-1 CaCl2,1.2 mmol L-1 MgCl2,5 mmol L-1 KCl,11 mmol L-1 glucose and 10 mmol L-1 HEPES,pH 7.2. The MEA grown with PC12 cells was placed into the device shown in Fig. 1c,which has sixty connection pads corresponding with sixty microelectrodes of the MEA and combines with HKEA amplifier. We chose the microelectrode occupied by a single cell as the target for monitoring quantal exocytosis,the microelectrode 22 (ch22) and others in Fig. 4a for example.
|
Download:
|
| Fig. 4. Detection of dopamine release from PC12 cells. (a) Sample photomicrographs indicate that 11 of 15 microelectrodes are occupied by PC12 cells following cell washing with cell buffer solution. (b) Eight microelectrodes occupied with PC12 cells captured spikes at the potential of 650 mV vs. Ag/AgCl, with 100 mmol L-1 K+ stimulation solution, and no spikes were observed on microelectrode 23 in the absence of 100 mmol L-1 K+ stimulation solution at the potential of 650 mV vs. AgjAgCl. (c) Two kinds of typical spikes (S1 and S2) separately from the microelectrode 22, microelectrode 29. (d) The models represent the processes of two kinds of vesicular exocytosis. S1 is a rapid and full exocytosis event. S2 is exocytosis which occurs in a step-wise fashion: the initial event observed is the foot signal recorded from the initial leakage of dopamine from the membrane fused vesicle, which may then progress into the sharp fusion pore and release of the major dopamine content. | |
No quantal exocytosis events described as a succession of amperometric spikes were recorded from the PC12 cells in the absence of an appropriate stimulus,as the trace of the ch23 shown in Fig. 4b. PC12 cell was triggered to release dopamine by adding a 100 mmol L-1 K+ stimulation solution which provokes cell depolarization and influx of Ca2+ through specific-binding ion channels. Except for ch23,8 microelectrodes among the 11 microelectrodes occupied with PC12 cells captured spikes with the signal to noise ratio larger than 3:1. The recorded results,presenting many spikes,are partially overlapping in ch27,ch36 and ch41,the reason for this phenomenon is that a large number of vesicles release at the same moment,or in a very short time (several milliseconds). Two typical kinds of spikes (S1 and S2) have been captured as shown in Fig. 4c and the possible exocytosis mechanisms for the two kinds are described in Fig. 4d. S1 is that a rapid and full exocytosis event,which is an instantaneous swelling of the membrane-fused vesicle and consequent rapid dopamine release. S2 is that exocytosis which occurs in a step-wise fashion. The initial event observed is the foot signal recorded from the initial leakage of dopamine from the membrane fused vesicle,which may then progress into the sharp fusion pore and release of the major dopamine content. The proposed mechanism is consistent with several researches on the exocytosis [30, 35].
3.5. Analysis of spikesEach spike characterizes one vesicular event,its morphology and intensity reveals the continuous kinetics of exocytosis from a single PC12 cell [54]. Consequently,rigorous investigations of the exocytosis can be provided for many cell models and contribute to the questions and debates about the exocytotic mechanism. Several parameters are calculated from amperometric spikes by the software of Igor Pro 6.1 as the algorithm has been reported [35]. The statistical distributions of T (pulse width for the current response of quantal exocytosis) represents the kinetics of the major DA content releasing from the membrane to the microelectrode surface,whereas Imax (the amplitude of the spike) and Q (the charge generated when neurotransmitter release each time) represent a measure of the vesicular DA content. The histograms in Fig. 5 illustrate the distribution of three parameters measured in the amperometric mode for individual spikes during various quantal exocytosis. Note that the population of all three parameters is approximate Gaussian distribution. The meant T,Imax and Q of the amperometric spikes from the DA release in a PC12 cell stimulated is 23.8 ±7.5 ms (mean ± SEM),61.1 ± 14.1 ms and 0.42 ± 0.03 pC,respectively. On average,2.63 × 106 ±1.85 × 105 oxidizable molecules are released per quantal exocytosis,which can be obtained by calculation of Q by use of Faraday’s law.

|
Download:
|
| Fig. 5. Statistical analysis of 592 amperometric spikes characteristics from 21 PC12 cells. The statistical distributions depict (a) the amplitude of the spike (Imax), (b) the charge generated when neurotransmitter release each time (Q), (c) pulse width for the current response of quantal exocytosis (T). | |
The distribution of each parameter reveals that the spikes released from single PC12 cell are probably with specific features,and therefore,the distribution of each spike measured by two parameters was investigated. 592 spikes released from 21 PC12 cells (diameters of 11 ± 3 mm) were statistically analyzed in order to find out the most common combinations on parameters from spikes produced by this type of cell. Fig. 6 shows the frequency distribution of spikes measured by parameters of T and Imax. The statistical distribution reveals that the spike measured by T and Imax,the number of fired spikes,whose T ranges from 25.6 ms to 35.4 ms correspond to Imax ranging from 45.6 pA to 65.2 pA,accounts for 68% of the total,which indicates that the spikes released from single cell exist inherent biostatistical regulation and steady-going electronic characterization.

|
Download:
|
| Fig. 6. The frequency distributions of spikes measured by two parameters (T and Imax). | |
4. Conclusion
In this paper,we constructed a MEA modified with a polymer film for real time detection of quantal exocytosis from individual PC12 cells. To promote adhesion of a single target cell,the microelectrode array was modified with a poly L-lysine and poly dopamine (PLL-PDA) film. Electrochemical methods were employed to evaluate the performance of the microelectrodes,and results revealed that the microelectrodes modified by PLL-PDA improved the sensitivity of dopamine. The microelectrodes were successfully used to culture PC12 cells and detected quantal release of dopamine from a single cell with high concentration of K+ stimulation and each resultant spike was analyzed by four parameters. The statistical results of parameters revealed the population of each parameter was an approximate Gaussian distribution and exhibited mostly common combinations of parameters from spikes produced by this type of cell. The device represents a potential advantage for detecting multichannel quantal exocytosis of a single cell and studying the neural communication of cells.
| [1] | A.A. Auerbach. Spontaneous and evoked quantal transmitter release at a vertebrate central synapse. Nat. New Biol. 234 (1971) 181–183 |
| [2] | R.M. Wightman, J.A. Jankowski, R.T. Kennedy, et al. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Nat. Acad. Sci. U.S.A. 88 (1991) 10754–10758 |
| [3] | L.C. You, R.S. Cox Ⅲ, R. Weiss, F.H. Arnold. Programmed population control by cell-cell communication and regulated killing. Nature 428 (2004) 868–871 |
| [4] | M.B. Byrne, L. Trump, A.V. Desai, et al. Microfluidic platform for the study of intercellular communication via soluble factor-cell and cell-cell paracrine signaling. Biomicrofluidics 8 (2014) |
| [5] | R.A. Wallingford, A.G. Ewing. Amperometric detection of catechols in capillary zone electrophoresis with normal and micellar solutions. Anal. Chem. 60 (1988) 258–263 |
| [6] | V. Carabelli, S. Gosso, A. Marcantoni, et al. Nanocrystalline diamond microelectrode arrays fabricated on sapphire technology for high-time resolution of quantal catecholamine secretion from chromaffin cells. Biosens. Bioelectron. 26 (2010) 92–98 |
| [7] | S. Barg, C.S. Olofsson, J. Schriever-Abeln, et al. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron 33 (2002) 287–299 |
| [8] | V. Krizhanovsky, M. Yon, R.A. Dickins, et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134 (2008) 657–667 |
| [9] | N.R. Gandasi, S. Barg. Contact-induced clustering of syntaxin and munc18 docks secretory granules at the exocytosis site,. Nat. Commun 5 (2014) 3914 |
| [10] | R. Heidelberger, C. Heinemann, E. Neher, G. Matthews. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371 (1994) 513–515 |
| [11] | S. Sugiura, S. Nishimura, S. Yasuda, Y. Hosoya, K. Katoh. Carbon fiber technique for the investigation of single-cell mechanics in intact cardiac myocytes. Nat. Protoc. 1 (2006) 1453–1457 |
| [12] | J. Bae, M.K. Song, Y.J. Park, et al. Fiber supercapacitors made of nanowire-fiber hybrid structures for wearable/flexible energy storage. Angew. Chem. Int. Ed. 50 (2011) 1683–1687 |
| [13] | R.A. Normann. Technology insight:future neuroprosthetic therapies for disorders of the nervous system. Nat. Clin. Pract. Neurol. 3 (2007) 444–452 |
| [14] | M.A. Lebedev, A.J. Tate, T.L. Hanson, et al. Future developments in brain-machine interface research. Clinics 66 (2011) 25–32 |
| [15] | R.A. Normann, D.J. Warren, J. Ammermuller, E. Fernandez, S. Guillory. Highresolution spatio-temporal mapping of visual pathways using multi-electrode arrays. Vision Res. 41 (2001) 1261–1275 |
| [16] | M. Seo, M.A. Hillmyer. Reticulated nanoporous polymers by controlled polymerization-induced microphase separation. Science 336 (2012) 1422–1425 |
| [17] | A.B. Kibler, B.G. Jamieson, D.M. Durand. A high aspect ratio microelectrode array for mapping neural activity in vitro. J. Neurosci. Methods 204 (2012) 296–305 |
| [18] | H. Charkhkar, G.L. Knaack, B.E. Gnade, et al. Development and demonstration of a disposable low-cost microelectrode array for cultured neuronal network recording. Sens. Actuators B:Chem. 161 (2012) 655–660 |
| [19] | T. Brezesinski, J. Wang, S.H. Tolbert, B. Dunn. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 9 (2010) 146–151 |
| [20] | D.M. Omiatek, Y. Dong, M.L. Heien, A.G. Ewing. Only a fraction of quantal content is released during exocytosis as revealed by electrochemical cytometry of secretory vesicles. ACS Chem. Neurosci. 1 (2010) 234–245 |
| [21] | A. Elhamdani, H.C. Palfrey, C.R. Artalejo. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron 31 (2001) 819–830 |
| [22] | L. Wang, H.R. Xu, Y.L. Song, et al. Highly sensitive detection of quantal dopamine secretion from pheochromocytoma cells using neural microelectrode array electrodeposited with polypyrrole graphene. ACS Appl. Mater. Interfaces 7 (2015) 7619–7626 |
| [23] | R.M. Wightman, C.L. Haynes. Synaptic vesicles really do kiss and run. Nat. Neurosci. 7 (2004) 321–322 |
| [24] | S.H. Joo, S.J. Choi, I. Oh, et al. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 412 (2001) 169–172 |
| [25] | Z. Zhou, S. Misler. Amperometric detection of stimulus-induced quantal release of catecholamines from cultured superior cervical ganglion neurons. Proc. Nat. Acad. Sci. U.S.A. 92 (1995) 6938–6942 |
| [26] | R. Borges, M. Camacho, K.D. Gillis. Measuring secretion in chromaffin cells using electrophysiological and electrochemical methods. Acta Physiol. 192 (2008) 173–184 |
| [27] | C.R. Artalejo, M.E. Adams, A.P. Fox. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature 367 (1994) 72–76 |
| [28] | P.S. Cahill, R.M. Wightman. Simultaneous amperometric measurement of ascorbate and catecholamine secretion from individual bovine adrenal medullary cells. Anal. Chem. 67 (1995) 2599–2605 |
| [29] | E.L. Ciolkowski, B.R. Cooper, J.A. Jankowski, J.W. Jorgenson, R.M. Wightman. Direct observation of epinephrine and norepinephrine cosecretion from individual adrenal medullary chromaffin cells. J. Am. Chem. Soc. 114 (1992) 2815–2821 |
| [30] | S. Barizuddin, X. Liu, J.C. Mathai, et al. Automated targeting of cells to electrochemical electrodes using a surface chemistry approach for the measurement of quantal exocytosis. ACS Chem. Neurosci. 1 (2010) 590–597 |
| [31] | C.D. James, A.J.H. Spence, N.M. Dowell-Mesfin, et al. Extracellular recordings from patterned neuronal networks using planar microelectrode arrays. IEEE Trans. Biomed. Eng. 51 (2004) 1640–1648 |
| [32] | J. Wang, M.Y. Su, J.Q. Qi, L.Q. Chang. Sensitivity and complex impedance of nanometer zirconia thick film humidity sensors. Sens. Actuators B:Chem. 139 (2009) 418–424 |
| [33] | A. Fujishiro, H. Kaneko, T. Kawashima, M. Ishida, T. Kawano. In vivo neuronal action potential recordings via three-dimensional microscale needle-electrode arrays. Sci. Rep 4 (2014) 4868 |
| [34] | L.Q. Chang, C.X. Liu, Y.Z. He, H.H. Xiao, X.X. Cai. Small-volume solution currenttime behavior study for application in reverse iontophoresis-based non-invasive blood glucose monitoring. Sci. China Chem. 54 (2011) 223–230 |
| [35] | F. Patolsky, B.P. Timko, G.H. Yu, et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313 (2006) 1100–1104 |
| [36] | C. Spé gel, A. Heiskanen, S. Pedersen, et al. Fully automated microchip system for the detection of quantal exocytosis from single and small ensembles of cells. Lab Chip 8 (2008) 323–329 |
| [37] | D. Atlas. The voltage-gated calcium channel functions as the molecular switch of synaptic transmission. Annu. Rev. Biochem. 82 (2013) 607–635 |
| [38] | F. Segura, M.A. Brioso, J.F. Gómez, J.D. Machado, R. Borges. Automatic analysis for amperometrical recordings of exocytosis. J. Neurosci. Methods 103 (2000) 151–156 |
| [39] | Y. Nam, B.C. Wheeler. In vitro microelectrode array technology and neural recordings. Crit. Rev. Biomed. Eng. 39 (2011) 45–61 |
| [40] | Y.X. Huang, D. Cai, P. Chen. Micro- and nanotechnologies for study of cell secretion. Anal. Chem. 83 (2011) 4393–4406 |
| [41] | W. Sun, X.Z. Wang, Y.H. Wang, et al. Application of graphene-SnO2 nanocomposite modified electrode for the sensitive electrochemical detection of dopamine. Electrochim. Acta 87 (2013) 317–322 |
| [42] | X.F. Yu, K. Yue, I.F. Hsieh, et al. Giant surfactants provide a versatile platform for sub-10-nm nanostructure engineering. Proc. Nat. Acad. Sci. U.S.A. 110 (2013) 10078–10083 |
| [43] | R. Salgado, R. del Rio, F. Armijo. Selective electrochemical determination of dopamine, using a poly(3,4-ethylenedioxythiophene)/polydopamine hybrid film modified electrode. J. Electroanal. Chem. 704 (2013) 130–136 |
| [44] | S.H. Ku, S. Palanisamy, S.M. Chen. Highly selective dopamine electrochemical sensor based on electrochemically pretreated graphite and nafion composite modified screen printed carbon electrode. J. Colloid Interface Sci. 411 (2013) 182–186 |
| [45] | P. Miao, B.D. Wang, Z.Q. Yu, J. Zhao, Y.G. Tang. Ultrasensitive electrochemical detection of microRNA with star trigon structure and endonuclease mediated signal amplification. Biosens. Bioelectron. 63 (2015) 365–370 |
| [46] | X.F. Wang, Z. You, H.L. Sha, et al. Sensitive electrochemical detection of dopamine with a DNA/graphene bi-layer modified carbon ionic liquid electrode. Talanta 128 (2014) 373–378 |
| [47] | W.H. Cai, T. Lai, H.J. Du, J.S. Ye. Electrochemical determination of ascorbic acid, dopamine and uric acid based on an exfoliated graphite paper electrode:a high performance flexible sensor. Sens. Actuators B:Chem. 193 (2014) 492–500 |
| [48] | P. Miao, L. Liu, Y.J. Nie, G. Li. An electrochemical sensing strategy for ultrasensitive detection of glutathione by using two gold electrodes and two complementary oligonucleotides. Biosens. Bioelectron. 24 (2009) 3347–3351 |
| [49] | F. Chen, X.P. Jiang, T.R. Kuang, et al. Polyelectrolyte/mesoporous silica hybrid materials for the high performance multiple-detection of pH value and temperature. Polym. Chem. 6 (2015) 3529–3536 |
| [50] | R. Suresh, K. Giribabu, R. Manigandan, et al. New electrochemical sensor based on Ni-doped V2O5 nanoplates modified glassy carbon electrode for selective determination of dopamine at nanomolar level. Sens. Actuators B:Chem. 202 (2014) 440–447 |
| [51] | F.S. Damos, M.P.T. Sotomayor, L.T. Kubota, S.M.C.N. Tanaka, A.A. Tanaka. Iron(Ⅲ) tetra-(N-methyl-4-pyridyl)-porphyrin as a biomimetic catalyst of horseradish peroxidase on the electrode surface:an amperometric sensor for phenolic compound determinations. Analyst 128 (2003) 255–259 |
| [52] | W.B. Zhang, X.F. Yu, C.L. Wang, et al. Molecular nanoparticles are unique elements for macromolecular science:from "nanoatoms" to giant molecules. Macromolecules 47 (2014) 1221–1239 |
| [53] | G.E. Flynn, J.P. Johnson, W.N. Zagotta. Cyclic nucleotide-gated channels:shedding light on the opening of a channel pore. Nat. Rev. Neurosci. 2 (2001) 643–651 |
| [54] | R. Zweigerdt, R. Olmer, H. Singh, A. Haverich, U. Martin. Scalable expansion of human pluripotent stem cells in suspension culture. Nat. Protoc. 6 (2011) 689–700 |
 2016, Vol. 27
2016, Vol. 27